Abstract
Purpose: The resection rate for liver metastases from gastric cancer is only 1.4–21.1%. This study aimed to evaluate the safety and therapeutic efficacy of microwave ablation (MWA) for liver metastases from gastric adenocarcinoma (LMGC).
Materials and methods: A database of 108 gastric adenocarcinoma patients with liver metastases who underwent MWA (n = 32) or systemic chemotherapy (n = 76) for LMGC between 2008 and 2016 was reviewed. Overall survival curves were assessed and compared based on different therapies.
Results: All the patients were followed up for a median of 15 months (range, 2–68 months). The median cumulative survival times of patients after MWA and systemic chemotherapy were 25 (95% confidence interval (CI) 16.5–33.5) months and 12 (95% CI 7.7–16.3) months, respectively (HR 1.751, 95% CI 1.077–2.845; p = .015). The 1-, 3-, and 5- year survival rates were 80.9%, 31.2%, and 16.7% (MWA group); and 50.0%, 18.8%, and 5.4% (chemotherapy group), respectively. In the MWA group, side effects were reported in eight patients who developed abdominal pain, transient fever, or nausea. Dominant size, number of liver metastases, therapeutic modalities, and presence of extrahepatic metastases showed significant prognostic value in univariate analyses; while the latter three were identified as independent prognostic factors in multivariate regression analysis.
Conclusions: MWA is a safe and useful alternative for liver metastases from gastric adenocarcinoma in selected patients.
Introduction
According to the latest worldwide estimates of cancer statistics, there were 951 594 new cases of gastric cancer diagnosed in 2012, as well as 723 073 deaths caused by this disease, making it the third leading cause of cancer deaths worldwide [Citation1]. It is reported that around 5–9% patients may develop liver metastases from gastric cancer (LMGC), and surgical resection is the optimal treatment [Citation2]. However, the resection rate for LMGC is just 1.4–21.1% [Citation3]. Several reasons may account for this low resection rate, including multiple and bilateral metastases, peritoneal dissemination, extensive lymph node metastases, and direct cancer invasion of other organs [Citation3]. Some patients with unresectable LMGC are thus treated by systemic chemotherapy, hepatic arterial infusion of chemotherapy, transcatheter arterial chemoembolisation, and molecular targeted therapy [Citation4–6]. Among these modalities, systemic chemotherapy is regarded as a first-line treatment. According to the statistics released by the Japan Clinical Oncology Group, the 5-year survival rate of patients with LMGC after systemic chemotherapy alone was just 1.7% [Citation7]. Hence, a safe and effective modality that can achieve complete tumour eradication is urgently needed for unresectable LMGC.
In recent years, ablative therapies have been developed for the treatment of primary and metastatic liver cancer with a curative intent [Citation8]. Radiofrequency ablation (RFA) has been demonstrated to be a safe and effective alternative for unresectable liver metastases, especially from colorectal cancer [Citation9]. Although different results were obtained when comparing RFA to surgical resection, it is evident that a potentially greater number of patients are eligible to be treated with RFA with a curative intent [Citation10,Citation11]. Compared with liver metastases from colorectal cancer, less data is available regarding RFA treatment of LMGC. Kim et al. reported a median overall survival of 30.7 months and an overall 5-year survival rate of 16.1% from 20 patients with LMGC after RFA [Citation12]. Chen et al. revealed a median survival of 14 months and an overall 5-year survival rate of 3% from 21 patients who underwent RFA for LMGC [Citation13].
More recently, microwave ablation (MWA) has developed into a popular modality to treat liver malignancies [Citation14,Citation15]. Compared with RFA, MWA has the advantage of larger ablation volumes, shorter treatment sessions, no charring or electrical insulation, less heat-sink effect, and lower rates of major complications [Citation16,Citation17]. A recent clinical study compared a matched cohort of 127 liver metastatic lesions and concluded that MWA resulted in lower ablation site recurrences at 2 years than RFA in the treatment of colorectal liver metastases [Citation18]. However, the efficacy of MWA in the treatment of LMGC remains unknown. In the present study, we aimed to retrospectively evaluate the feasibility, safety, and efficacy of ultrasound-guided percutaneous MWA for the treatment of LMGC after curative resection of primary gastric cancer. Additionally, comparison was made between patients who received MWA and systemic chemotherapy for LMGC in order to provide context for the outcomes observed after MWA.
