Abstract
Purpose: To retrospectively compare the local tumour response and survival rates in patients with non-colorectal cancer lung metastases post-ablation therapy using laser-induced thermotherapy (LITT), radiofrequency ablation (RFA) and microwave ablation (MWA).
Material and methods: Retrospective analysis of 175 computed tomography (CT)-guided ablation sessions performed on 109 patients (43 males and 66 females, mean age: 56.6 years). Seventeen patients with 22 lesions underwent LITT treatment (tumour size: 1.2–4.8 cm), 29 patients with 49 lesions underwent RFA (tumour size: 0.8–4.5 cm) and 63 patients with 104 lesions underwent MWA treatment (tumour size: 0.6–5 cm). CT scans were performed 24-h post-therapy and on follow-up at 3, 6, 12, 18 and 24 months.
Results: The overall-survival rates at 1-, 2-, 3- and 4-year were 93.8, 56.3, 50.0 and 31.3% for patients treated with LITT; 81.5, 50.0, 45.5 and 24.2% for patients treated with RFA and 97.6, 79.9, 62.3 and 45.4% for patients treated with MWA, respectively. The mean survival time was 34.14 months for MWA, 34.79 months for RFA and 35.32 months for LITT. In paired comparison, a significant difference could be detected between MWA versus RFA (p = 0.032). The progression-free survival showed a median of 23.49 ± 0.62 months for MWA,19.88 ± 2.17 months for LITT and 16.66 ± 0.66 months for RFA (p = 0.048). The lowest recurrence rate was detected in lesions ablated with MWA (7.7%; 8 of 104 lesions) followed by RFA (20.4%; 10 of 49 lesions) and LITT (27.3%; 6 of 22 lesions) p value of 0.012. Pneumothorax was detected in 22.16% of MWA ablations, 22.73% of LITT ablations and 14.23% of RFA ablations.
Conclusion: LITT, RFA and MWA may provide an effective therapeutic option for non-colorectal cancer lung metastases with an advantage for MWA regarding local tumour control and progression-free survival rate.
Introduction
The role of surgical resection of non-colorectal cancer lung metastases is mainly determined by the location and number of lesions, comorbidity or the presence of other systemic metastases [Citation1–3]. Systemic chemotherapy is a therapeutic option in non-surgical cases of multiple lung metastases [Citation4–6].
Minimally invasive image-guided therapies became more recognised within the last decade. Thermal ablation methods were designated as therapeutic tool for both non-resectable primary and secondary lung tumours [Citation7–10]. Percutaneous ablation methods, such as microwave ablation (MWA), radiofrequency ablation (RFA) and laser-induced thermotherapy (LITT) were described in the literature as safe and effective therapy options for patients who are not eligible for surgical resection [Citation11].
The purpose of the study was to retrospectively evaluate and compare the local tumour control, time to progression and survival rates in patients with pulmonary metastases from non-colorectal cancer origin, which were treated by LITT, RFA or MWA techniques.
Material and methods
Patient selection and tumour criteria
This study was approved by the ethical committee of the university hospital. All patients provided a written informed consent with the acceptance of the ablation therapy as well as the possible anonymous usage of their data for research purposes. The study was retrospectively performed to include all thermal ablations performed between May 2000 and May 2014. The study included 175 ablation sessions for 175 non-colorectal cancer pulmonary metastases in 109 patients (43 males and 66 females). Seventeen patients [mean age: 53.5 years standard deviation (SD): ±10.2 range: 38–72] underwent LITT (22 ablations), 29 patients (mean age: 57.1 years SD: ±12.8 range: 40–71) underwent RFA (49 ablations) and 63 patients (mean age: 59.6 SD: ±11.9 range: 39–74) underwent MWA (104 ablations) (). The eligibility for ablation therapy was determined by a multidisciplinary team consisting of clinical oncologists, thoracic surgeons, pneumologists, radiation oncologists and interventional radiologists. The inclusion criteria for the ablation therapy were: (i) patients with surgically inoperable pulmonary metastases, (ii) poor candidates for surgery because of medical reasons including limited cardiopulmonary reserve, (iii) patients with five or fewer lesion and (iv) lesions with a maximal axial diameter ≤5 cm, and patients who refused the surgical option.
