Abstract
The gadolinium-doped iron oxide nanoparticles (GdIONP) with greater specific power adsorption rate (SAR) than Fe3O4 was developed and its potential application in tumour therapy and particle tracking were demonstrated in transgenic adenocarcinoma of the mouse prostate C1 (TRAMP-C1) tumours. The GdIONPs accumulated in tumour region during the treatment could be clearly tracked and quantified by T2-weighted MR imaging. The therapeutic effects of GdIONP-mediated hyperthermia alone or in combination with radiotherapy (RT) were also evaluated. A significant increase in the tumour growth time was observed following the treatment of thermotherapy (TT) only group (2.5 days), radiation therapy only group (4.5 days), and the combined radio-thermotherapy group (10 days). Immunohistochemical staining revealed a reduced hypoxia region with vascular disruption and extensive tumour necrosis following the combined radio-thermotherapy. These results indicate that GdIONP-mediated hyperthermia can improve the efficacy of RT by its dual functions in high temperature (temperature greater than 45 °C)-mediated thermal ablation and mild-temperature hyperthermia (MTH) (temperature between 39 and 42 °C)-mediated reoxygenation.
Introduction
Magnetic nanoparticles are widely used for a variety of biomedical application, including targeted delivery of therapeutic agents, hyperthermia treatment of cancers, magnetic resonance (MR) imaging, and biomagnetic separation [Citation1–4]. Magnetic nanoparticles have been suggested as a suitable mediator in conventional thermotherapy (TT) for achieving intratumour hyperthermia and reducing side effects [Citation5–7]. Magnetic nanoparticles-mediated TT involves a coupling of an external magnetic field to heat the tumour areas surrounding the magnetic particles [Citation8–10]. The magnetic particles interact with the magnetic field and dissipate energy to their surroundings, causing a heating effect in deep tissues. The mechanisms that the energy is dissipated are strongly related to the physical and chemical properties of the particles and can be expressed by their specific power adsorption rate (SAR) [Citation11]. We have developed gadolinium-doped iron oxide nanoparticles (GdIONP) with superparamagnetic characteristics and a higher SAR value than the reported ones of Fe3O4 by four times [Citation12,Citation13]. Radiation therapy has been employed to treat approximately 50% of various cancer patients. The ionising radiation dosage given to the tumour is determined based on the radiosensitivity of the surrounding normal tissue [Citation14]. Therefore, the tolerant radiation dose is frequently not sufficient to eliminate all loco-regional reoccurrences or cure localised cancers [Citation15]. The success rate of local tumour control by radiation therapy is determined primarily by the number of clonogens [Citation16] and their intrinsic cellular radioresistance, and the microenvironmental factors such as hypoxia. The hypoxia induced by cellular and micro-environmental changes of tumour contribute to tumour aggressiveness and resistance to radiation therapy [Citation15,Citation17]. Several therapeutic strategies have been developed to overcome the problem of tumour hypoxia [Citation18]. One of the most highly effective therapeutic adjuncts for radiation therapy is mild hyperthermia, which provides both direct anti-tumour [Citation19,Citation20] as well as the microenvironment effects [Citation21, Citation22]. Several preclinical or clinical studies have demonstrated improved responses when tumours were treated with a combination of localised hyperthermia and radiotherapy (RT) compared with RT alone [Citation19,Citation23,Citation24]. However, several issues still need to be resolved before hyperthermia can be utilised as a radiation enhancer in routine clinical practices, including a sufficient delivery of thermal doses, the heating duration required, and the problem resulted from the instability of local temperatures. Nanoparticle-induced magnetic field hyperthermia has shown its potential in solving these issues by providing a non-invasive and localised treatment. In the previous studies, we have shown the biocompatibility, thermal profile under alternating magnetic field and superparamagnetic characters of the GdIONP [Citation12,Citation13]. Herein we further demonstrate the effects of GdIONP-induced magnetic field hyperthermia combined with a RT and the interaction mechanism in an intramuscular TRAMP-C1 tumour model.
This study demonstrates that the magnetic nanoparticle-mediated hyperthermia caused mixed thermotherapeutic effects such that thermal ablation close to the GdIONP localisation while mild hyperthermia at a distant was observed. The results suggest that GdIONP-induced magnetic field hyperthermia enhances the efficacy of radiation therapy by two possible mechanisms: (1) reducing the fraction of hypoxic cells that are resistant to radiation cytotoxic effect and (2) inducing localised tumour-specific vascular disruption and necrosis. This study also presents a simple approach to incorporate the advantages of these two radiation-modifying effects using a single strategy, GdIONP-mediated hyperthermia.
