Abstract
Objective: The purpose of this study was to evaluate the outcomes of a novel technique consisting of ultrasound-guided percutaneous arterial embolisation (PAE) of the tumour-feeding artery before radiofrequency ablation (RFA) for hypervascular hepatocellular carcinoma (HCC).
Methods: Twenty-three HCC patients with hypervascular tumours who were non-TACE candidates were enrolled in the study. The mean size of the tumours was 4.6 ± 1.2 cm (3.2–6.0 cm). PAE was performed using 20-gauge catheter needles under greyscale and colour ultrasound guidance. Regular follow-up was performed to evaluate the outcomes and safety of this novel method.
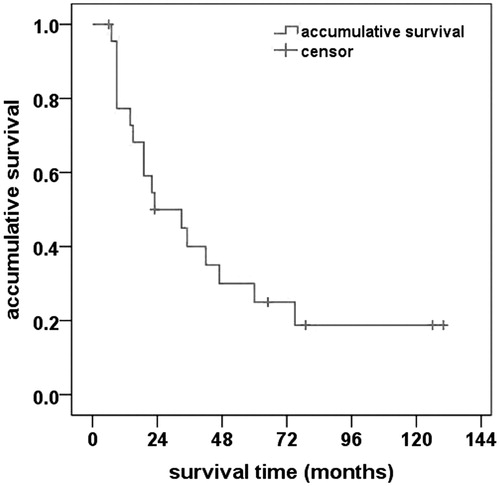
Results: Post-PAE colour Doppler ultrasound revealed an immediate embolising effect in 29 feeding arteries, including 21 arteries that became invisible (72.4%) and 8 arteries that exhibited reduced flow velocity (27.6%). Based on the one-month enhanced CT/MRI, complete necrosis was achieved in 24 of 25 tumours (96.0%). The mean follow-up period was 42.7 ± 9.8 months. Local tumour recurrence was observed in 3 tumours (12.0%), and new intrahepatic tumours developed in 9 patients (39.1%). The probabilities of overall survival at 1, 3, 5 and 10 years were 77.3%, 40.0%, 25.0% and 18.8%, respectively. No major complications occurred in this group.
Conclusions: The PAE method could help effectively to reduce or inhibit the blood supply of HCC. RFA followed by PAE treatment achieved 25.0% 5-year survival with no major complications. As a minimally invasive approach, PAE may provide a novel local therapy for hypervascular HCC patients who are non-TACE candidates.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumours in the world, causing almost 800 000 new liver cancer cases and 700 000 deaths every year [Citation1]. In recent years, various local therapies for HCC, especially radiofrequency ablation (RFA), have been proven safe and effective [Citation2–6]. One limitation of RFA is that its therapeutic impact is partly compromised by the blood flow cooling effect, especially in hypervascular HCC [Citation7,Citation8]. Recent studies have shown that TACE and RFA combined therapy decreases HCC blood supply and increases the ablated volume, thereby improving the outcome [Citation9,Citation10]. However, TACE is limited in patients who cannot tolerate this therapy due to the side effects of repeated TACE [Citation11,Citation12], have poor liver function or have previously undergone hepatic artery ligation. The present study focussed on “difficult-to-treat” HCC patients who were neither surgical candidates nor TACE candidates. The purpose of this study was to evaluate the feasibility, efficiency and safety of a novel technique consisting of ultrasound-guided percutaneous arterial embolisation (PAE) of the tumour-feeding artery before RFA for hypervascular HCC.
Patients and methods
Patients
The institutional review board of the Peking University Cancer Hospital approved this study. A detailed written description of the procedure was provided to all patients before enrolment, and informed consent was obtained before treatment. We retrospectively analysed the clinical and follow-up data.
The patient inclusion criteria for PAE were as follows: (1) non-surgical candidate; (2) non-TACE candidate due to previous TACE failure (a lack of radiologic response post-procedure), previous ligation of hepatic artery or poor liver function; (3) hypervascular HCC with obvious feeding vessels and a maximal flow velocity >50 cm/s; (4) absence of significant arterio-venous shunting; (5) tumour size 3.0–6.0 cm, tumour number <3 and at least one tumour confirmed as HCC by pathology; and (6) patient able to control his breath during the procedure.
In addition, the criteria for treatment with percutaneous RFA included the following: (1) accessibility of tumours via a percutaneous approach, (2) platelet count ≥50 000/ml and INR <1.6, (3) absence of tumour thrombus in the main portal veins and branches or main hepatic veins and (4) absence of extensive extrahepatic metastasis.
