Abstract
Introduction: Salvage treatment options for recurrent unilateral prostate cancer (PCa) after primary radiation are limited and associated with severe complications and poor quality of life measures. Salvage focal cryotherapy (SFC) has shown success in biochemical recurrence (BCR) free survival. We aim to determine if SFC can delay use of androgen deprivation therapy (ADT) in recurrent PCa with low morbidity.
Methods: A retrospective review of patients who underwent SFC at our institution from 2007 to 2015 was performed. Patients with <2 follow-up prostate-specific antigen (PSA) values, metastatic disease, and a history of radical prostatectomy were excluded. Age at treatment, prior treatment history, PSA nadir, complications, BCR status (nadir +2 ng/ml), and follow-up data were obtained/analysed. ADT was commenced if patient experienced BCR and had a PSA doubling time <6 months or positive confirmatory biopsy or positive imaging. Cox regression and survival analysis were used to assess confounding and time to BCR respectively.
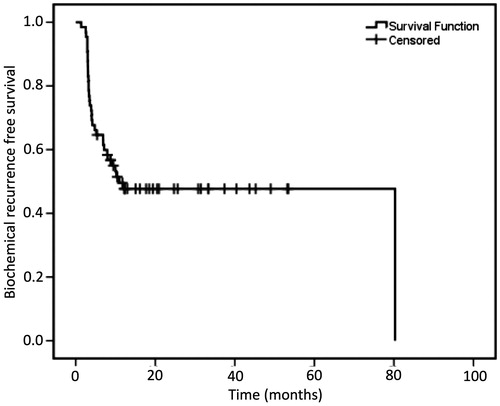
Results: A total of 65 patients were included and followed for a median of 26.6 (8.0–99.0) months. Thirty-one (47.7%) patients did not experience BCR. An even higher number of patients (52/65, 80.0%) are yet to receive ADT. Of those who experienced BCR [median time to BCR, 17.1 [interquartile range (IQR):11.4–23.3] months], 22/34 (64.7%) are currently carefully monitored without ADT. Survival analysis showed a biochemical recurrence-free survival of 48.1 at 1- and 3-year follow up. No patient died/experienced major complications.
Conclusions: SFC may be used to delay the use of ADT. Further assessment of our findings with high-powered studies and longer follow-up is required.
Introduction
Despite the intent to achieve a cure, patients may experience a recurrence following primary treatment for localised prostate cancer (PCa), including10–60% of patients treated with radiation therapy (XRT) [Citation1,Citation2]. Salvage treatment options with curative intent include radical prostatectomy, cryotherapy, high-intensity focussed ultrasound, and XRT [Citation3–6]. Androgen deprivation therapy (ADT) is an alternative treatment option with no curative potential and can significantly alter quality of life [Citation4,Citation5]. Patients treated with salvage radical prostatectomy incur significant post-operative morbidity [Citation7]. Currently, the optimal treatment strategy for biochemical or local recurrence following minimally invasive primary treatment remains controversial.
Advancement in new technology and better patient selection is paving the path to cryotherapy, a minimally invasive treatment option for patients with organ confined PCa [Citation8,Citation9]. Cryotherapy has been well studied in both primary and salvage settings and can involve partial (hemiablation or focal lesion ablation) or total ablation of the prostate [Citation10,Citation11]. Focal and total salvage cryotherapy has comparable oncologic outcomes but focal ablation has been shown to minimise the morbidities associated with radical surgery [Citation4,Citation12]. Salvage focal cryotherapy (SFC) may allow patients to delay or negate the use of androgen deprivation therapy (ADT) or other treatments which may impact quality of life. We aim to determine if SFC can delay use of ADT in recurrent PCa patients status-post primary minimally invasive treatment.
Methods
After IRB approval, we retrospectively reviewed data of patients treated by a single surgeon (A. E. K.) with SFC of the prostate from January 2010 to October 2015. Exclusion criteria included patients with a history of radical prostatectomy, metastatic disease and having <2 post-SFC follow-up prostate-specific antigen (PSA) values. All patients had pathologically proven recurrent PCa with no evidence of metastasis on imaging. The study population also included patients who had adjuvant ADT at time of primary treatment and neoadjuvant ADT prior to SFC. Patient demographics and pre-treatment clinical parameters are reported.
Surgical technique
All procedures (hemiablation) were performed by a single surgeon (AEK) at an outpatient ambulatory surgical centre. The operative procedure has been previously reported by Ullal et al. [Citation13]. Spinal anaesthesia was used for all patients. Depending on prostate volume, 4–6 17-gauge ice rod needles (Galil Medical Inc., Arden Hills, MN) were used to freeze prostate tissue, along with a temperature-monitoring device to ensure that a minimum temperature of −40 °C was reached in the area of the cancer. A urethral warmer was utilised during the procedure to avoid urethral injury. Patients were sent home with an 18 French Foley Catheter for 4 d. On the 5th post-operative day, an active trial of void was given.