Materials and methods
Synthesis of GdIONP
GdIONP were synthesised as described by Drake et al. [Citation12]. The detailed synthesis and characterisation can be seen in the supplementary material associated with this paper. The nanoparticle (NPs) composition was Gd0.02Fe2.98O4 as determined by inductive coupled plasma with atomic emission spectroscopy (ICP–AES). The nanoparticle (NPs) composition was Gd0.02Fe2.98O4 as determined by ICP–AES. The GdIONPs are 13.2 ± 3.1 nm in diameter and superparamagnetic with a magnetisation value (Ms) of 65.67 emu g−1. The magnetisation curve, X-ray diffraction pattern (XRD) and TEM images can be seen in the Figures S1, S2 and S3, respectively. For use in this study the GdIONPs were given a dextran coating. To achieve this the nanoparticles were dispersed in deionised water and dextran (10 000 Mw). After ultrasonic mixing, NH4OH was added to bring the pH to 10. The mixture was then continuously stirred while being heated to 75 °C and was held at this temperature for 75 min. To remove excess dextran the suspension was dialysed using a membrane with a molecular weight cut-off (MWCO) at 10 000 Daltons. The suspension was then centrifuged at 4000g for 30 min to remove any large aggregates. Finally, the suspension was filtered through a 0.2 mm filter.
Cells and animals
All experiments were done with the use of six-to-eight-weeks-old male C57BL/6 J mice. The TRAMP-C1 prostate cancer cell line was derived from transgenic mice with adenocarcinoma of the mouse prostate [Citation25] and was purchased from the ATCC (CRL-2730). During the experiments, all mice were cared for in accordance with the guidelines approved by the Institutional Animal Care and Use Committee (IACUC) of National Tsing Hua University, Taiwan (approved number: IACUC:09705). Tumours were generated by intramuscular injection (i.m.) of 3 × 106 viable cells into the thigh. Mice with tumours with 4–6 mm in diameter were selected and randomly allocated to groups for experimentation (tumour diameter was defined as the diameter of tumour-bearing leg – the diameter of control mouse leg, in which the diameter is defined as ((the length of long axis at the tumour region + the length of short axis at the tumour region)/2), and the tumour volume in mm3 was calculated by the formula: volume = 4/3 × 3.1416 × (diameter/2)3, in which at least five mice were evaluated at each time point.
Radiotherapy and thermotherapy
The mice were randomly divided into four groups for the different treatments: (1) no treatment as control, (2) a single dose radiation therapy (RT) of 25 Gy, (3) twice of the 40-min GdIONP-mediated TT using magnetic field hyperthermia (500 Ampere and 200 Gauss) separated by 16-h interval, (4) a combined treatment (RT + TT) using a 25 Gy radiation therapy followed by a TT (radio-thermotherapy) 30 min afterward (see Figure S4). The mice in TT group and RT + TT group were injected with 0.05 ml GdIONP in saline before the magnetic field hyperthermia. The mice in control group and RT group were injected with 0.05 ml saline without hyperthermia treatment. For mice receiving two injections of either nanoparticles or saline solutions for the blank control group, the first injection were conducted at the start of treatment or after RT, and the second injection were carried out 16 h after the first injection. This combined therapeutic protocol was designed according to the suggested best combined sequence [Citation14]. Mice were anaesthetised by a mixture of ketamine and xylazine and restrained by adhesive tape during irradiation, TT, and MRI imaging. A single dose irradiation of 25 Gy was delivered to the tumour site using 6 MV X-rays from a linear accelerator with a dose rate of 2.3 Gy/min and a 1.5 cm bolus on the surface, with the rest of body shielded. GdIONP (1 mg) prepared in 0.05 mL saline was intratumorally injected into the tumours. The groups receiving TT were then subjected to an induction heating operated at 52 kHz and 246 Oe. The tumour size was then monitored by daily recording the diameter measured by Vernier calliper. The temperature read by the thermocouple needle positioned at the tumour core (or injection site) was continuously recorded during the magnetic hyperthermia. The effect of comparison among groups was made with tumour growth delay that was defined as the time taken for a treated tumour to grow to 350 cubic mm after treatment and compared with control.