From July 2004 to September 2006, 25 consecutive HCC patients met the above criteria and were enrolled in this study. Among them, PAE failed in 3 arteries of two patients because the vessel was too small to puncture or the punctured vessel could not be differentiated from hepatic vein branches. The remaining 23 patients received successful PAE in 29 feeding arteries before RFA and were considered the PAE group. This group included 18 males and 5 females aged 30–79 years (average: 56.5 ± 14.9 years). The average tumour size was 4.6 ± 1.2 cm (range 3.2–6.0 cm). The mean number of tumours was 1.2 ± 0.7 (range: 1–2 tumours). Eleven patients had Child-Pugh class A liver function, and 12 patients had Child-Pugh class B liver function. Eleven patients had elevated AFP before the procedure, and four of them had levels greater than 400 ng/ml. The reasons these patients were not candidates for TACE included the following: previous TACE failure (n = 12), history of hepatic resection (n = 5), history of liver transplantation (n = 3) or poor liver function (n = 3). These patients received PAE with Lipiodol to block the tumour-feeding artery and subsequently underwent RFA. Based on different treatment plans, these 23 patients were then divided into two subgroups. The first 11 patients had RFA 7–10 days following PAE (subgroup A). The remaining 12 patients received RFA immediately after PAE (subgroup B). summarises the characteristics of these 23 patients. Extrahepatic metastasis was observed in four patients (18.4%), including bone metastasis (n = 2) and lung metastasis (n = 2). These 4 patients had local control (resection of local bone metastasis) and stable disease (chemotherapy for lung metastasis) before RFA treatment.
Table 1. Clinical characteristics of the patient population.
Ultrasound-guided RFA instruments
Ultrasound systems Logiq 9 (GE, Milwaukee, WI), Technos DU8 (Esaote, Milan, Italy), and IU22 (Philips, Amsterdam, Netherlands) were used for examination, PAE guiding and patient monitoring. The Logiq 9 ultrasound system (GE) was used to guide the RFA procedure. The probe frequency ranged from 3 to 5 MHz.
For RFA treatment, the RITA-1500 (Model 1500; RITA Medical System, Mountain View, CA) ablation system with a 460-KHz generator was used. Expandable electrodes consisted of an outer insulated needle with a 14-gauge calibre and a length of 15–20 cm. Nine prongs were deployed and retracted by a movable hub, and the deployment diameter ranged from 3 to 5 cm. The time to produce a 5-cm ablation sphere was approximately 20 min.
PAE procedure
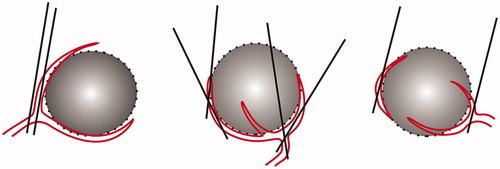
PAE was performed via a collaboration of two radiologists with at least 8 years of experience in interventional ultrasound (C.M.H. and Y. K.). Pre-PAE ultrasound of the liver with colour Doppler imaging was conducted for each patient after confirmation of the HCC location and nearby vascular structures. Spectral Doppler ultrasound was used to identify the feeding arteries as PAE-target vessels. The flow velocities of these vessels were measured by placing the sample volume into the main feeding artery of the tumour. The velocity curves were analysed using the commercial analysis software supplied with the ultrasound system. The maximal velocity was measured at the tallest peak of the flow curve. The hepatic artery–venous shunt was also assessed using spectral Doppler ultrasound (blood flow curves have both arterial and venous patterns). The target vessel had a high flow velocity and was located far from the hepatic vein and the accompanying portal vein branches. The arterial puncture site was typically proposed on the lateral or posterior side of the tumour, the entrance of feeding artery where the artery entered the tumour and away from centre of the tumour. Arterial puncture was performed at its largest segment with an angle of 0–60 degrees between the needle and vessel ().
Figure 1. Schema of the needle puncture for PAE in HCC patients. The arterial punctual site was usually proposed on the lateral or posterior side of the tumour, away from the tumour centre. The arterial puncture was made at its largest segment with angle of 0–60 degrees between the needle and vessel. PAE: percutaneous artery embolisation.
The patient was in the supine or lateral position according to the puncture angle. After local anaesthesia of 10–15 ml of 1% lidocaine (Liduokayin; Yimin, Beijing, China), a 20- to 21-gauge catheter needle (<45 degree tip slope, 0.8–0.9 mm outer diameter, 15–20 cm long) (Hakko, Tokyo, Japan) was inserted under real-time ultrasound guidance with the patients holding their breath. When the artery was punctured, colour ultrasound revealed that the flow was temporally disturbed or disappeared. Backflow of a flesh red colour from the 2-ml glassy injector helped confirm the puncture of the artery. Next, 1 ml of shaken saline was injected to confirm the location of the needle tip. The needle was rotated so that its tip sloped toward the tumour, and the embolising agent was injected slowly with pressure of approximately 1 ml/30–60 s. Repeated puncture was performed if the first attempt failed.
