Abstract
Background and aims: Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) has high morbidity and mortality. In this study, the safety and efficacy of a modification of ALPPS (radiofrequency-assisted ALPPS, RALPPS) were assessed in patients with hepatocellular carcinoma (HCC).
Materials and methods: Patients who were diagnosed with HCC and were considered to have an insufficient future liver remnant (FLR) were enrolled. In stage I, a radiofrequency ablation (RFA) device was used to cauterise along the planned transection plane to form a coagulum avascular area. When the FLR reached above 40%, hepatectomy was performed in stage II along the coagulum area established previously. After two stages, operative morbidity, mortality, per cent increase in FLR, operative time and blood loss were evaluated.
Results: Between July 2014 and September 2015, 10 patients with HCC (9 with hepatitis-related cirrhosis) were treated with the RALPPS procedure. The incidence of severe complications (Clavien-Dindo ≥ IIIb) was 20% (2/10). One patient died. No biliary leakage, intraperitoneal infection or post-hepatectomy liver failure (PHLF) occurred after both stages. The median FLR before stage I was 31% (364 ml). This increased to 47% (632 ml) before stage II after a median interval of 28 days. The median percentage increase in FLR was 53% (210 ml). Additionally, the median operative time during the first and second stages was 214 and 281 min, respectively. The corresponding median blood loss was 200 and 550 ml, respectively.
Conclusions: RALPPS has a potential advantage in eliminating serious complications of biliary leakage and PHLF associated with classic ALPPS. On the basis of rigorous patient selection criteria, RALPPS may achieve the same effect of promoting significant growth of the FLR in patients with cirrhosis-related HCC and insufficient FLR volume, albeit at the cost of a longer interval time.
Introduction
Curative surgical resection, whenever possible, remains one of the best therapies to gain long-term benefits for liver tumour patients [Citation1,Citation2]. However, the future liver remnant (FLR) is a primary limiting factor for extensive hepatectomy. In normal livers, the FLR should be above 30%. For cirrhotic livers, it should be 40% or greater [Citation3]. Conventional methods, including portal vein embolisation or portal vein ligation (PVL) are used to induce the regeneration of FLR before extensive hepatectomy. However, they have the risk of poor regeneration and subsequent tumour progression [Citation4]. Studies have demonstrated the effect of cirrhosis on liver regeneration [Citation5], which further increased the risks mentioned above. As it could promote rapid regeneration in a short time, associating liver partition and portal vein ligation for staged hepatectomy (ALPPS) was considered as one of the creative breakthroughs in hepatobiliary surgery [Citation6,Citation7]. However, classic ALPPS has high morbidity and mortality [Citation8,Citation9]. Some researchers have optimised the approach of ALPPS with the use of radiofrequency ablation (RFA). This innovation has been labelled radiofrequency-assisted ALPPS (RALPPS) [Citation10]. However, case reports of RALPPS are few and all have been applied in treating metastatic liver cancer without accompanying cirrhosis [Citation10]. This report was to evaluate the safety and efficacy of RALPPS in patients with cirrhosis-related hepatocellular carcinoma (HCC).
Materials and methods
Patients
Clinical data were obtained from patients with primary HCC who underwent RALPPS at the Institute of Hepatobiliary Surgery of Southwest Hospital of Third Military Medical University between July 2014 and September 2015. This study complied with the Declaration of Helsinki and the research protocol was approved by the hospital ethics committee. All patients provided written informed consent before participating in the study.
All enrolled patients had a primary diagnosis of HCC according to the criteria issued by the American Association for the Study of Liver Disease (AASLD) [Citation11]. All patients had insufficient FLR volume (FLR <30% in healthy livers and <40% in injured livers, such as those with cirrhosis and cholestasis) [Citation3] and could not tolerate extensive liver resection. Treatment decisions were made by a multidisciplinary team of hepatobiliary surgeons, hepatologists, radiologists, oncologists and anaesthesiologists. Patients underwent preoperative contrast-enhanced 64-row computed tomography (CT) of the chest and abdomen to determine tumour stage. Positron emission tomography-CT was performed to exclude extrahepatic metastasis if necessary.