Table 1. Demographic data of patients’ groups, histopathology of primary tumours and previous treatment.
The exclusion criteria were as follows: (i) uncontrolled primary malignancy, (ii) extrathoracic spread, (iii) more than five lesions, (iv) lesions with a maximal axial diameter >5 cm, (v) radiologic evidence of lymph node metastases, (vi) tumours infiltrating the chest wall or mediastinal structures and (vii) uncontrolled coagulopathy [platelet count <75 000/ml, international-normalised ratios (INR) ≥ 1.5].
Pre-ablation assessment
Pre-ablation images were revised to evaluate the number and size of lesions. A physical examination was performed and a comprehensive clinical history of each patient was taken. Pre-interventional treatment such as systemic chemotherapy, surgical resection of lung metastases and radiation is summarised in .
The coagulation profile was controlled including platelet count, partial thromboplastin time and INR. Because of the risk of bleeding, patients were asked to stop taking anticoagulant or antiplatelet medication for a period ranging between 3 days and 1 week before ablation.
Ablation procedure
LITT
Patients treated with LITT received an intravenous combination of sedative and analgesic medication with Piritramid (0.05–0.2 mg/kg body weight; Dipidolor®, Janssen-Cilaq, Neuss, Germany) and during the entire ablation process blood pressure, electrocardiographic and pulse oximetry were monitored. After injecting local anaesthesia with 10 ml of 0.5% Mepivacain (Scandicain), a 9-F sheath of a laser application kit (SOMATEX Medical Technologies, Berlin, Germany) was inserted under computed tomography (CT) guidance. Ablation procedure was performed by using an Nd:YAG laser (Medilas Dornier, Sunnydale, CA) emitting laser light at an effective distance of 20–30 mm. To monitor temperature changes during ablation, patients were transferred to the magnetic resonance (MR) imaging unit (Privileg, 0.5-T, General Electrics, Milwaukee, WI) where T1-weighted thermal sequences were performed. Routinely after LITT, unenhanced and contrast media-enhanced [0.1 mmol/kg of body weight of gadopentetate dimeglumine (Magnevist, Bayer, Leverkusen, Germany)] MR imaging (Symphony Quantum, 1.5-T, Siemens, Erlangen, Germany) was performed to evaluate the laser-induced necrosis and exclude associated complications, if any.
MWA and RFA
Each thermal ablation was performed by using CT-fluoroscopic guidance (Somatom Sensation 64; Siemens Healthcare GmbH, Erlangen, Germany) with the following parameters: 5-mm collimation, 30 mAs, 120 kV, and 5-mm section thickness. Lung ablation was done under aseptic conditions by two interventional radiologists (with an experience in thoracic intervention of more than 10 and 17 years, respectively).
During ablation all patients received a combination of sedative and analgesic medication with fentanyl citrate (1 μg/kg body weight) and midazolam hydrochloride (0.010–0.035 mg/kg body weight) as long as the ablation was tolerated by the patient. During the procedure, blood pressure and pulse oximetry were monitored continuously.
RFA was done using a RITA® StarbursTM XL 14-gauge/6.4-French nine arrays plus active trocar tip 5 thermocouples with shaft length: 10, 15 and 25 cm and a RITA RF Generator (AngioDynamics, Manchester, GA) with a power of 100–150 W. The mean ablation time of RFA was 10.3 ± 6.7 min (range 2–30 min).