Lipiodol (Guerbet, Roissy France) was used as the embolising agent for the PAE procedure. In total, 3–12 ml of Lipiodol was injected into the feeding arteries for each patient. Depending on the tumour size, a dosage of 4–6 ml per artery was planned for <5-cm tumours and 7–10 ml for 5- to 6-cm tumours. The injection was stopped when the intra-artery injection exhibited high resistance. Then, the remaining Lipiodol in the injector was deposited around the artery in the tumour. The Lipiodol dosage was decreased in HCC patients who had Child-Pugh B liver function.
During the injection, the patient breathed calmly and repeated confirmation of the needle position was needed. Real-time ultrasound was used to monitor the Lipiodol distribution (hyperechoic) in the tumour and liver. The patients’ responses, including cough and choking, were closely monitored, and care was taken to ensure that the embolisation agent did not flow into hepatic veins and cause lung embolisation. After the injection, the needle was withdrawn, taking care to avoid drug leakage. Each of the two operators conducted ultrasound monitoring, needle handling and injection. Each procedure was completed in approximately 20–30 min.
The patient’s vital signs were monitored during the procedure. Colour ultrasound immediately after PAE was performed in all patients, and the lack of flow or decreased flow (decreased value >25 cm/s) on spectral Doppler ultrasound in the same section was considered to indicate effective embolisation. In subgroup A, all patients underwent 24-h post-PAE CT to assess Lipiodol deposits in the tumour and liver. In subgroup B, contrast-enhanced ultrasound (CEUS) was used to evaluate tumour perfusion after PAE. SonoVue (Bracco, Milan, Italy) was used as the contrast agent.
RFA procedure
RFA was conducted by the same two radiologists in the subsequent 7–10 days (subgroup A) or in the subsequent 30 min (subgroup B). The patient was under conscious sedation during the procedure, and an anaesthetist monitored the patient’s vital signs. Moderate sedation anaesthesia was induced with the intravenous administration of 2.5–5.0 mg of midazolam (Roche; Basel, Switzerland) and 50–100 μg of fentanyl (Fentaini; Renfu, Yichang, China). Some patients with tumours adjacent to the diaphragm, hepatic hilum or ligament felt obvious local and right shoulder pain when the ablation was extended. Intravenous infusion of propofol (Diprivan; Zeneca, Macclesfield, United Kingdom) (1–2 mg/kg) was used to temporarily enhance anaesthesia.
The patients were conscious when the electrode was placed. Their vital signs, including blood pressure, heart rate, respiration rate and oxygen saturation, were continuously monitored during the procedure. These patients were moved to inpatient rooms 1–2 h after treatment if there was no evidence of active bleeding visible on the ultrasound scans. Generally, the patients were hospitalised for 1–3 days after the RFA procedure.
Treatment efficacy evaluation and follow up
All patients underwent contrast-enhanced CT/MRI/ultrasound 1 month after RFA. The tumour was considered to exhibit complete necrosis if no abnormal enhancement or wash-out was observed in or around the tumour. Complete blood cell counts (CBCs), hepatic function tests and AFP tests were examined 3 days, 1 week and 1 month after treatment. Subsequently, the patients were monitored regularly for intrahepatic recurrence in the outpatient clinic according to a follow-up protocol including serum AFP, abdominal ultrasound and enhanced CT/MRI every 2–3 months for the first year and then AFP every 2–3 months and abdominal ultrasound and enhanced CT/MRI every 4–6 months after the first year. All patients were followed up for 6–130 months after the initial treatment (mean: 42.7 ± 9.8 months). Local recurrence was diagnosed when abnormal enhancement and wash-out tissue was observed in or around the ablated area on the enhanced imagines. New nodules at other sites of the liver were considered new tumours.
Statistical analysis
The significance of differences in the baseline characteristics and treatment results was assessed using Chi-squared test, Fisher’s exact probability test and independent-sample t test. Overall survival duration was counted in months from the date of RFA to death. Survival curves were evaluated using a Kaplan–Meier model and were compared using the log-rank test. SPSS statistical analysis software (SPSS, Chicago, IL) was used to perform statistical analyses.
Results
PAE results
PAE was successfully performed in 23 of 25 patients (92.0%). Among them, 3 of the arteries (10.3%) were punctured twice. The arteries had a maximum flow velocity of 71.4 ± 15.7 cm/s (range: 53.0–116 cm/s) and RI = 0.75 ± 0.1 (range: 0.55–0.92). Colour Doppler immediate post-PAE revealed effective embolisation in the 29 arteries, including 21 arteries (72.4%) lacking flow and 8 arteries (27.6%) exhibiting reduced flow velocity.