Follow up and morbidity
All SFC patients were routinely monitored with serial PSAs and digital rectal exams every 3 months for the first 2 years and every 6 months thereafter. Nadir PSA was determined using the first 2–3 PSA values obtained within 12 months of SFC. Biochemical recurrence was determined using the Phoenix criteria (nadir +2 ng/ml). Patients who recurred were administered ADT if they had a positive confirmatory biopsy. Other BCR patients with PSA doubling time (PSADT) < 6 months and/or positive MRI [Prostate Imaging Reporting and Data System (PIRADS) > 3) or bone scan were also administered ADT. Complications (haematuria, fistulas, infection, urethral strictures, and retention) associated with the procedure were recorded. Side effects such as incontinence – defined as the use of >1 pad per day – and erectile dysfunction – defined as impotency 9 months after the procedure – were also recorded.
Statistical analysis
Analysis was performed using JMP 11.0 (SAS Institute Inc., Gary, NC) and IBM SPSS (Version 21, SPSS Inc., Chicago, IL). Kaplan–Meier estimates were used to analyse Biochemical Recurrence-Free Survival (BCRFS). The chi-square test was used to test the statistical significance between the independent groups: patients who received neoadjuvant ADT prior to SFC, patients with no prior history of ADT, and patients who received ADT during the time of primary treatment. Cox proportional regression analysis was used in predictive analysis to determine if prior ADT history was a predictor of BCR.
Results
A total of 65 of 88 patients who underwent SFC met the study inclusion criteria. Patient demographics are summarised in . The median patient age was 71.0 [interquartile range (IQR): 65.0–74.3] years with a median follow-up of 26.6 (range: 8.0–99.0) months. The majority of our patients were initially diagnosed with Gleason >3 + 4 (66.2%) and 86.2% of them were treated with XRT as their primary treatment. Forty-one (63.1%) of our patients had no prior history of ADT.
Table 1. Focal salvage cryotherapy patient demographics.
A drop in PSA of >50% was recorded in the majority (47/65, 71.2%) of the patients after SFC. The median nadir PSA following SFC was 0.5 (IQR: 0.1–1.7) ng/ml. Thirty-one (47.7%) patients have yet to experience BCR and an even greater number 52 (80.0%) are still not on ADT. Of those who experienced BCR [median time to BCR, 17.1 (IQR: 11.4–23.3) months], 22/34 (64.7%) have at least a negative confirmatory biopsy, a PSADT >6 months or negative MRI or bone scan are currently being carefully monitored without ADT.
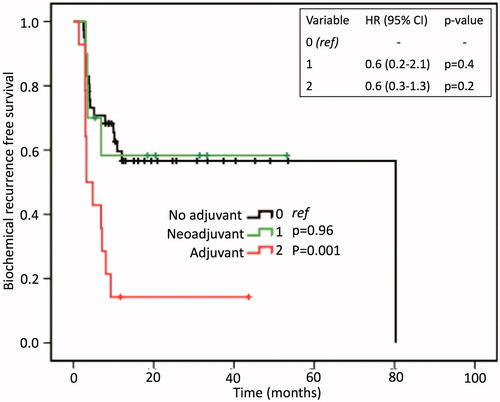
Kaplan–Meir curves () showed patients who received ADT at time of primary treatment to have a significantly different BCRFS trend compared to those with no prior history of ADT (p = 0.001). However, on Cox regression analysis, prior ADT history was not a predictor of BCR, ruling it out as a potential confounder to oncologic outcomes (specifically BCR). Survival analysis showed that 50.0% of the patients had not recurred at 10.9 months. The BCRFS survival curve plateaued at ∼1 year of follow-up leading to a 1- and 3-year BCR free survival (BCRFS) of 48.1% ().
Figure 1. Biochemical recurrence-free survival analysis and Cox regression analysis between patients with (0) no prior history of ADT, (1) neoadjuvant prior to salvage focal cryotherapy, and (2) adjuvant hormones at time of primary treatment. The Cox regression table rules out prior ADT history as a potential confounder of biochemical recurrence. HR: hazards ratio.
A total of eight (12.3%) patients experienced minor complications (Clavien–Dindo Grade I). Two (3.1%) patients experienced transient haematuria. Three (4.1%) patients had urethral strictures, none of which required surgical intervention. No patients were hospitalised. Three patients (4.1%) had prolonged (>4 d) catherisation. Four (6.1%) patients reported having incontinence and 14 (21.5%) patients reported erectile dysfunction (ED) following the procedure.