The insertion of the microwave antennas/radiofrequency electrodes was accomplished by a single pleural puncture without using guiding needles. To confirm correct positioning of the antennas/electrodes, the lesion was monitored by using CT. Throughout the ablation procedure CT fluoroscopic imaging was performed to reassess adequate ablation area, to locate the antennas/electrodes and to exclude possible complications. Depending on the severity of peri-procedural complications and the necessity of following interventional procedures, termination of ablation was decided as required. At the end of each session, the needle track had to be coagulated to prevent seeding of tumour cells in the needle track while removing the electrode.
MWA was performed by using Covidien microwave antennas (shaft length: 12, 17 or 22 cm; active tip: 3.7 cm) and microwave generators (Tyco Healthcare Group, Boulder, CO) with power settings at 35–45 W. Thermal ablation times were recorded for all procedures with a mean ablation time of 13.3 ± 7.9 min (range: 3–36 min). A single antenna was used for lesions up to 3 cm in diameter; and two antennae were used for lesions more than 3 cm in diameter. All ablation procedures were performed according to the manufacturer’s recommendations, provided that the total time of ablation and amount of energy were tolerated by the patient.
Follow-up protocol
The local tumour control rate was determined by using unenhanced and contrast-enhanced CT images at 3, 6, 12, 18 and 24 months after LITT, RFA and MWA. The determining factor of success or failure of tumour ablation was the contrast enhancement pattern. Irregular focal soft-tissue enhancement (>15 HU) was considered to be a sign of residual or recurrent disease. A thin symmetric rim of peripheral enhancement of <5 mm wide observed up to 6 months after ablation was considered to be a sign of benign peritumoural enhancement. This was based on the parameters used by different scientific institutions in previous studies [Citation7–11].
Follow-up was performed by CT scans at 24 h after each therapy to exclude delayed post-ablation complications and to assess the morphologic changes. In case of pneumothorax or pulmonary haemorrhage, a chest X-ray after 2–3 h was performed as well. Post-ablation survival rates were also recorded.
Statistical analysis
The data provided included the tumour response from the primary ablation and did not include the re-do lesions to allow homogenous evaluation of the results. The evaluation of pre-, intra- and post-procedural CT images was performed by radiologists, who were not involved in the thermal ablation process of the patients. Tumour volume was calculated on the basis of the evaluated diameters with the following ellipsoidal volume equation: volume = 4/3π × length × width × height. The percentage change of tumour volume post-ablation at follow-up was measured by determining the ratio between: (i) the difference between the basic control volume and the tumour volume post-ablation and (ii) to the basic control volume multiplied by 100.
Patients were classified into three groups, depending on the ablation procedure used (LITT, RFA or MWA). The evaluation included the following variables: (i) technical success, which was defined as treating the tumour according to the recommended manufacturer protocol and covering the target tumour completely by the ablation zone; (ii) ablated region volume changes, which were measured by Kruskal–Wallis method; (iii) side effects and complications; (iv) local tumour control; (v) time to progression and (vi) survival data, including overall and progression-free survival rates.
All patients were included in the calculation of the survival data. Survival rates were calculated from first ablation using Kaplan–Meier and log-rank tests and compared between MWA, LITT and RFA. For the volumetric changes, the volume of ablation zone at 24 h was considered as the control value and to which all other values in follow-up periods were compared. A p values <0.05 was considered statistically significant. Statistical software (Bias for Windows, Version 8.4, Frankfurt, Germany) was used.