Immunohistochemistry and image analysis
For detecting hypoxia, pimonidazole hydrochloride (Hypoxyprobe-1 Kit; Hydroprobe, Burlington, MA) was administered as 4 mg in 0.1 mL by intravenous injection (i.v.). One hour after administration of the hypoxia reagent [Citation26], the tumours were removed and stored in optimal cutting temperature compounds at −80 °C. For immunohistochemical staining, 10-mm cryostat sections were fixed in methanol at −20 °C for 10 min and then rehydrated in PBS. Nonspecific binding was blocked by incubating the sections in 1% bovine serum albumin in PBS for 30 min. Tumour sections were double stained for pimonidazole (PIMO) and CD31. Pimonidazole was detected with the mouse antibody (Hydroprobe, Burlington, MA) and goat anti-mouse IgGγ1 Alexa 488 (Invitrogen, Carlsbad, CA). For labelling endothelial cells, rat anti-CD31 antibody (BD Biosciences, San Jose, CA) and then a secondary goat anti-rat antibody conjugated with Alexa 594 (Invitrogen, Carlsbad, CA) was utilised. Slides were rinsed in PBS and mounted with ProLong Gold antifade reagent with DAPI (P-36931; Invitrogen, Carlsbad, CA). Sections stained by standard H&E reagent (Merck, Kenilworth, NJ) were used to identify the necrotic areas.
Immunofluorescent images from each tumour section were captured with the use of an external digital camera (DXM-1200C; Nikon, Melville, NY) on a Nikon fluorescence microscope (Nikon Eclipse TE 2000-S). Scanning at ×100 magnification yielded composite images of endothelial cells with the perfusion marker and pimonidazole. Composite images were obtained at ×200 or ×630 magnification. The images were analysed by Image-Pro version 4 software (Media Cybernetics, Rockville, MD). Microvascular density (MVD) was determined as the number of pixels positive for CD31 divided by the total tumour area. Regions of gross necrosis were visually identified on the H&E images and were outlined with a multiple-area-of-interest tool to determine the percentage in total tumour area. The hypoxia fraction was defined as the area positive for pimonidazole divided by the total tumour area (necrosis excluded). Data are presented as means with standard deviation. Statistical analyses used GraphPad Prism version 3 (GraphPad Software, La Jolla, CA). For all comparisons, assessment of statistical significance was by unpaired t-tests or one-way ANOVA with significance set at p ≦ 0.05.
MR imaging
All magnetic resonance imaging (MRI) scans were acquired on a 7-T animal MRI scanner (Bruker ClinScan 70/30, Karlsruhe, Germany). The imaging parameters for the T1-weighted images of pre-GdIONP injection were as follows: repetition time (TR)/echo-time (TE)/flip angle = 600 ms/3.81 ms/25°, slice thickness = 0.5 mm, matrix size = 176 × 256 and field-of-view (FOV) = 27 × 40 mm. About 1 mg GdIONPs in 0.05 mL of saline were intratumorally injected. The T1-weighted imaging parameters were used: TR/TE/flip angle = 600 ms/3.81 ms/25°, slice thickness = 0.5 mm, matrix size = 176 × 256 and FOV = 34 × 50 mm. T2-weighted images were then acquired to confirm no anatomical change after GdIONPs injection. The following parameters were used: TR/TE/Flip angle = 3140 ms/38 ms/180°, slice thickness = 1 mm, matrix size = 228 × 320, FOV = 25 × 35 mm.
Results
Combined GdIONPs-mediated hyperthermia with radiation therapy enhances tumour growth delay
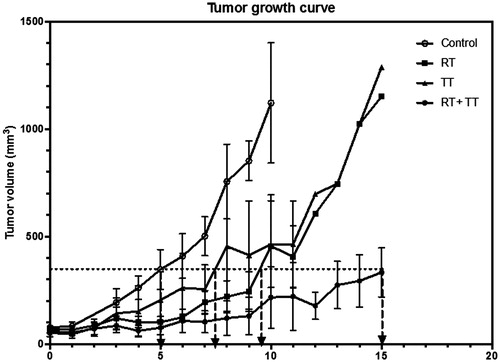
In order to assess the influences of GdIONPs-mediated hyperthermia on radiation therapy-induced tumour growth delay, the tumour size of individual mouse in each treatment group was measured daily by calliper (). The tumour growth curve shows that the RT and TT had similar effect on tumour growth delay (2.5 and 4.5 days, respectively), while tumour growth were delayed more than 10 days by the combined therapy (RT + TT). This result suggests that the GdIONPs-mediated hyperthermia could significantly increase the efficacy of radiation therapy for TRAMP-C1 tumour.