Materials and methods
Patients
This study received approval from our Institutional Review Board, and informed consent for treatment procedures was obtained from each patient prior to treatment. Between March 2008 and March 2016, patients who underwent MWA for unresectable LMGC at our department were enrolled. Liver metastases were determined to be unresectable owing to the location, distribution, and number of liver metastases, insufficient liver remnants, and/or comorbidities. Indication criteria for percutaneous MWA included (1) resection of primary gastric cancer and histologic diagnosis of gastric adenocarcinoma; (2) presence of three or fewer unresectable liver metastases; (3) a single metastatic lesion with a largest diameter of 5 cm or less, or multiple lesions with a largest diameter of 3 cm or less; (4) patients who could achieve a curative intent after MWA; (5) absence of peritoneal dissemination; and (6) an anticipated life-expectancy of 6 months or more. In the meantime, we reviewed database of patients who received systemic chemotherapy for LMGC at Department of Oncology of our hospital during the same period. The age, sex, Karnofsky performance scale (KPS) score, type of gastrectomy, location, TNM stage, and histologic grade of the primary gastric cancer, carcinoembryonic antigen level, timing, number, size, and distribution of intrahepatic metastases, and the type of systemic chemotherapy of each patient were documented. The medical records, imaging studies, and blood tests of each patient were reviewed.
Therapeutic modalities
All MWA treatments were performed at our department. In the preprocedural stage, we always performed a two-dimensional ultrasound scan, contrast-enhanced ultrasound (CEUS), contrast-enhanced three-phase computed tomography/dynamic contrast-enhanced magnetic resonance imaging of the abdomen to display the number, size, and distribution of liver metastases. All ablations were performed using a cooled-shaft microwave system (KY-2000; Kangyou Medical Instruments, Nanjing, China) that produces a maximum power of 100 W at 2450 MHz. All ablation procedures were performed under the guidance of two-dimensional ultrasound or CEUS if an inconspicuous image was displayed on conventional ultrasound scanning as we previously described [Citation19]. One antenna was inserted at the designated location for tumours with diameters less than 1.7 cm, whereas two antennae were used for tumours with diameters of 1.7 cm or more. Antennae tips of 0.5 or 1.1 cm were chosen according to tumour diameter. After intravenous anaesthesia with a combination of propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, DE) and ketamine (Shuanghe Pharmaceuticals, Beijing, China), antennae insertion was performed. Under ultrasound guidance, 20-gauge thermocouple needles were percutaneously placed 0.5 cm outside the tumour margins to monitor the real-time temperature throughout the procedure [Citation20]. The mean power and time of MWA for each metastases in this study were 50 W (range, 40–60 W) and 496 s (range, 150–960 s), respectively. Microwave emission was stopped if the measured temperature remained above 54 °C for at least 3 min or reached 60 °C. A tumour-free margin of at least 5 mm from the periphery was aimed for during MWA. The needle track was coagulated during withdrawal of the antenna to avoid bleeding and tumour seeding. Immediately after MWA treatment, CEUS was performed on each patient to determine whether complete ablation of tumours was achieved. If the inactivation of lesions was complete with a tumour-free margin of at least 5 mm, MWA treatment was stopped. Otherwise, additional MWA sessions were required. Technical success was defined as no contrast enhancement of ablated tumours on CEUS one month after the MWA procedure. Complications and side effects were recorded according to the standardisation of terminology and reporting criteria for image-guided tumour ablation proposed by Ahmed et al. [Citation21]
For patients who received systemic chemotherapy after detection of liver metastases, the treatment regimen was determined by the consensus of the multidisciplinary tumour panel, which consisted of experienced surgeons, physicians, and radiologists, following clinical assessment and radiological findings. The patients received systemic chemotherapy based on the location and extent of the primary cancer, as well as the patients’ physical condition.
Follow-up
All the enrolled patients received CEUS and contrast-enhanced magnetic resonance imaging 1, 3, 6, 9, and 12 months after initial treatment and at 3–6-month intervals thereafter. Laboratory tests including blood routine, liver function, and tumour markers were performed at the same time. Follow-up time was defined as the time from initial treatment to the most recent imaging follow-up. Local recurrence was recorded if viable tumour inside or adjacent to the ablated tumour was observed at CEUS or contrast-enhanced magnetic resonance imaging at least 1 month after MWA.