In subgroup A, 24-h post-PAE CT indicated intratumor iodised oil deposits. Seven patients (63.6%) had dense Lipiodol deposits inside the tumour, and 4 patients (36.4%) had Lipiodol deposits inside the tumour and in the normal liver tissue. Ultrasound examination 3–10 days after PAE revealed reopening of 5 treated arteries (35.7%). Of these, two (14.3%) feeding arteries had full recovery and were subject to repeated PAE successfully. CEUS immediately after PAE exhibited various degrees of intratumor perfusion defects in all 12 patients in subgroup B, including no enhancement in 3 tumours (23.1%) and a greater than 50% enhancing defect in the remaining 10 tumours (76.9%).
Tumor response
There was an average of 4.0 ± 2.2 overlapping ablations per tumour (range, 2–10 ablations) within a single session for the PAE group. An average of 4.3 ± 2.2 overlapping ablations was noted for subgroup A, and an average of 3.6 ± 2.1 overlapping ablations was noted for subgroup B (p = 0.121). Post-RFA enhanced CT/MRI at 1 month revealed completed tumour necrosis in 24/25 patients (96.0%) in the PAE group. A residual tumour was observed in 1 patient due to the tumour being close to the clone and the limited safety margin. This patient then received a second RFA session, and the secondary ablation was successful (100%).
During the follow-up period, local recurrence developed in 3 tumours 4–12 months after RFA. The local recurrence rate and intrahepatic new tumour rate were 12.0% (3/25 tumours) and 39.1% (9/23 patients), respectively ( and , ).
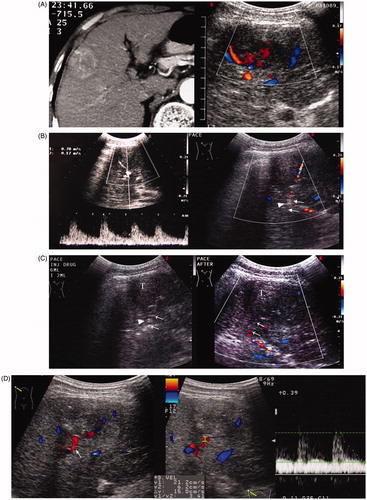
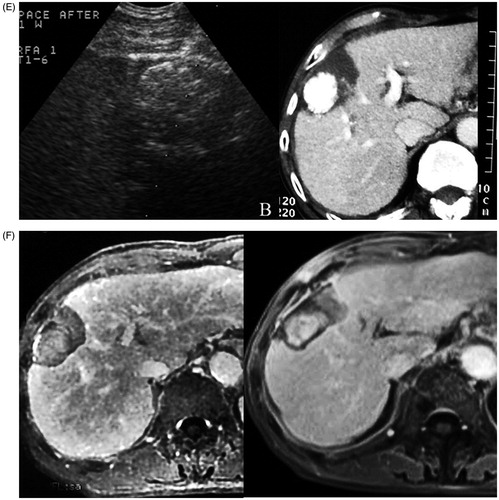
Figure 2. A 69-year-old man with HCC recurrence after TACE failure. (A) Contrast CT showed a solid mass in the right lobe of the liver with heterogeneous enhancement in the arterial phase (Left). Ultrasound showed a 5.2 × 4.8 cm mass with a rich internal flow signal on colour Doppler (Right). (B) The flow velocity of the bifurcate segment of the feeding artery was measured (70 cm/s) (Left). Colour Doppler guided puncture of main segment of feeding artery (arrow) with a 21-gauge needle. The needle tip (arrow head) passed the artery at first and then was slowly withdrawn followed by confirmation of the presence of the needle tip in the vessel (Right). (C) Saline injection confirmed the flow direction from the needle tip (arrow head). Injection of Lipiodol solution under ultrasound monitoring (Left). Post-PAE colour Doppler ultrasound showed no flow signal in the target feeding vessel (arrow) and within the mass (T) (Right). (D) Ultrasound at the sixth day after PAE: HCC was smaller, measuring 3.0 × 3.1 cm, and heterogeneous. Colour Doppler imaging showed reopening of the embolised artery (arrow). The flow velocity in the feeding artery decreased to 21.2 cm/s (Right). (E) Ultrasound-guided ablation of the mass 1 week after PAE (Left). Contrast CT at 1 month after RFA showed necrosis and dense Lipiodol deposits in the mass (Right). (F) Contrast MRI at 6 months after RFA showed no viability of HCC (Left). Contrast MRI at 123 months after RFA showed no viability of HCC (Right).