Results
Survival data
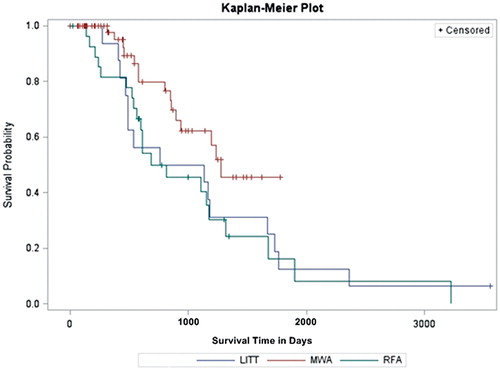
The Kaplan–Meier method showed 1-, 2-, 3- and 4-year overall-survival rates of 93.8, 56.3, 50.0 and 31.3% for patients treated with LITT, 81.5, 50.0, 45.5 and 24.2% for patients treated with RFA and 97.6, 79.9, 62.3 and 45.4% for patients treated with MWA, respectively. The mean survival time was 34.14 month for MWA with a standard deviation of 1.87 month (95% confidence: 29.54 month), 34.79 month for RFA with a standard deviation of 6.14 month (95% confidence: 17.71–38.54 month) and 35.32 month for LITT with a standard deviation of 5.85 month (95% confidence: 15.44–54.93 month).
A Kaplan–Meier curve () of the overall-survival rates within the follow-up period of the study showed no significant difference between LITT, RFA and MWA at log-rank test analysis (p = 0.078). However, in paired comparison, a significant difference could be seen between MWA versus RFA (p = 0.032).
Progression-free survival
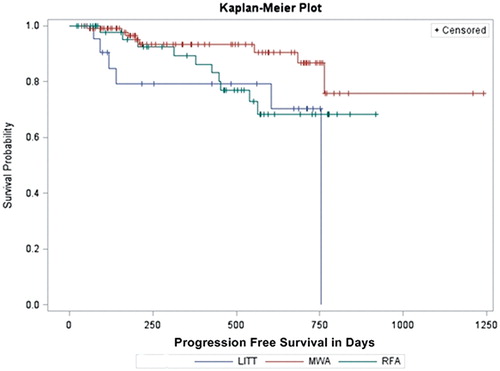
The progression-free survival () showed a median of 23.49 ± 0.62 month for MWA, 19.88 ± 2.17 month for LITT and 16.66 ± 0.66 month for RFA (p = 0.048). In paired comparison, a significant difference could be seen for MWA versus LITT (p = 0.007). The 1- and 2-years of progression-free survival was 93.4 and 86.6% for MWA, 79.2 and 70.4% for LITT, and 89.4 and 68.2% for RFA.
Local tumour control rate
Local tumour control without tumour recurrence or residue detection was achieved in 90.5% (57 of 63 patients) of patients treated with MWA, in 79.3% (23 of 29 patients) of patients treated with RFA and in 70.6% (12 of 17 patients) of patients treated with LITT () ().
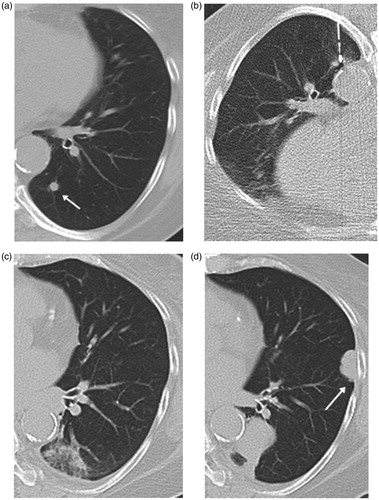
Figure 3. Axial CT images from 60-year-old man with solitary pulmonary metastasis from renal cell carcinoma in the left lower lobe treated by MWA. (a) CT image prior to ablation showing a sub-pleural-rounded pulmonary metastasis of 8 mm axial diameter located on the lateral aspect of left lower lobe (arrow). (b) Image obtained during the ablation therapy. (c) Image obtained 3 months post-ablation showing reticular retraction of the ablation due to progressive scarring. (d) Axial CT image 18 months post-ablation showing a residual scarring in the ablation bed denoting complete ablation of the lesion.
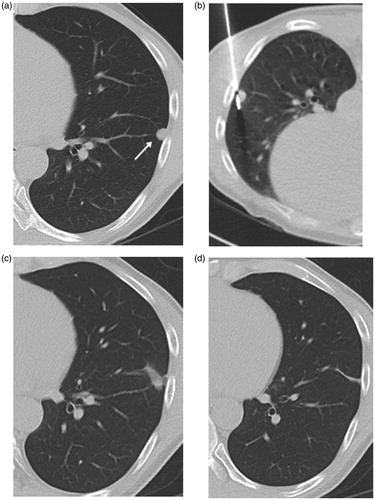
Table 2. Time to tumour recurrence or residual detection.