Liver assessment
The volume of total liver and FLR was determined by software Amira 4.1 (VSG Inc., Hillsboro, OR) preoperatively after contrast-enhanced CT scan, followed by weekly postoperative follow-up examinations to evaluate the changes in liver volume and FLR. FLR volume was assessed before (baseline; Vol 0) and after surgery (postoperative; Vol 1). The increase in FLR volume was calculated as (Vol 1 − Vol 0)/Vol 0 × 100%. The degree of fibrosis was classified according to the Metavir scoring system [Citation12].
Surgical approaches
Surgical strategy
Standard right hepatectomy was conducted with the planned future transection plane on the right side of the middle hepatic vein when the entire tumour was located within the right lobe of the liver. Extended right hepatectomy was conducted with the future transection plane on the left side of the middle hepatic vein when the tumour invaded the middle hepatic vein. Right trisectionectomy was conducted with the planned transection plane on the right of the falciform ligament when the tumour invaded the left medial lobe of the liver.
First-stage operation
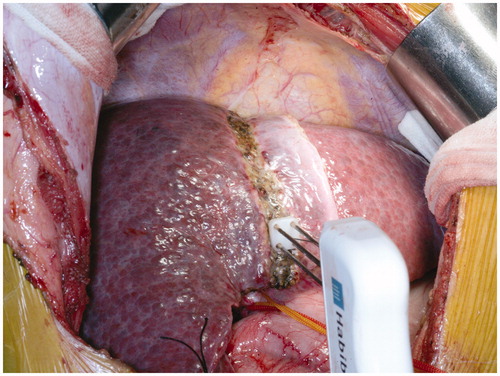
An inverted L-shaped incision was made from the right upper quadrant subcostal to the abdomen. Next, intraoperative ultrasound (IOUS) was performed to determine tumour conditions. After cholecystectomy, the right hepatic artery, right branch of portal vein and right hepatic duct were dissected without mobilising the liver. Then, the branch of the portal vein was ligated. After performing the liver hanging manoeuvre, a RFA device (Habib 4X, RITA 4401L, AngioDynamics Inc., Manchester, GA) was used to cauterise along the future transection plane to the front wall of the elastic band. In this way, an avascular area 2–3 cm in width was established between the FLR and contralateral lobe (. An absorbable suture encircled the right hepatic artery and right hepatic duct. This replaced the elastic band as a marker for stage II operation. No T-tube or adhesion barrier films were placed. A peritoneal drain was placed, followed by abdominal wall closure.
Assessment and treatment intervals
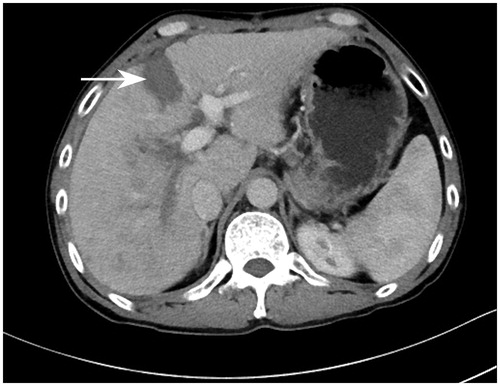
Assessment of FLR. After stage I, FLR volume was assessed weekly using contrast-enhanced CT to estimate the FLR volume until it reached ≥40% (. When the FLR was sufficient and patients were in stable condition, patients were eligible to undergo the second-stage operation.
Figure 2. Contrast-enhanced computer tomography (CT) on day 28 after stage I of RALPPS depicted a clear avascular area between the diseased hemiliver and FLR, which was formed by RFA (white arrow).
Tumour assessment. Tumour growth, necrosis and metastasis were evaluated according to contrast-enhanced CT or ultrasound. Patients with metastasis in the FLR or extrahepatic metastasis were not eligible to undergo the second-stage operation. Further interventional therapy or molecularly targeted drugs, such as sorafenib, were administered instead.