Statistical analysis
Overall survival (OS) was defined as the interval from MWA treatment (initiation of systemic chemotherapy) to death. The OS curves were described using the Kaplan–Meier method and compared with the log-rank test. Univariate analysis and multivariable analyses were performed to determine the independent predictors for OS. A p value <.050 was inferred as statistically significant. All statistical analyses were performed by using the SPSS 19.0 software package (SPSS Inc., Chicago, IL) and GraphPad Prism 5 software (GraphPad, La Jolla, CA).
Results
Demographics
From March 2008 to March 2016, a total of 108 patients who underwent MWA/systemic chemotherapy for LMGC were included in this study after exclusion of missing follow-up cases. Among these patients, 32 patients with 46 liver metastases were treated with percutaneous MWA. Detailed information regarding patient demographics and intrahepatic metastases is shown in . The mean number and largest diameter of intrahepatic metastases were 1.5 (range, 1–3) and 3.8 cm (range, 1.6–4.8 cm). Thirteen patients had extrahepatic metastases at initial detection of liver metastases, including metastases to distant lymph nodes (n = 5), lung (n = 3), adrenal gland (n = 3), bone (n = 1), and brain (n = 1). In the MWA group, 26 patients (81.25%) received adjuvant chemotherapy, and the remaining six patients declined chemotherapy. Chemotherapy regimens included DCF (docetaxel, cisplatin, 5-fuorouracil), FOLFOX (leucovorin, 5-fuorouracil, oxaliplatin), and XELOX (capecitabine, oxaliplatin) regimens, similar with that of the chemotherapy-only group.
Table 1. Clinicopathological characteristics and tumour parameters of the study group.
In systemic chemotherapy group (n = 76), multiple intrahepatic metastatic lesions were detected in 33 patients and the mean largest diameter of liver metastases was 4.7 cm (range, 1.2–6.7 cm). Twenty-eight patients had extrahepatic metastases at initial detection of liver metastases, including peritoneal dissemination (n = 11) and metastases to distant lymph nodes (n = 10), lung (n = 4), adrenal gland (n = 3), mesentery (n = 2), bone (n = 2), brain (n = 1), and ovary (n = 1). Detailed clinicopathological characteristics are summarised in . XELOX, DCF, FOLFOX, and EOX (epirubicin, oxaliplatin, capecitabine) regimens were commonly administered in this group. Comparison between groups was performed regarding clinicopathological characteristics and no significant differences between groups were detected except the KPS score ().
Ablation results
Technical success was achieved in 31 of 32 patients (96.78%) based on CEUS results one month after MWA. One patient with a remaining tumour on CEUS scan received additional MWA treatment and subsequently obtained complete ablation. Fifteen patients underwent MWA under two-dimensional ultrasound guidance, and 16 patients under CEUS guidance. In 14 patients, only one MWA procedure was performed, whereas 16 patients underwent two MWA procedures and two patients had three procedures. There was no MWA-related mortality in the 32 patients. No ablation-related major or minor complications such as intrahepatic abscess, biliary obstruction, intestinal fistula, intraperitoneal bleeding, parietal abscess, pneumothorax, or tumour seeding were reported. Side effects after MWA were recorded in eight patients, including abdominal pain (n = 4), transient fever (n = 3), and nausea (n = 1). All the side effects resolved with supportive care.
Recurrence results
After a median follow-up of 19 months (range, 4–62 months), 24 patients presented with intrahepatic metastases (new foci at other site of remnant liver, while no recurrence at the ablation site was detected), while 19 patients experienced extrahepatic metastases. Thirteen of the 24 patients received repeat MWA treatment for recurrent liver metastases, and the remaining patients with recurrence received systemic chemotherapy (n = 11).
Survival and prognostic factors
All the patients were followed up for a median of 15 months (range, 2–68 months). The median OS was 16 (95% confidence interval (CI) 11.3–20.7) months and the 1-, 3-, and 5- year OS rates were 59.1%, 22.3%, and 7.9%, respectively.
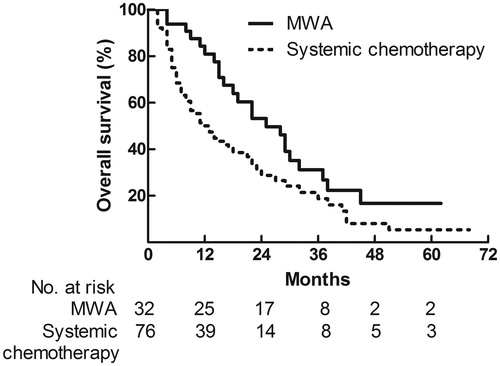
In the MWA group, the median OS from the date of initial ablation was 25 (95% CI 16.5–33.5) months after a median follow-up of 19 months (range, 4–62 months). The 1-, 3-, and 5- year OS rates were 80.9%, 31.2%, and 16.7%, respectively. In the systemic chemotherapy group, the median OS was 12 (95% CI 7.7–16.3) months after a median follow-up of 13 months (range, 2–68 months). The 1-, 3-, and 5- year OS rates were 50.0%, 18.8%, and 5.4%, respectively. OS curves of the two groups are shown in . OS result of MWA group was superior in comparison with that of systemic chemotherapy group (HR 1.751, 95% CI 1.077–2.845; p = .015).