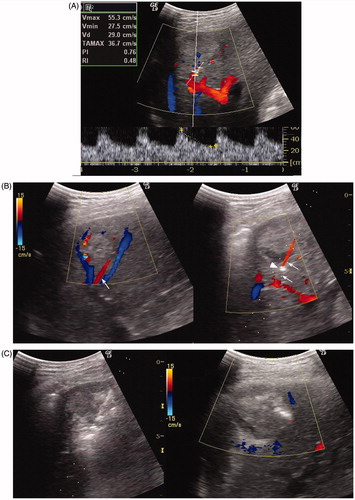
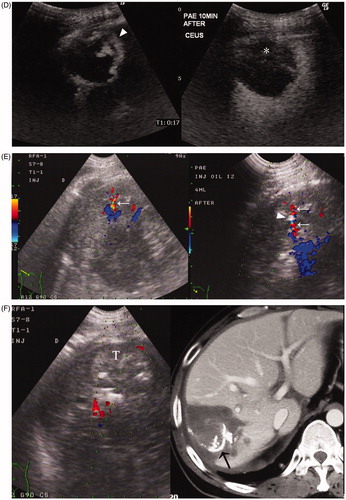
Figure 3. A 37-year-old man with HCC recurrence 5 months after liver transplantation. The mass measured 3.8 cm in diameter six months ago and increased to 5.0 cm now. (A) Colour Doppler ultrasound showed the feeding vessel of the mass. The feeding artery flow velocity measured 55.3 cm/s. (B) Colour Doppler imaging showed two feeding arteries with the accompanying portal vein and hepatic vein; the larger artery (arrow) was targeted for PAE (Left). Puncture was performed in the direction parallel to the artery (arrow), and the vessel became thinner and interrupted on Colour Doppler imaging after the needle tip (arrow head) entered the artery (Right). (C) Lipiodol was slowly injected under ultrasound monitoring. There was a local increased echogenicity area around the needle tip that was considered as extravasation of little embolisation agent. After the slow injection of 8 ml of Lipiodol, the tumour echogenicity was significantly increased (Left). Immediate post-PAE colour Doppler imaging showed no flow signals in the targeted vessel (Right). (D) CEUS at 10 min after PAE showed no enhancement in most parts of the mass, irregular enhancement can only be seen at its anterior aspect (▴) (Left). CEUS in the portal phase demonstrated a wash-out region in the enhanced area (*), suggesting viable tumour tissue (Right). (E) Pre-RFA colour Doppler showed another feeding artery (arrow) anterior to the mass (Left). Therefore, a second PAE puncture (needle tip: arrow head) was performed under colour Doppler guidance. A total of 4 ml of iodised oil was injected to embolise this vessel (Right). (F) Post-PAE, RFA was performed for this tumour (T) (Left). Contrast CT 1 month after RFA showed the ablated region over-covers the mass area and had no enhancement, and Lipiodol deposits in the feeding arteries (arrow) and branches. Laboratory tests showed normal liver function.
Table 2. Comparison of the tumor response post-RFA in 23 HCC patients.
In the PAE group, 1-week post-RFA follow-up laboratory tests revealed elevated liver function enzymes in 18 patients (78.3%) with a maximal ALT level of 452 U/L and an AST level of 561 U/L. One-month follow up indicated that liver function enzymes returned to normal levels in 16 patients (88.9%). No CBC abnormality was observed after RFA. Post-RFA AFP tests at 1–3 months were normal in 90.9% (10/11) of the patients in the PAE group.
Survival
Follow up ranged from 6 to 130 months with an average duration of 42.7 ± 9.8 months. At the time of the last follow up, 17 patients died and 5 patients were alive. The remaining one patient (6.7%) was lost to follow up (23 months before lost) due to geographical change. Overall, 15 deaths were related to HCC progression, and two deaths were unrelated to liver disease. The probabilities of overall survival at 1, 3, 5 and 10 years were 77.3%, 40.0%, 25.0% and 18.8%, respectively (. Based on treatment protocol, subgroup analysis identified similar overall survival rates in subgroup A (70.7%, 40.4%, 33.3% and 20.0%) and subgroup B (83.3%, 38.1%, 30.0% and 15.4%) (p = 0.971, log-rank test).
Complications
Seven patients (30.4%) experienced side effects of pain, fever, an elevated heart rate or blood pressure after the PAE procedure. There were two cases of right moderate pleural effusion and one case of post-treatment haemorrhage in front of the liver after RFA treatment. No severe surgical complication or acute liver failure was observed. No seeding metastasis of the needle track was observed during follow up.
Discussion
RFA is a promising, minimally invasively treatment method that can cause complete coagulation necrosis of the local tumour without surgical exposure [Citation13–18]. However, the effect of focal ablation is partly limited in hypervascular HCC due to the cooling effect of the blood flow. To solve this problem, TACE combined with RFA exhibited improved outcomes [Citation19,Citation20]. The results of animal and clinical trials also demonstrated that pre-RFA vessel embolisation improved therapy outcomes [Citation8,Citation9,Citation19–21]. However, some patients had a poor response to TACE or were non-TACE candidates, such as HCC patients after surgery or liver transplantation. In addition, repeated TACE may further impair the liver function of the patient and compromise the survival of patients with poor liver function [Citation22]. For these cases, we report the first attempt to introduce the PAE technique to decrease the blood supply before RFA. This technique used a smaller dose of embolising agent (3–12 ml) and caused less damage to liver function than TACE (10–25 ml) but achieved a similar effect of blocking the feeding artery.