Liver function and systemic assessment. Postoperative liver function assessment included daily clinical assessment and blood tests, such as alanine transaminase (ALT), aspartate aminotransferase (AST), total bilirubin (TBIL) and prothrombin time (PT) assessments.
Second-stage operation
The second stage of the operation was performed using the previous incision to reach the abdomen. IOUS was performed to evaluate liver conditions, especially those of the FLR. After lifting the liver using the absorbable suture remaining in the retrohepatic tunnel during stage I, following the anterior approach, a clamp-crushing technique was used to partition the FLR and contralateral lobe along with the avascular area coagulated by RFA in stage I as well as to reach the front of the inferior vena cava. An endo-GIA linear vascular stapler was used to clip the right hepatic pedicle (hepatic artery, right branch of the portal vein and right hepatic ducts) and right hepatic vein. Then, the right hepatic ligament was severed to dissect the tumour-bearing liver tissue.
During liver resection, central venous pressure was maintained below 5 cm H2O. The residual liver was fixed to the anterior wall of the abdominal cavity using the falciform ligament. A drainage tube was placed and then the abdominal wall was closed.
Postoperative complications, mortality and follow-up
The Clavien-Dindo classification was used to evaluate postoperative complications [Citation13]. Grades ≥ IIIb were defined as severe complications. A “50–50 criteria” was used to identify post-hepatectomy liver failure (PHLF) [Citation14]. All deaths during hospitalisation were included in mortality, even if they did not appear related to the operation. After discharge, patients underwent monthly follow-up in the outpatient clinic of the Clinical Research Center of Southwest Hospital, including routine laboratory tests, tumour marker examination and imaging (upper abdominal CT or contrast ultrasound scanning).
Statistical analyses
Categorical variables were described in percentages. Normally distributed variables were presented as medians (range) and further analysed using the Student’s t-test. p < .05 was considered to be statistically significant. SPSS 19.0 software (SPSS, Chicago, IL) was used for the statistical analyses.
Results
Between July 2014 and September 2015, 10 patients with HCC underwent the RALPPS procedure. Nine patients were men. The median age was 41 years (range 33–60 years). All 10 patients had hepatitis B virus infection. Nine patients had liver cirrhosis and one had F3 class fibrosis according to the Metavir scoring system. All patients were initially diagnosed with HCC according to AASLD criteria. Except for one patient with two lesions, all tumours were single and located in the right lobe of the liver. The median maximum tumour diameter was 9.2 cm (range 6.4–15.4 cm). Five patients had macrovascular invasion: portal vein thrombosis (4) and hepatic vein thrombosis (2). All patients had Class A Child-Pugh scores. The median MELD (model for end-stage liver disease) score was 7 (range 0–19) [Citation15]. One patient had liver cancer resection and interventional treatment 20 weeks before surgery. Seven weeks before surgery, another patient underwent transcatheter arterial chemoembolization (TACE) treatment ().
Table 1. Clinical characteristics of patients undergoing RALPPS.
Postoperative morbidity and mortality
Two patients did not proceed to the second-stage operation. One patient had liver dysfunction and massive ascites after stage I. The other patient had metastasis in FLR tissue during the waiting period before stage II and did not proceed to the second-stage operation. The incidence of severe complications was 20%. Excluding these two patients, there was no > grade III complication after stage I of RALPPS. After stage II, 4 patients had complications including pleural effusion (3) and reversible liver dysfunction (1). All complications improved after conservative treatment. One patient who had previous chronic renal dysfunction prior to the operation died from kidney failure and severe pulmonary infection after stage II (). Hepatic recovery parameters after each stage are shown in .
Table 2. Postoperative morbidity and mortality of RALPPS.
Table 3. Postoperative liver function parameters after each stage of RALPPS.
Volumetric changes in the liver
The median preoperative FLR volume was 364 ml (range 234–606 ml). It increased to 632 ml (range 498–736 ml) before stage II. The median increase was 210 ml (161–377 ml), corresponding to a median increase of 53% (range 35–133%). The median preoperative FLR was 31% (range 19–37%). This increased to 47% (40–58%) before the RALPPS stage II. The median interval between the two operations was 28 days (range 13–31 days) ().