Figure 1. Overall survival curves of patients who had microwave ablation (n = 32) and systemic chemotherapy (n = 76) for liver metastases from gastric adenocarcinoma.
The parameters included in univariate and multivariate analyses for OS included age, gender, KPS score, type of surgery, TNM stage and histologic grade of primary cancer, carcinoembryonic antigen, dominant size, distribution, number, time of liver metastases, therapeutic strategy, and presence of extrahepatic metastases. In univariate analyses, dominant size, number of liver metastases, treatment for LMGC, and presence of extrahepatic metastases showed significant prognostic value (). Multivariate regression analysis identified number of liver metastases, treatment and presence of extrahepatic metastases as prognostic factors for OS ().
Table 2. Univariate and multivariate analyses of prognostic factors for overall survival in gastric cancer patients after microwave ablation for liver metastases.
Discussion
In China, 404 996 (42.6% of global statistics) new gastric cancer cases were diagnosed in 2012, and 325 116 (45.0% of global statistics) deaths occurred as a result of this disease [Citation1]. The high incidence and mortality rates in China are mainly due to different dietary patterns in comparison with Western countries, as well as chronic infection with Helicobacter pylori [Citation22]. Approximately 5–9% of gastric cancer patients develop synchronous or metachronous liver metastases, leading to a 5-year survival rate of just 0–10% [Citation23].
Surgery is regarded as the treatment of choice for resectable LMGC, and carries a 5-year survival rate of 0–42% [Citation24]. A recent study which enrolled 256 patients reported a median OS of 31.1 months and 1-, 3-, and 5- year OS rates of 77.3%, 41.9%, and 31.1%, respectively, after surgical resection of LMGC [Citation24]. For unresectable LMGC, systemic chemotherapy is regarded as first-line treatment with a median survival time of 11.0–13.8 months [Citation25]. Yoshida et al. investigated 643 patients with LMGC and reported a 5-year survival rate of just 1.7% after systemic chemotherapy [Citation7].
Nowadays, thermal ablation is considered a promising therapy for liver metastases due to its minimal invasiveness, repeatability, low rate of complications, and applicability in patients with relatively poor physical conditions [Citation20,Citation26]. In colorectal cancer liver metastases, some studies have reported the potential of RFA to achieve comparable overall and disease-free survival rates with surgical resection meanwhile with fewer complications [Citation27]. In LMGC, the efficacy of RFA has been reported in some studies with a median survival of 10–30.7 months ().
Table 3. Literature review regarding ablative therapy for liver metastases from gastric adenocarcinoma.
MWA is another type of thermal ablation that is now widely used. The advantages of MWA over RFA are a faster increase in temperature, less heat-sink effect, higher local temperature, larger ablation volume in a shorter ablation time, ability to better treat cystic tumours, and the lack of a need for grounding pads [Citation34]. To our knowledge, there is still no published data regarding the safety and efficacy of MWA for the treatment of LMGC. The results of this study indicated that MWA is a safe and effective treatment for unresectable LMGC in selected patients. No MWA-related mortality, major, or minor complications were reported and only eight patients experienced side effects including abdominal pain, transient fever, and nausea. To better investigate the efficacy of MWA, we reviewed the data of patients who received systemic chemotherapy for LMGC during the same period. Results showed improved survival of MWA group in comparison with that of chemotherapy group (median survival: 25 months vs. 12 months, p = .015). Several reasons may account for the improved survival of MWA group. First, complete ablation was achieved as a tumour-free margin of at least 5 mm was achieved during the procedure. Second, the patients in MWA group were strictly selected. Gannon et al. [Citation35] reported that due to tumour biology and behaviour, patients would not benefit from ablation if number of liver metastases was more than five. In this study, we enrolled patients whose liver metastases were three or fewer. Additionally, patients in MWA group had a largest LMGC of 4.8 cm. In comparison, 43.4% (33/76) patients in chemotherapy group had multiple liver metastases, 36.8% (28/76) with extrahepatic metastases, and the largest size was 6.7 cm. In univariate analysis, size, number, and extrahepatic metastases were identified as prognostic factors for survival, which was in consistent with previous studies regarding RFA for LMGC [Citation2,Citation13,Citation29]. Additionally, studies regarding hepatectomy for LMGC also revealed prognostic value of size and number of LMGC [Citation24,Citation36].