The lowest recurrence rate was detected in lesions ablated with MWA (7.7%; 8 of 104 lesions) followed by RFA (20.4%; 10 of 49 lesions) and LITT (27.3%; 6 of 22 lesions) p values of 0.012.
The histopathology of the primary had insignificant impact on the local tumour control in all patients groups (p values >0.05).
New metastases developed in 15.9% (10 out of 63) of patients treated with MWA, in 17.2% (5 out of 29) of patients treated with RFA and in 17.6% (3 out of 17) of patients treated with LITT (). The difference in incidence of new metastases development was statistically insignificant between the three ablation groups (p values >0.05)
Figure 4. Axial CT images in 62-year-old female patient with solitary metastasis in the left lower lobe from breast cancer treated with RFA. (a) CT image prior to ablation showing a nodular metastatic lesion in the left lower lobe (arrow). (b) Image obtained during the ablation session showing the RFA-electrode in situ. (c) CT image obtained 24 h after ablation showing the perilesional ground glass opacification induced by ablation therapy. (d) CT image 18 months after ablation shows local tumour progress as well as the appearance of metachronous pulmonary metastatic lesion (arrow).
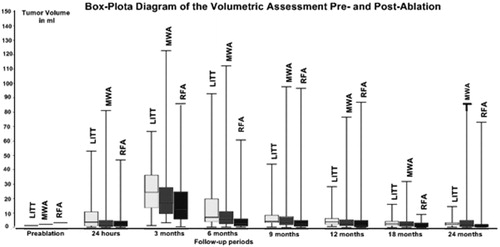
Volume changes of the ablated area
The overall absolute and percentage volume changes for ablated lesions by MWA, RFA and LITT are demonstrated in and . The mean pre-ablation tumour volume was lowest in lesions treated with RFA with 5.66 ± 15.24 ml, followed by those lesions treated with MWA (6.62 ± 14.60 ml) and LITT (8.75 ± 12.77 ml) ().
Figure 5. Axial CT images in 62-year-old male patient with metastatic tumour from malignant melanoma in right lower lobe and treated with LITT. (a) Image obtained before ablation showing a nodular metastatic lesion in the right lower lobe with axial diameter of 2 cm. (b) CT image obtained during the process of ablation showing the LITT-applicator within the lesion. (c,d) CT images 6 and 12 months post-LITT ablation, respectively showing atelectatic changes in the ablation zone followed by regression of the scarring changes and resolution of the post-ablation changes.
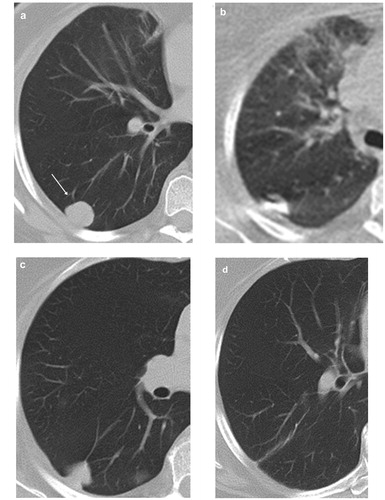
Table 3. Volumetric changes of lesions post-ablation.
The initial tumour volume of lesions with local tumour control for the MWA was 5.52 ± 12.67 ml, for RFA it was 2.82 ± 4.44 ml and for the LITT it was 6.09 ± 8.15 ml. Compared to the initial tumour volume of lesions with recurrence, there was a significant difference for lesions treated with MWA with p = 0.015. The p values for lesions treated with RFA was 0.072 and for those lesions treated with LITT it was 0.228, this also showed no significance.