Table 4. Volumetric changes of the FLR and intraoperative data.
Intraoperative data
During stage I of RALPPS, the median blood loss was 200 ml (range 50–400 ml), and no patient underwent a red blood cell (RBC) transfusion. The median operative time was 214 min (range 166–270 min). The median RFA time was 10 min (range 8–15 min). One case was treated laparoscopically. Eight patients completed both stage operations, resulting in a study completion rate of 80%. During stage II, 4 patients underwent a right hepatectomy, 1 had an extended right hepatectomy and 3 had a right trisectionectomy. The median blood loss was 550 ml (range 300–1000 ml). One patient needed a blood transfusion (2 units of RBCs). The median operative time of stage II was 281 min (range 210–487 min). Regarding pathology, all eight cases had HCC. Three had poorly differentiated HCC, four had moderately differentiated HCC and one had well-differentiated HCC ( and ). The eight patients underwent complete surgical resection which revealed negative margins. The completeness of resection (R0) rate was 100%.
Follow-up
All patients, except for one who died in the hospital after stage II, were followed up for an average of 7 months (range 3–15 months). The two patients who did not complete stage II operations died within 3 months of discharge. Another two cases had tumour recurrence within 3 months of discharge. Of these, one died and the other underwent TACE with sorafenib. Another patient subsequently underwent TACE when local tumour recurrence was observed 9 months after discharge. Four cases have had no disease recurrence as of this publication.
Discussion
ALPPS is a promising procedure for patients with insufficient FLR volume after removal of large amounts of liver parenchymal tissue. It has improved the resection rate of patients with nonresectable disease previously. Unfortunately, it also has 22–87.5% operative morbidity and 12–28.7% mortality [Citation6,Citation16–18]. Complications of ALPPS mainly involved biliary leakage and resultant intraperitoneal infection [Citation19], primarily due to three reasons. (1) In stage I, two large raw surfaces were created during in situ dissection of the liver, which increased the incidence of biliary leakage. For patients with HCC complicated by cirrhosis, postoperative infection is often fatal [Citation20]. (2) Followed by in situ dissection, stage I of ALPPS included mobilisation of the liver [Citation21]. Postoperative abdominal adhesions were usually extremely widespread, which increased the difficulty of the second-stage operation. (3) During parenchymal transection in stage I, patients may have experienced bleeding or needed repeated portal occlusion, which may have greatly affected liver function and postoperative recovery. Previous work confirmed that using the Pringle manoeuvre in stage I affected FLR growth [Citation22].
In ALPPS, the FLR could regenerate rapidly, mainly secondary to (1) the redistribution of hepatic inflow after PVL; (2) an inflammatory-like reaction stimulated by liver surgical trauma to mediate the release of several hepatotrophic factors and accelerate FLR growth and (3) transection of bilateral lobes of the liver, preventing collateral circulation formation and further increasing the hepatic inflow to the FLR [Citation3,Citation6,Citation7]. Recent studies have demonstrated that in situ liver partitioning in ALPPS is not necessary for the rapid growth of FLR. Partial liver transection (50–80%, partial-ALPPS) could create a more pronounced parallel effect in promoting FLR rapid regeneration than complete transaction could [Citation23]. Another animal experiment also confirmed that in addition to liver parenchyma transection, injury to other organs (lung, kidneys or spleen) also stimulated the production of hepatotrophic factors. Combined with PVL, this also promoted rapid growth of the FLR [Citation24]. For this reason, theoretically and practically speaking, in situ partitioning of the liver that produces two large raw surfaces in ALPPS during stage I operation is not necessary for FLR regeneration.