At our centre with the experience of more than 20 000 cases of ultrasound-guided needle biopsies and intrahepatic bile duct punctures, we applied PAE in HCC patients with a high success rate (92.0%). Among them, 89.7% (26/29 arteries) required only 1 attempt. According to pathological studies, there is less elastic and smooth muscle tissue in the tumour artery wall [Citation23,Citation24]. Therefore, the vessel wall is thin and easily punctured. There is pressure in the artery, and the blood easily flows into catheter, aiding the PAE procedure. We also deposited the embolising agent around the feeding artery when the resistance increased during intra-artery injection. Following the treatment protocol and with the cooperation of the operators, PAE can be completed with a high success rate. In addition, multiple advantages are noted, including a lower dose of embolising agent, less injury to the patient and no radiation, compared with conventional TACE.
TACE plus RFA provides a method to treat post-TACE residual tumour with improved results [Citation8,Citation9,Citation25,Citation26]. In our study, patients were non-surgical candidates or underwent previous surgery or liver transplantation. In these patients, liver function was typically poor, but the HCC was comparatively large with rich blood supply. In the clinic, treatment options for these "difficult-to-treat" HCC patients are limited.
Studies have shown that the feeding artery supply plays an important role in post-RFA HCC recurrence [Citation7,Citation27]. Blocking the feeding artery is the key step to improve the local efficacy of RFA. Colour ultrasound provided accurate information to display the tumour-feeding artery during ultrasound scanning. Our previous experience involved first ablating the sites of the feeding arteries entering the tumour to increase the coagulation effect [Citation20]. However, we found that it was difficult to block the feeding arteries with a flow velocity of greater than 50 cm/s by RFA alone. Even if the vessel was partially blocked, the development and dilation of collateral vessels were typically observed. Therefore, we used PAE to embolise the main feeding artery when the flow velocity was greater than 50 cm/s.
The optimal injection method included turning the needle tip slope toward the tumour, injecting shaken saline and intermittently assessing blood backflow to confirm the needle position during PAE. During injection, monitoring of the echo texture change in the tumour helped evaluate the process. A local increased echogenicity area noted around the needle tip during the initial seconds of injection was considered to be extravasation of minimal embolisation agent. Reduction of the injection speed was helpful to prevent continued extravasation. There were three cases in the PAE-A group with an injection velocity greater than 1 ml/10 s. Follow-up CT revealed reflux of embolisation agents into the normal liver region. Thus, steady and slow injection of the embolising agent was particularly important for successful embolisation of the tumour vessel.
Colour Doppler imaging was used immediately after PAE to determine that the tumour blood supply was significantly decreased after the feeding arteries were embolised. CEUS immediately after PAE also exhibited a greater than 50% perfusion-deficient region in the tumour, suggesting that the liver tumour was rendered ischaemic due to the embolisation effect of PAE. This finding was similar to the Pringle strategy [Citation28]. In addition, this effect helped minimise the thermal loss during RFA and obtain better coagulation results. There were target iodised oil depositions inside the tumours on post-PAE CT, indicating that PAE could achieve a similar effect of super-selective embolisation for liver tumours as TACE. In addition, ultrasound real-time monitoring is very important for successful PAE, and ultrasound involves no radiation. In addition, the flow patterns on spectral Doppler ultrasound help identify the artery. Therefore, we performed both PAE and RFA under ultrasound guidance. Furthermore, the laboratory tests one week post-RFA revealed elevated liver function enzymes. However, these levels returned to normal after one month in 88.9% of the patients. There were 3 patients with a Child-Pugh score of 9 who had successful ablation and survived 9–24 months after the treatment. This finding may suggest that PAE can be used as an alternative option for patients with significantly impaired liver function.
The appropriate timing for TACE combined with RFA for HCC is controversial [Citation29–31]. Bharadwaz et al. [Citation32] suggested performing TAE one to two weeks prior to RFA to allow liver function recovery. However, Wang [Citation33] recommended that TACE could be performed with RFA on the same day to obtain a better coagulation effect. Recanalization or reopening of the embolised vessels near HCC can be observed a few weeks after TACE [Citation34]. The strategy for PAE-B can be considered a modified method based on the clinical experience of PAE-A therapy. Our data showed that the strategy used in subgroup B had several advantages compared with that used in subgroup A. Subgroup A exhibited various degrees of vessel reopening in 35.7% of the vessels 3 to 10 days after PAE, and 2 of the cases required repeat PAE. Subgroup B received immediate RFA, which reduced vessel reopening and other complications. In addition, the procedure employed in subgroup B was easier and faster with a shorter hospitalisation time.