Procedure-related complications
No mortality was recorded within 30 days of the ablation procedure in all patients groups. Pneumothorax was detected in 22.16% (23/104) of MWA ablations, 22.73% (5/22) of LITT ablations and 14.23% (7/49) of RFA ablations. The incidence of pneumothorax was statistically insignificant among the three groups. Chest tube was indicated to drain pneumothorax in: 4.8% (5/104) of MWA group, 13% (3/22) of LITT group and 7.5% (3/40) of RFA group. Mild reactive pleural effusion developed in 44.23% of MWA, 50% of LITT and 38.78% of RFA without need for therapeutic drainage. Subcutaneous emphysema was detected in 33.65% of MWA, 9.10% of LITT and 4.10% of RFA.
Discussion
Pulmonary metastasectomy is the treatment of choice for lung metastases being the most predictive factor for prolonged survival [Citation1–6]. The criteria for surgical eligibility for metastasectomy are: (i) the primary tumour is controlled, (ii) no extrathoracic lesions are present, (iii) the metastases seem technically resectable and (iv) the general and functional risks are tolerable. Numerous studies in the literature demonstrated 5-year survival rates of 30–50% post-pulmonary metastasectomy for a large variety of primary tumours in highly selected patients [Citation1–6]. However, up to 70% of patients with pulmonary metastases are not suitable for surgery due to the bilaterality or multiplicity of the metastases, uncontrolled primary tumour, low cardio-pulmonary reserve, existing comorbidities, poor lung function, impaired/poor general performance or because of the patient refusing the surgical option [Citation1–6].
Recently, thermal ablation of pulmonary metastases had attracted more attention as an alternative treatment option for patients not eligible for or refusing surgery. The advantages of ablation therapy include selective damage of the tumour tissue, adequate intraprocedual monitoring by CT-imaging control, protection of healthy lung tissue, repetition of treatment is possible and the treatment does not require general anaesthesia [Citation12–16].
In the current study, three different thermal ablation methods (MWA, RFA and LITT) were compared with regards to their effectiveness in the treatment of non-colorectal cancer lung metastases. The efficacy of thermal ablation in general is predominantly governed by ablating the tumour with a surrounding safety margin, to ensure necrosis of microscopic tumour satellites [Citation11–22]. When comparing MWA and LITT on one side and RFA on the other side; RFA has its restrictions in form of impedance and smaller ablation volumes. The physical background of RFA is dependent on the electrical current transmission between the active electrodes through the tissues. This would result in heat production locally around the active electrode. The heat-related dehydration results in a progressive coagulative necrosis of the tissue, but at the same time will lead loss of conductibility of the tissues to the RF current, followed by rising tissue impedance (resistance to the current flow). Additionally, most RFA systems use the relative increase of impedance as a parameter for controlling the ablative process, while ascending impedance down regulates the delivered power. This would limit the ablation volume [Citation21]. MWA induces higher intratumoural temperatures and wider ablation zone resulting in reduced treatment times and an improved tumour control rate [Citation8,Citation14,Citation17]. The current results showed that MWA had a better local tumour control in comparison to LITT and RFA. Theoretical advantages of LITT are the lower cooling effects, the absence of impedance problems and the provocation of large ablation volumes. The most valuable advantage of LITT is the real-time monitoring of ablation under MRI guidance, where thermal MR sequences illustrate increasing temperature in the ablated area, permitting accurate evaluation of the growing coagulative necrosis area [Citation15,Citation19]. Considerable differences were noticed in volume changes of the ablated region, complications, local tumour control and survival data.
Volumetric assessment of lesions post-ablation demonstrated an increase in size of all ablated lesions in all patient groups at 24 h, which could be a result of the ablation-induced oedema and inflammatory response surrounding the ablated lesions [Citation14]. In patients treated with MWA and LITT, a higher mean volume was recorded at 24 h in comparison to RFA. This higher volume could add to the technical benefits of these methods as reported in several studies [Citation14]. The wide ablation zone induced by MWA and LITT is most likely due to the higher intratumoural temperatures, decreased “heat sink” effect, and increased affinity for water-based tissue [Citation14,Citation17].