To eliminate the aforementioned drawbacks of classic ALPPS, we used RFA, a widely used technique [Citation25], to modify ALPPS: in situ partitioning was replaced with RFA to form a coagulum avascular area between the FLR and collateral lobe in stage I, which could achieve the same purpose of preventing bilateral blood supply. The coagulum area was not must to the level of vena cava, just like the partial-ALPPS. Data showed that RALPPS was better than ALPPS in stage I () with regard to (1) prevention of hepatic transection formation and resultant biliary leakage; (2) reduction of intraoperative blood loss and operative time; (3) limitation of the invasiveness and the adhesion of the peritoneal cavity () and (4) prevention of the need for the Pringle manoeuver, to prevent further damage to liver function. These advantages reduced the complexity of surgery and the incidence of complications, thus creating a stable foundation for RALPPS stage II. In this study, the absence of biliary leakage or subsequent intraperitoneal infection after either stage of the operation confirmed the aforementioned advantages of RALPPS.
Figure 3. During stage II of RALPPS, the peritoneal inflammatory response decreased and adhesions were few because the liver was not mobilised in stage I.
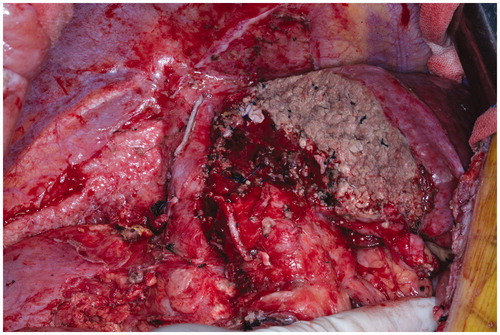
Table 5. Literature review.
According to the first report of the International ALPPS Registry, which covered 202 cases, the two most important independent risk factors associated with severe complications and that could be artificially controlled were blood transfusion and the >300 min operative time in stage I (OR values were 5.2 and 4.4, respectively) [Citation22]. Compared with the literature, we found that RALPPS involved reduced operative time (214 min vs. 327 min) and conditions for blood transfusion (0% vs. 28%; 2 units vs. 3 units). The morbidity of RALPPS was slightly lower than reported in the literature (20% vs. 27%, ≥grade III b) as one patient succumbed to his pre-existing underlying disease rather than any direct complication of the operation.
The RALPPS procedure resembles the partial-ALPPS to some extent [Citation23]. Similar to the results of the RALPPS, partial-ALPPS was not only associated with zero operation-related mortality, but also with a favourable postoperative morbidity profile after stage I operation. The results of both procedures confirmed the hypothesis that the systemic and local infection reaction and the resultant inflammatory factors after liver trauma play a more important role than the liver parenchyma per se in promoting the regeneration of the FLR [Citation24]. However, in the RALPPS, there was no need to transect the liver parenchyma, which could further reduce the risk of biliary leakage and subsequent intraperitoneal infection.
Additionally, not mobilising the liver in stage I and following the anterior approach for surgical resection in stage II complied with the “no touch” principle. Reducing intraoperative blood loss and perioperative RBC transfusion also could increase long-term survival after hepatic resection [Citation29].
To date, only two studies have reported on the RALPPS surgical treatment. They covered a total of seventeen patients with colorectal cancer liver metastases (CRLM) [Citation10,Citation26]. To the best of our knowledge, this is the first study to offer an evaluation using RALPPS to treat HCC with accompanying liver cirrhosis.
To date, ALPPS is commonly performed in patients with CRLM, whereas ALPPS for HCC with accompanying cirrhosis has rarely been reported [Citation28,Citation30–34]. There were nine patients with cirrhosis-related HCC in this study. The FLR growth rate herein was lower than in other studies (53% vs. 75–89.7%) [Citation6,Citation22,Citation35] and the interval time was longer (28 days vs. 7–9 days). The reasons for these differences are illustrated as follows: (1) FLR should be at a recommended safer level of least above 40%; (2) cirrhosis affects the rate and degree of liver regeneration, resulting in slower growth than in livers without cirrhosis and (3) patients had poor liver reserve function and higher PHLF incidence and mortality, which required a more conservative decision on time to perform the stage II of RALPPS. Additionally, the rate of drop-off was higher than that in cases of metastatic hepatic carcinoma without cirrhosis (20% vs. 0–5%) as two patients did not complete the operations because of either insufficient FLR growth during the interval period or hepatic dysfunction [Citation6,Citation36].