The problems with ablating a large tumour are not trivial. Livraghi et al. described their experience treating 126 primary liver lesions >3 cm in diameter (mean: 5.4 cm) in 114 consecutive patients. They reported achieving complete tumour necrosis in 60 (47.6%) of 126 lesions [Citation7]. Solbiati et al. [Citation35] reported that 13 (68%) of 19 liver metastases >4 cm in diameter (mean: 4.7 ± 0.9 cm) had local recurrence during a follow-up period of 6 to 52 months. In our previous study, we treated 121 hepatic tumours >3.5 cm; according to one-month CT results, the initial tumour necrosis rate was obtained in 106 (87.6%) of the 121 tumours [Citation16]. Our current study indicated PAE plus immediate RFA for HCC obtained a satisfactory outcome of 96.0% for tumour necrosis rate and 12.0% for local recurrence during the 10-year follow up. Compared with our previous study, PAE before RFA increased the tumour necrosis rate from 87% to 96% and decreased the local recurrence rate from 24% to 12% [Citation16]. No severe surgical complication or acute liver failure was observed in our patients. Optimal selection of the indication and super-selective embolisation of the feeding artery might have contributed to the absence of severe complications in our study.
There are some limitations in the present study. First, the patients were not candidates for TACE treatment. Therefore, it is difficult to establish a control group of TACE treatment for comparison with the novel technique. However, randomised studies are needed to determine whether performing PAE actually improves the effectiveness of RFA compared with that of RFA alone. Second, compared with TACE, PAE required more experience in ultrasound-guided procedures and the concise identification of liver anatomic structures. Third, the inability to identify all supplying arteries to an HCC is a limitation of this technique. In addition, the standardised and optimised injection of the embolisation agent and selection of the embolisation agent in PAE are under further investigation. We believe the adoption of this novel technique provides a useful starting point to promote this type of technique to further improve the efficiency of tumour ablation.
In conclusion, our experience with the 23 cases suggested that using PAE before RFA can efficiently block the blood supply of HCC and minimise thermal loss during RFA with the advantages of a small dose of embolising agent, target embolisation of the tumour-feeding artery, no radiation, no major complications and better patient tolerance. This therapy is a promising option, especially for non-TACE candidate patients with a rich blood supply and HCC tumours >3 cm in size.
Acknowledgements
This research was supported by the Natural Science Foundation of Beijing Municipality [7152031], Beijing Municipal Science & Technology Commission [Z161100000516061] and the National Natural Science Foundation of China [81471768].
Disclosure statement
The authors declare that they have no conflicts of interest concerning this article.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65:87–108.
- Curley SA, Izzo F, Delrio P, et al. (1999). Radiofrequency ablation of unresectable primary and metastatic hepatic malignancies: results in 123 patients. Ann Surg 230:1–8.
- Gazelle GS, Goldberg SN, Solbiati L, et al. (2000). Tumor ablation with radio-frequency energy. Radiology 217:633–46.
- Choi D, Lim HK, Rhim H, et al. (2007). Percutaneous radiofrequency ablation for early-stage hepatocellular carcinoma as a first-line treatment: long-term results and prognostic factors in a large single-institution series. Eur Radiol 17:684–92.
- Seror O, N'Kontchou G, Nault JC, et al. (2016). Hepatocellular carcinoma within Milan criteria: No-Touch multibipolar radiofrequency ablation for treatment-long-term results. Radiology 280:611–21.
- Yang W, Yan K, Goldberg SN, et al. (2016). Ten-year survival of hepatocellular carcinoma patients undergoing radiofrequency ablation as a first-line treatment. World Journal of Gastroenterology 22:2993–3005.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. (2000). Hepatocellular carcinoma: radiofrequency ablation of medium and large lesions. Radiology 214:761–8.
- Dodd GD, 3rd, Dodd NA, Lanctot AC, et al. (2013). Effect of variation of portal venous blood flow on radiofrequency and microwave ablations in a blood-perfused bovine liver model. Radiology 267:129–36.
- Tang C, Shen J, Feng W, et al. (2016). Combination therapy of radiofrequency ablation and transarterial chemoembolization for unresectable hepatocellular carcinoma: a retrospective study. Medicine (Baltimore)95:e3754.
- Kitamoto M, Imagawa M, Yamada H, et al. (2003). Radiofrequency ablation in the treatment of small hepatocellular carcinomas: comparison of the radiofrequency effect with and without chemoembolization. AJR Am J Roentgenol 181:997–1003.
- Ernst O, Sergent G, Mizrahi D, et al. (1999). Treatment of hepatocellular carcinoma by transcatheter arterial chemoembolization: comparison of planned periodic chemoembolization and chemoembolization based on tumor response. AJR Am J Roentgenol 172:59–64.
- Murakami R, Yoshimatsu S, Yamashita Y, et al. (1994). Transcatheter hepatic subsemental arterial chemoembolization therapy using iodized oil for small hepatocellular carcinomas. Correlation between lipiodol accumulation pattern and local recurrence. Acta Radiol 35:576–80.