In the current study, lesions treated with MWA showed significantly the lowest local recurrence rate of 7.7%, as compared to RFA (20.4%) and LITT (27.3%). Additionally, concerning the overall-survival times, the highest rates were seen for MWA, even though the difference was not statistically proven. The progression-free survival rates showed here a significant benefit for MWA.
The results published by Simon et al. in 2007 [18], regarding the efficacy of RFA in the treatment of Stage 1 non-small cell lung cancer (NSCLC) and pulmonary metastasis from colorectal carcinoma, supports our results. They reported an overall 1-, 2-, 3-, 4- and 5-year survival rates of 78, 57, 36, 27 and 27%, respectively for Stage I NSCLC; while the rates for colorectal pulmonary metastases were 87, 78, 57, 57 and 57%, respectively. They reported that local tumour progression-free rates were significantly related to the tumour size whether larger or smaller than 3 cm. The 1-, 2-, 3-, 4- and 5-year local tumour progression-free rates in their study were 83, 64, 57, 47 and 47%, respectively for tumours 3 cm or smaller and were 45, 25, 25, 25 and 25% for tumours larger than 3 cm.
This study showed comparable data to literature regarding local tumour control and progression-free survival rates in MWA. Lu et al. [Citation22] reported in their study, which included 69 patients treated with MWA, that the progress-free period for all patients was 22.0 months. The overall-survival rates in 1, 2 and 3 years were 66.7, 44.9 and 24.6%, respectively; the overall-survival rates for NSCLC patients in 1, 2 and 3 years were 75.0, 54.2 and 29.2%, respectively, and the overall-survival rates for pulmonary metastatic tumour patients in 1, 2 and 3 years were 47.6, 23.8 and 14.3%, respectively. The recurrence free-survival rates for NSCLC patients in 1, 2 and 3 years were 72.9, 50.0 and 27.1%, respectively; and the mortality rates for pulmonary metastatic tumour patients in 1, 2 and 3 years were 47.6, 19.0 and 14.3%, respectively [Citation21]. In their study, Wolf et al. [Citation17] demonstrated a tumour control of 74% (37 of 50 patients) with an overall-survival rate for 1-, 2- and 3-years of 65, 55 and 45.4%, respectively and a progression-free survival for 1 year of 67%. In their study, Rosenberg et al. [Citation19] reported a local tumour control rate of 48.44% (31 of 64 patients) post-LITT therapy. They quantified a progression-free survival of 7.4 month and a survival-rate of 81, 59, 44, 44 and 27% for 1, 2, 3, 4 and 5 years, respectively. This shows discrepancy to our presented data which could be attributed to the different applicator system used for ablation therapy in both institutes.
Pneumothorax was detected in 22.16% of MWA ablations, 22.73% of LITT ablations and 14.23% of RFA ablations. The range of pneumothorax incidences in the literature varies also according to the method of ablation, being 3.5–18% for RFA, 25–39% for MWA and up-to 38% for LITT treatment.
This study was limited by a number of factors, including the non-randomised non-controlled retrospective design, the relatively short imaging follow-up of 24 months, the element of selection bias of the modality of ablation and the absence of histopathological correlation after ablation. The selection of method was driven by breakthroughs in the field of pulmonary ablation therapy. Another factor is the difference in number of lesions treated by each modality.
Conclusion
MWA, RFA and LITT could be used as therapeutic options for lung metastases from non-colorectal cancer. Statistically significant differences were seen in local tumour control and progression-free survival representing a potential advantage for MWA over RFA and LITT.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of this article.
References
- Pagès PB, Serayssol C, Brioude G, et al. (2016). Risk factors for survival and recurrence after lung metastasectomy. J Surg Res 203:293–300.