As this is an initial report, the work is limited by being an uncontrolled study with a small sample size. As a learning curve existed, the criteria of patients enrolled in the early period of this study were not very strict. As a result, there was one case of in-hospital death. The two cases who did not complete both stages were also in the early period. It is important and necessary to determine the greatest risk factors for poor regeneration of FLR leading to dropouts before stage II in cirrhosis-related HCC patients. Additionally, more studies are needed to investigate the differences between RFA-based and conventional hepatic transection in partitioned liver techniques. We are conducting this work and have registered it at www.clinicaltrial.gov (NCT 02299843).
Conclusion
This initial report demonstrated that the modified RALPPS procedure may have an advantage in eliminating the serious classic ALPPS complications of biliary leakage and PHLF. Using rigorous patient selection criteria, RALPPS may achieve the same effect of promoting significant growth of FLR when applied to patients with cirrhosis-related HCC and insufficient FLR volume, albeit at the cost of longer interval time.
Acknowledgements
The authors would like to thank Yunhua Tan and Yujun Ji for their technical support and valuable discussion.
Disclosure statement
The authors declare no conflicts of interest.
Additional information
Funding
References
- Hocquelet A, Balageas P, Laurent C, et al. (2015). Radiofrequency ablation versus surgical resection for hepatocellular carcinoma within the Milan criteria: a study of 281 Western patients. Int J Hyperthermia 31:749–57.
- Tang T, Feng X, Yan J, et al. (2014). Predictive value of indocyanine green retention rate with respect to complications of radiofrequency ablation in 878 patients with hepatocellular carcinoma. Int J Hyperthermia 30:402–7.
- Alvarez FA, Ardiles V, Sanchez Claria R, et al. (2013). Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): tips and tricks. J Gastrointest Surg 17:814–21.
- Schadde E, Malago M, Hernandez-Alejandro R, et al. (2015). Monosegment ALPPS hepatectomy: extending resectability by rapid hypertrophy. Surgery 157:676–89.
- van Lienden KP, van den Esschert JW, de Graaf W, et al. (2013). Portal vein embolization before liver resection: a systematic review. Cardiovasc Interv Radiol 36:25–34.
- Schnitzbauer AA, Lang SA, Goessmann H, et al. (2012). Right portal vein ligation combined with in situ splitting induces rapid left lateral liver lobe hypertrophy enabling 2-staged extended right hepatic resection in small-for-size settings. Ann Surg 255:405–14.
- de Santibanes E, Clavien PA. (2012). Playing Play-Doh to prevent postoperative liver failure: the “ALPPS” approach. Ann Surg 255:415–17.
- Aloia TA. (2015). Insights into ALPPS. Eur J Surg Oncol 41:610–11.
- Figueras J, Belghiti J. (2014). The ALPPS approach: should we sacrifice basic therapeutic rules in the name of innovation? World J Surg 38:1520–1.
- Gall TM, Sodergren MH, Frampton AE, et al. (2015). Radio-frequency-assisted Liver partition with portal vein ligation (RALPP) for liver regeneration. Ann Surg 261:e45–6.
- Bruix J, Sherman M. (2005). Management of hepatocellular carcinoma. Hepatology 42:1208–36.
- Goodman ZD. (2007). Grading and staging systems for inflammation and fibrosis in chronic liver diseases. J Hepatol 47:598–607.
- Clavien PA, Barkun J, de Oliveira ML, et al. (2009). The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–96.
- Balzan S, Belghiti J, Farges O, et al. (2005). The “50-50 criteria” on postoperative day 5: an accurate predictor of liver failure and death after hepatectomy. Ann Surg 242:824–9.
- Kamath PS, Wiesner RH, Malinchoc M, et al. (2001). A model to predict survival in patients with end-stage liver disease. Hepatology 33:464–70.
- Dokmak S, Belghiti J. (2012). Which limits to the “ALPPS” approach? Ann Surg 256:e6; author reply e16–17.
- Li J, Girotti P, Konigsrainer I, et al. (2013). ALPPS in right trisectionectomy: a safe procedure to avoid postoperative liver failure? J Gastrointest Surg 17:956–61.