- Zhang FS, Wu GD, Sun H, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma in elderly patients fitting the Milan criteria: A single center with 13 years experience. Int J Hyperthermia 30:471–9.
- Lin CC, Cheng YT, Chen M WT, et al. (2016). The Effectiveness of multiple electrode radiofrequency ablation in patients with hepatocellular carcinoma with lesions more than 3 cm in size and Barcelona Clinic Liver Cancer Stage A to B2. Liver Cancer 5:8–20.
- Yang W, Chen MH, Yin SS, et al. (2006). Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early and late phase recurrence. Am J Roentgenol 186:S275–83.
- Chen MH, Yang W, Yan K, et al. (2004). Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients- mathematic model, overlapping mode, and electrode placement process. Radiology 232:260–71.
- Chen MH, Yang W, Yan K, et al. (2006). Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol 17:671–83.
- Levy AE, Kowdley KV. (2001). Unresectable hepatocellular carcinoma: the need for an individualized multidisciplinary approach. J Clin Gastroenterol 33:180–2.
- Bloomston M, Binitie O, Fraiji E, et al. (2002). Transcatheter arterial chemoembolization with or without radiofrequency ablation in the management of patients with advanced hepatic malignancy. Am Surg 68:827–31.
- Rossi S, Garbagnati F, De Francesco L, et al. (1999). Relationship between the shape and size of radiofrequency induced thermal lesions and hepatic vascularization. Tumori 85:128–32.
- Shi F, Zhang L, Li S, et al. (2016). Chemolipiodolization with or without embolization in transcatheter arterial chemoembolization combined with radiofrequency ablation for hepatocellular carcinoma-propensity score matching analysis. Oncotarget 24:31311–21.
- Ryu M, Shmamura Y, Kinoshita T, et al. (1997). Therapeutic results of resection, tanscatheter arterial embolization and percutaneous transhepatic ethanol injection in 3225 patients with hepatocellular carcinoma: a retrospective multicenter study. Jpn J Clin Oncol 27:251–7.
- Siemann DW, Chap lin DJ, Horsman MR. (2004). Vascular-targeting therapies for treatment of malignant disease. Cancer 100:2491–9.
- Ferrara N, Kerbgel RS. (2005). Angiogenesis as a therapeutic target. Nature 438:967–74.
- Palma LD. (1998). Diagnostic imaging and interventional therapy of hepatocellular carcinoma. Br J Radiol 71:808–18.
- Yu MH, Kim JH, Yoon JH, et al. (2013). Role of C-arm CT for transcatheter arterial chemoembolization of hepatocellular carcinoma: diagnostic performance and predictive value for therapeutic response compared with gadoxetic acid-enhanced MRI. Am J Roentgenol 201:675–83.
- Poch FG, Rieder C, Ballhausen H, et al. (2016). The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int J Hyperthermia32:749–56.
- Nikfarjam M, Muralidharan V, Malcontenti-Wilson C, et al. (2006). Impact of blood flow occlusion on liver necrosis following thermal ablation. ANZ J Surg 76:84–91.
- Pleguezuelo M, Marelli L, Misseri M, et al. (2008). TACE versus TAE as therapy for hepatocellular carcinoma. Expert Rev Anticancer Ther 8:1623–41.
- Liu H, Wang ZG, Fu SY, et al. (2016). Randomized clinical trial of chemoembolization plus radiofrequency ablation versus partial hepatectomy for hepatocellular carcinoma within the Milan criteria. Br J Surg 103:348–56.
- Azuma S, Asahina Y, Nishimura-Sakurai Y, et al. (2016). Efficacy of additional radiofrequency ablation after transcatheter arterial chemoembolization for intermediate hepatocellular carcinoma. Hepatol Res 46:312–19.
- Bharadwaz A, Bak-Fredslund KP, Villadsen GE, et al. (2016). Combination of radiofrequency ablation with transarterial chemoembolization for treatment of hepatocellular carcinoma: experience from a Danish tertiary liver center. Acta Radiol 57:844–51.
- Wang ZJ, Wang MQ, Duan F, et al. (2013). Transcatheter arterial chemoembolization followed by immediate radiofrequency ablation for large solitary hepatocellular carcinomas. World J Gastroenterol 19:4192–9.
- Kubota K, Hisa N, Nishikawa T, et al. (2001). Evaluation of hepatocellular carcinoma after treatment with transcatheter arterial chemoembolization: comparison of Lipiodol-CT, power Doppler sonography, and dynamic MRI. Abdom Imaging 26:184–90.
- Solbiati L, Livraghi T, Goldberg SN, et al. (2001). Percutaneous radio-frequency ablation of hepatic metastases from colorectal cancer: long-term results in 117 patients. Radiology 221:159–66.