- Kaifi JT, Gusani NJ, Deshaies I, et al. (2010). Indications and approach to surgical resection of lung metastases. J Surg Oncol 102:187–95.
- McGarry RC, Song G, des Rosiers P, Timmerman R. (2002). Observation-only management of early stage, medically inoperable lung cancer: poor outcome. Chest 121:1155–8.
- Davidson RS, Nwogu CE, Brentjens MJ, Anderson TM. (2001). The surgical management of pulmonary metastasis: current concepts. Surg Oncol 10:35–42.
- Smith R, Pak Y, Kraybill W, Kane JM III. (2009). Factors associated with actual long-term survival following soft tissue sarcoma pulmonary metastasectomy. Eur J Surg Oncol 35:356–61.
- Friedel G, Pastorino U, Buyse M, et al. (1999). Resection of lung metastases: long-term results and prognostic analysis based on 5206 cases. Int Registry Lung Metastases 124:96–103.
- Cheng M, Fay M, Steinke K. (2016). Percutaneous CT-guided thermal ablation as salvage therapy for recurrent non-small cell lung cancer after external beam radiotherapy: a retrospective study. Int J Hyperthermia 32:316–23.
- Sidoff L, Dupuy DE. (2016). Clinical experiences with microwave thermal ablation of lung malignancies. Int J Hyperthermia 25–33. Available form: https://doi.org/http://dx.doi.org/10.1080/02656736.2016.1204630 [Epub ahread of print].
- Sofocleous CT, Sideras P, Petre EN, Solomon SB. (2011). Ablation for the management of pulmonary malignancies. AJR Am J Roentgenol 197:581–9.
- Fanucchi O, Ambrogi MC, Aprile V, et al. (2016). Long-term results of percutaneous radiofrequency ablation of pulmonary metastases: a single institution experience. Interact Cardiovasc Thorac Surg 23:57–64.
- McTaggart RA, Dupuy DE. (2007). Thermal ablation of lung tumors. Tech Vasc Interv Radiol 10:102–13.
- Thanos L, Mylona S, Ptohis N, et al. (2009). Percutaneous radiofrequency thermal ablation in the management of lung tumors: presentation of clinical experience on a series of 35 patients. Diagn Interv Radiol 15:290–6.
- Bojarski JD, Dupuy DE, Mayo-Smith WW. (2005). CT imaging findings of pulmonary neoplasms after treatment with radiofrequency ablation: results in 32 tumors. AJR Am J Roentgenol 185:466–71.
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. (2011). Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 261:643–51.
- Vogl TJ, Straub R, Lehnert T, et al. (2004). Percutaneous thermoablation of pulmonary metastases. Experience with the application of laser-induced thermotherapy (LITT) and radiofrequency ablation (RFA), and a literature review. Rofo 176:1658–66.
- Baisi A, De Simone M, Raveglia F, Cioffi U. (2013). Thermal ablation in the treatment of lung cancer: present and future. Eur J Cardiothorac Surg 43:683–6.
- Wolf FJ, Grand DJ, Machan JT, et al. (2008). Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 247:871–9.
- Simon CJ, Dupuy DE, DiPetrillo TA, et al. (2007). Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 243:268–75.
- Rosenberg C, Puls R, Hegenscheid K, et al. (2009). Laser ablation of metastatic lesions of the lung: long-term outcome. AJR Am J Roentgenol 192:785–92.
- Carrafiello G, Mangini M, Fontana F, et al. (2012). Complications of microwave and radiofrequency lung ablation: personal experience and review of the literature. Radiol Med 117:201–13.
- Vogl TJ1, Naguib NN, Lehnert T, Nour-Eldin NE. (2011). Radiofrequency, microwave and laser ablation of pulmonary neoplasms: clinical studies and technical considerations. Eur J Radiol 77:346–57.
- Lu Q, Cao W, Huang L, et al. (2012). CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol 10:80.