Discussion
The panic of a rising PSA after curative treatment may lead to the introduction of ADT, radical prostatectomy (RP) or Radiotherapy (XRT). Treatment of recurrent PCa is comprised mainly of RP, ADT, and XRT per the National Comprehensive Cancer Network (NCCN) guidelines [Citation14]. All these salvage treatment options are associated with severe complications and comorbidities [Citation3–6]. Previous studies have shown that SFC provides disease control in select patients with acceptable risk of morbidity [Citation3–6]. Also, SFC is gaining popularity in the management of recurrent PCa [Citation11]. We sought to evaluate whether or not SFC could safely delay the use of salvage hormones in recurrent PCa patients who were initially treated with minimally invasive primary treatment options.
Salvage focal cryotherapy delayed the use of salvage ADT or other treatment options in our patient cohort. While a significant number of patients experienced BCR, many had high PSADTs, negative confirmatory biopsies and or negative MRI or bone scan which warrant further delay in ADT use with closer monitoring. In addition, using the Phoenix criteria to determine BCR in focal cryotherapy patients is controversial and there is yet to be a focal cryotherapy-specific BCR criterion for clinical use [Citation15].
The current body of literature together with our results provides supportive data to consider use of SFC to delay ADT. Li et al. [Citation5] in their study of 91 SFC patients from the Cryo On-line Data (COLD), the largest study to date, show a BCRFS survival of 95.3, 72.4, and 46.5 at 1, 3, and 5 years, respectively. Our brief literature review, a 10-year span, found five studies that show a 5-year BCRFS ranging from 46.5% to 54.4%; many of these studies are small single-centre studies [N range: 10–91] [Citation4–6,Citation16,Citation17]. Our study is the largest single-centre study to date and shows a 3-year BCRFS of 48.1%. The time to BCR serves as the window of delay of use of ADT. These numbers are not robust, however, with the growing acceptance of use of cryotherapy in the treatment of organ confined or locally recurrent PCa, high-powered studies, ideally randomised clinical trials, would better characterise this window of delay of ADT use [Citation11].
Interestingly enough, prior treatment history (ADT vs. no ADT) did not impact oncologic outcomes (particularly BCRFS) in our study. This finding is vastly different from what has been reported in the literature. The use of ADT, particularly before salvage total cryotherapy (STC), leads to lower BCRFS [Citation18–20]. Li et al. [Citation19] in a study of 740 patients who underwent STC showed a 5-year BCRFS of 39.3% and 63.3% for the pre-STC ADT and no ADT groups, respectively. The literature postulates that ADT may be delaying and masking the window of opportunity for definitive therapy of hormone independent clonal population of PCa cells [Citation18,Citation19]. To date, none of the three SFC studies have addressed the effect of ADT on BCRFS [Citation21]. Our study, with a longer follow up, may trend or show the association of pre-SFC ADT with lower BCFS.
One of the major reasons the patients and their providers may pursue SFC is to avoid the side effects and complications associated with salvage ADT, XRT, and RP. Unlike the COLD Registry study where the fistula complication rate was 3.3%, we did not experience any rectal fistulas in our cohort [Citation5]. Similarly, in the Wenske et al. study, 5.5% of the SFC cohort experienced a fistula complication [Citation6]. The incontinence rate (6.2%) of our study was comparable with that (5.5%) reported in the COLD registry study [Citation5]. These comparisons might not be accurate due to different BCR definitions, surgical systems, patient inclusion criteria, and a host of other potential confounders. However, the complication rates are low imparting a better quality of life until ADT at a later date.
This retrospective study has limitations. The main limitation to this study is the lack of long-term follow up to track survival outcomes. While the follow up time is comparable with other studies, a longer follow-up time will further our understanding of the curative nature of SFC treatment. This study also lacks a concrete end point, as we are using PSA as a surrogate; the Phoenix criterion is designed to monitor PSA post radiotherapy [Citation22]. The use of an MRI and biopsy findings as end points should be analysed in future studies. As this was a retrospective study, selection bias may have influenced which patients received SFC versus whole gland cryotherapy versus immediate ADT. Furthermore, objective measures of pre-treatment quality of life domains using validated questionnaires were lacking.
Conclusions
Salvage focal cryotherapy may delay the use of ADT in recurrent PCa patients who might otherwise have fewer curative options and are at high risk of major treatment associated morbidities and complications. High-powered studies with longer-term follow-up are required to assess the potential for durable oncologic control.
Disclosure statement
The authors report no declarations of interest.
References
- Agarwal PK, Sadetsky N, Konety BR, et al. (2008). Treatment failure after primary and salvage therapy for prostate cancer: likelihood, patterns of care, and outcomes. Cancer 112:307–14.