- Nadalin S, Capobianco I, Li J, et al. (2014). Indications and limits for associating liver partition and portal vein ligation for staged hepatectomy (ALPPS). Lessons learned from 15 cases at a single centre. Z Gastroenterol 52:35–42.
- Bertens KA, Hawel J, Lung K, et al. (2015). ALPPS: challenging the concept of unresectability – a systematic review. Int J Surg 13:280–17.
- Yang T, Tu PA, Zhang H, et al. (2014). Risk factors of surgical site infection after hepatic resection. Infect Control Hosp Epidemiol 35:317–20.
- Gauzolino R, Castagnet M, Blanleuil ML, Richer JP. (2013). The ALPPS technique for bilateral colorectal metastases: three “variations on a theme”. Updates Surg 65:141–8.
- Schadde E, Ardiles V, Robles-Campos R, et al. (2014). Early survival and safety of ALPPS: first report of the International ALPPS Registry. Ann Surg 260:829–36; discussion 836–8.
- Petrowsky H, Gyori G, de Oliveira M, et al. (2015). Is partial-ALPPS safer than ALPPS? A single-center experience. Ann Surg 261:e90–2.
- Schlegel A, Lesurtel M, Melloul E, et al. (2014). ALPPS: from human to mice highlighting accelerated and novel mechanisms of liver regeneration. Ann Surg 260:839–46. discussion 846–7.
- Zhang F, Wu G, Sun H, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma in elderly patients fitting the Milan criteria: a single centre with 13 years experience. Int J Hyperthermia 30:471–9.
- Edmondson MJ, Sodergren MH, Pucher PH, et al. (2016). Variations and adaptations of associated liver partition and portal vein ligation for staged hepatectomy (ALPPS): many routes to the summit. Surgery 159:1058–72.
- Schadde E, Ardiles V, Slankamenac K, et al. (2014). ALPPS offers a better chance of complete resection in patients with primarily unresectable liver tumors compared with conventional-staged hepatectomies: results of a multicenter analysis. World J Surg 38:1510–19.
- D’Haese JG, Neumann J, Weniger M, et al. (2016). Should ALPPS be used for liver resection in intermediate-stage HCC? Ann Surg Oncol 23:1335–43.
- Quesada R, Poves I, Berjano E, et al. (2017). Impact of monopolar radiofrequency coagulation on intraoperative blood loss during liver resection: a prospective randomized controlled trial. Int J Hyperthermia 33:135–141.
- Chan AC, Poon RT, Chan C, Lo CM. (2016). Safety of ALPPS procedure by the anterior approach for hepatocellular carcinoma. Ann Surg 263:e14–16.
- Xiao L, Li JW, Zheng SG. (2015). Totally laparoscopic ALPPS in the treatment of cirrhotic hepatocellular carcinoma. Surg Endosc 29:2800–1.
- Vennarecci G, Laurenzi A, Levi Sandri GB, et al. (2014). The ALPPS procedure for hepatocellular carcinoma. Eur J Surg Oncol 40:982–8.
- Cai X, Peng S, Duan L, et al. (2014). Completely laparoscopic ALPPS using round-the-liver ligation to replace parenchymal transection for a patient with multiple right liver cancers complicated with liver cirrhosis. J Laparoendosc Adv Surg Tech A 24:883–6.
- Cavaness KM, Doyle MB, Lin Y, et al. (2013). Using ALPPS to induce rapid liver hypertrophy in a patient with hepatic fibrosis and portal vein thrombosis. J Gastrointest Surg 17:207–12.
- Alvarez FA, Ardiles V, de Santibanes M, et al. (2015). Associating liver partition and portal vein ligation for staged hepatectomy offers high oncological feasibility with adequate patient safety: a prospective study at a single center. Ann Surg 261:723–32.
- Torres O, Fernandes E, Oliveira C, Lima C. (2013). Associating liver partition and portal vein ligation for staged hepatectomy (ALPPS): the Brazilian experience. Arq Bras Cir Dig 26:40–3.