Previous studies [Citation4,Citation37] reported that approximately 66.7% LMGC patients may develop recurrent liver metastases after curative resection of LGMC. This high recurrence rate made it a challenge for surgeons to perform repeat surgery due to severe anatomical adhesion, potential morbidity and mortality. In this study, 75% (24/32) of the patients experienced recurrent liver metastases and 13 patients received repeat MWA. Consequently, for patients with recurrent liver metastases, MWA can be resorted to obtain local control after comprehensive clinical assessment.
This study has some limitations. First, the retrospective nature of this study makes selection bias in this study unavoidable. Thus, more caution should be paid when interpreting this result to all LMGC patients. Second, the inclusion criteria in this study were based on published articles and our own experience. Third, patients in MWA group and chemotherapy group are heterogenous in various aspects, such as KPS score. Thus, randomised controlled trials are needed to obtain a more unbiased result.
Conclusions
In conclusion, our results revealed 25-month median survival in patients who received MWA for LMGC. MWA can serve as a safe and feasible option in properly selected patients who developed liver metastases originated from gastric adenocarcinoma.
Acknowledgements
The authors would like to thank the participants and staff of the Department of Interventional Ultrasound, Chinese PLA General Hospital, for their valuable contributions.
Disclosure statement
The authors report no conflicts of interest.
Additional information
Funding
References
- Ferlay J, Soerjomataram I, Dikshit R, et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–86.
- Hwang JE, Kim SH, Jin J, et al. (2014). Combination of percutaneous radiofrequency ablation and systemic chemotherapy are effective treatment modalities for metachronous liver metastases from gastric cancer. Clin Exp Metastases 31:25–32.
- Komeda K, Hayashi M, Kubo S, et al. (2014). High survival in patients operated for small isolated liver metastases from gastric cancer: a multi-institutional study. World J Surg 38:2692–7.
- Kakeji Y, Morita M, Maehara Y. (2010). Strategies for treating liver metastasis from gastric cancer. Surg Today 40:287–94.
- Lordick F, Kang YK, Chung HC, et al. (2013). Capecitabine and cisplatin with or without cetuximab for patients with previously untreated advanced gastric cancer (EXPAND): a randomised, open-label phase 3 trial. Lancet Oncol 14:490–9.
- Vogl TJ, Gruber-Rouh T, Eichler K, et al. (2013). Repetitive transarterial chemoembolization (TACE) of liver metastases from gastric cancer: local control and survival results. Eur J Radiol 82:258–63.
- Yoshida M, Ohtsu A, Boku N, et al. (2004). Long-term survival and prognostic factors in patients with metastatic gastric cancers treated with chemotherapy in the Japan Clinical Oncology Group (JCOG) study. Jpn J Clin Oncol 34:654–9.
- Li D, Kang J, Madoff DC. (2014). Locally ablative therapies for primary and metastatic liver cancer. Expert Rev Anticancer Ther 14:931–45.
- Stang A, Oldhafer KJ, Weilert H, et al. (2014). Selection criteria for radiofrequency ablation for colorectal liver metastases in the era of effective systemic therapy: a clinical score based proposal. BMC Cancer 14:500.
- Aliyev S, Agcaoglu O, Aksoy E, et al. (2013). Efficacy of laparoscopic radiofrequency ablation for the treatment of patients with small solitary colorectal liver metastases. Surgery 154:556–62.
- Agcaoglu O, Aliyev S, Karabulut K, et al. (2013). Complementary use of resection and radiofrequency ablation for the treatment of colorectal liver metastases: an analysis of 395 patients. World J Surg 37:1333–9.
- Kim HR, Cheon SH, Lee KH, et al. (2010). Efficacy and feasibility of radiofrequency ablation for liver metastases from gastric adenocarcinoma. Int J Hyperthermia 26:305–15.
- Chen J, Tang Z, Dong X, et al. (2013). Radiofrequency ablation for liver metastasis from gastric cancer. Eur J Surg Oncol 39:701–6.