- Zelefsky MJ, Kuban DA, Levy LB, et al. (2007). Multi-institutional analysis of long-term outcome for stages T1–T2 prostate cancer treated with permanent seed implantation. Int J Radiat Oncol Biol Phys 67:327–33.
- Chang X, Liu T, Zhang F, et al. (2015). Salvage cryosurgery for locally recurrent prostate cancer after primary cryotherapy. Int Urol Nephrol 47:301–5.
- de Castro Abreu AL, Bahn D, Leslie S, et al. (2013). Salvage focal and salvage total cryoablation for locally recurrent prostate cancer after primary radiation therapy. BJU Int 112:298–307.
- Li YH, Elshafei A, Agarwal G, et al. (2015). Salvage focal prostate cryoablation for locally recurrent prostate cancer after radiotherapy: initial results from the cryo on-line data registry. Prostate 75:1–7.
- Wenske S, Quarrier S, Katz AE. (2013). Salvage cryosurgery of the prostate for failure after primary radiotherapy or cryosurgery: long-term clinical, functional, and oncologic outcomes in a large cohort at a tertiary referral centre. Eur Urol 64:1–7.
- Chiang PH, Liu YY. (2016). Comparisons of oncological and functional outcomes among radical retropubic prostatectomy, high dose rate brachytherapy, cryoablation and high-intensity focused ultrasound for localized prostate cancer. Springer Plus 5:1905.
- Donaldson IA, Alonzi R, Barratt D, et al. (2015). Focal therapy: patients, interventions, and outcomes – a report from a consensus meeting. Eur Urol 67:771–7.
- Shaikh AM, Srivastava A, Atrey MD. (2015). Next generation design, development, and evaluation of cryoprobes for minimally invasive surgery and solid cancer therapeutics: in silico and computational studies. Omics: J Integr Biol 19:131–44.
- Habibian DJ, Katz AE. (2016). Emerging minimally invasive procedures for focal treatment of organ-confined prostate cancer. Int J Hyperthermia 32:795–800.
- Kongnyuy M, Halpern DM, Kosinski KE, Katz AE. (2017). Cryosurgery, an alternative treatment option for organ-confined prostate cancer: current beliefs and practice patterns of urologists. Int Urol Nephrol 49:43–8.
- Bahn D, de Castro Abreu AL, Gill IS, et al. (2012). Focal cryotherapy for clinically unilateral, low-intermediate risk prostate cancer in 73 men with a median follow-up of 3.7 years. Eur Urol 62:55–63.
- Ullal AV, Korets R, Katz AE, Wenske S. (2013). A report on major complications and biochemical recurrence after primary and salvage cryosurgery for prostate cancer in patients with prior resection for benign prostatic hyperplasia: a single-center experience. Urology 82:648–52.
- Network NCC. NCCN Guidelines: Prostate Cancer Version 3; 2016.
- Kongnyuy MLM, Islam S, Robins DJ, et al. (2017). Predictors of biochemical recurrence after primary focal cryosurgery for localized prostate cancer: a multi-institutional analytic comparison of phoenix and stuttgart criteria. AUA 2017 Annual Conference.
- Bomers JG, Yakar D, Overduin CG, et al. (2013). MR imaging-guided focal cryoablation in patients with recurrent prostate cancer. Radiology 268:451–60.
- Eisenberg ML, Shinohara K. (2008). Partial salvage cryoablation of the prostate for recurrent prostate cancer after radiotherapy failure. Urology 72:1315–18.
- Izawa JI, Madsen LT, Scott SM, et al. (2002). Salvage cryotherapy for recurrent prostate cancer after radiotherapy: variables affecting patient outcome. J Clin Oncol: Off J Am Soc Clin Oncol 20:2664–71.
- Li R, Ruckle HC, Schlaifer AE, et al. (2015). The effect of androgen deprivation therapy before salvage whole-gland cryoablation after primary radiation failure in prostate cancer treatment. Urology 85:1137–42.
- Pisters LL, Rewcastle JC, Donnelly BJ, et al. (2008). Salvage prostate cryoablation: initial results from the cryo on-line data registry. J Urol 180:559–63; Discussion 63-4.
- Duijzentkunst DA, Peters M, van der Voort van Zyp JR, et al. (2016). Focal salvage therapy for local prostate cancer recurrences after primary radiotherapy: a comprehensive review. World J Urol 34:1521–31.
- Truesdale MD, Cheetham PJ, Hruby GW, et al. (2010). An evaluation of patient selection criteria on predicting progression-free survival after primary focal unilateral nerve-sparing cryoablation for prostate cancer: recommendations for follow up. Cancer J (Sudbury, Mass) 16:544–9.