- Leung U, Kuk D, D'Angelica MI, et al. (2015). Long-term outcomes following microwave ablation for liver malignancies. Br J Surg 102:85–91.
- Engstrand J, Nilsson H, Jansson A, et al. (2014). A multiple microwave ablation strategy in patients with initially unresectable colorectal cancer liver metastases – a safety and feasibility study of a new concept. Eur J Surg Oncol 40:1488–93.
- Pathak S, Jones R, Tang JM, et al. (2011). Ablative therapies for colorectal liver metastases: a systematic review. Colorectal Dis 13:e252–65.
- Vogl TJ, Farshid P, Naguib NN, et al. (2014). Thermal ablation of liver metastases from colorectal cancer: radiofrequency, microwave and laser ablation therapies. Radiol Med 119:451–61.
- Correa-Gallego C, Fong Y, Gonen M, et al. (2014). A retrospective comparison of microwave ablation vs. radiofrequency ablation for colorectal cancer hepatic metastases. Ann Surg Oncol 21:4278–83.
- Liu F, Yu X, Liang P, et al. (2011). Contrast-enhanced ultrasound-guided microwave ablation for hepatocellular carcinoma inconspicuous on conventional ultrasound. Int J Hyperthermia 27:555–62.
- Liang P, Wang Y, Yu X, Dong B. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation-complications among cohort of 1136 patients. Radiology 251:933–40.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology 273:241–60.
- Siegel R, Naishadham D, Jemal A. (2013). Cancer statistics, 2013. CA Cancer J Clin 63:11–30.
- Wang W, Liang H, Zhang H, et al. (2014). Prognostic significance of radical surgical treatment for gastric cancer patients with synchronous liver metastases. Med Oncol 31:258.
- Kinoshita T, Saiura A, Esaki M, et al. (2015). Multicentre analysis of long-term outcome after surgical resection for gastric cancer liver metastases. Br J Surg 102:102–7.
- Takemura N, Saiura A, Koga R, et al. (2012). Long-term outcomes after surgical resection for gastric cancer liver metastases: an analysis of 64 macroscopically complete resections. Langenbecks Arch Surg 397:951–7.
- Liu C, Wang Y, Yu X, et al. (2011). Is percutaneous microwave ablation of liver tumor safe for patients with renal dysfunction. Eur J Radiol 79:e103–7.
- Minami Y, Kudo M. (2013). Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver 7:1–6.
- Oki E, Tokunaga S, Emi Y, et al. (2016). Surgical treatment of liver metastases of gastric cancer: a retrospective multicenter cohort study (KSCC 1302). Gastric Cancer 19:968–76.
- Guner A, Son T, Cho I, et al. (2016). Liver-directed treatments for liver metastases from gastric adenocarcinoma: comparison between liver resection and radiofrequency ablation. Gastric Cancer 19:951–60.
- Lee CW, Kim JH, Won HJ, et al. (2015). Percutaneous radiofrequency ablation of hepatic metastases from gastric adenocarcinoma after hysterectomy. J Vasc Interv Radiol 26:1172–9.
- Kim HO, Hwang SI, Hong HP, Yoo CH. (2009). Radiofrequency ablation for metachronous hepatic metastases from gastric cancer. Surg Laparosc Endosc Percutan Tech 19:208–12.
- An JY, Kim JY, Choi MG, et al. (2008). Radiofrequency ablation for hepatic metastasis from gastric adenocarcinoma. Yonsei Med J 49:1046–51.
- Yamakado K, Nakatsuka A, Takaki H, et al. (2005). Prospective study of arterial infusion chemotherapy followed by radiofrequency ablation for the treatment of liver metastases of gastric cancer. J Vasc Interv Radiol 16:1747–51.
- Yamakado K. (2014). Image-guided ablation of adrenal lesions. Semin Intervent Radiol 31:149–56.
- Gannon CJ, Curley SA. (2005). The role of focal liver ablation in the treatment of unresectable primary and secondary malignant liver tumors. Semin Radiat Oncol 15:265–72.
- Shinohara T, Maeda Y, Hamada T, Futakawa N. (2015). Survival benefit of surgical treatment for liver metastases from gastric cancer. J Gastrointest Surg 19:1043–51.
- Cheon SH, Rha SY, Jeung HC, et al. (2008). Survival benefit of combined curative resection of the stomach (D2 resection) and liver in gastric cancer patients with liver metastases. Ann Oncol 19:1146–53.