Figure 1. Tumour growth curves after various treatments. Combined therapy (RT + TT) has resulted in longer tumour growth delay (∼10 days) than the radiotherapy (RT, ∼4.5 days) or the GdIONP-mediated thermotherapy (TT, ∼2.5 days) only. TRAMP-C1 cells (1 × 106) were injected intramuscularly into the tights of male C57BL/6 J mice. Mice with a palpable tumour of approximately 60 mm3 in volume were randomly separated into four groups (n = 5) and were given two times intratumour injections of GdIONP or saline before thermotherapy. Mice were anaesthetised before treatments. For the control and RT group, 0.05 ml saline alone was injected. For TT and RT + TT group, 1 mg GdIONP prepared in 0.05 ml saline was injected. TT group were subjected to induction heating at 52 kHz and 246 Oe. In the end of each experiment, mice were sacrificed and the tumours were excised and weighed.
GdIONP-mediated hyperthermia
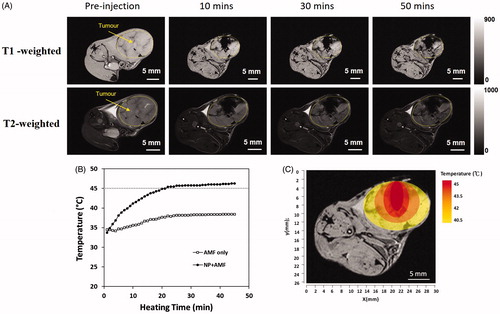
The distribution of the injected GdIONPs was investigated using T1 and T2 MRI as shown in . Images showed that the injected GdIONPs remained concentrate in TRAMP-C1 tumours after the hyperthermia treatment for 10, 30, and 50 min. The temperature profile () recorded by thermocouple needle showed that the temperature of at the cord of tumour tissue exceeded 45 °C after hyperthermia treatment for more than 40 min. The simulated temperature distribution profile () [Citation27] showed that the tumour temperature decreases 1.5 °C for each 2 mm. This indicates that GdIONP-mediated thermal effect may not be the same at the different regions, which depends on the distance to the injection site.
Figure 2. (A) The retention of GdIONPs in the TRAMP-C1 tumour shown by T1 and T2-weighted MR imaging taken at different times. The injected GdIONPs were remained in the TRAMP-C1 tumours (highlighted by the yellow outline) for 10, 30, and 50 min. (B) The temperature distribution profile changes across heating time measured by a thermocouple needle at the GdIONP injection site. The thermotherapy was conducted by an induction heating at an external alternating magnetic field (AMF, 52 kHz and 246 Oe) for 40 min. Mice were injected with PBS (AMF only) or GdIONP (AMF + NP) under external alternating magnetic field (AMF). (C) The simulated temperature distribution within tumour. The tumour temperature decreased by 1.5-°C for every 2 mm away from the TT core.
Anti-tumour and microenvironmental effects
Hematoxylin and Eosin (H&E) and PIMO staining were used to examine the pattern of necrosis and hypoxia, respectively, of tissues at day 1 and week 1 post GdIONP-induced thermotherapy, and the necrotic regions were highlighted by the yellow outlines in . While there is no regular necrotic pattern in control tumours at day 1, the necrotic regions in thermotherapy-treated tissue were mainly located in the areas with intensive signal of T2 image (). This is in consistent with our previous report that the necrotic region induced by TT was co-localised with GdIONP deposition from the analysis obtained by the laser ablation/inductively coupled plasma (LA–ICP–MS) mapping technique [Citation28]. PIMO staining revealed large continuous hypoxia zones surrounding the necrotic regions. When the areas of the necrotic and hypoxic regions were calculated (), it appeared that the ratios of necrosis and hypoxia in the control tumours were increased as the tumours grew. However, the necrotic region was significantly increased one day after TT, and the ratio was maintained high for one week after treatment. It is worth noting that the ratio of hypoxia was not increased with the increase of necrotic region at week one after TT as observed in the control group. This supports the hypothesis that two working mechanisms were involved in the GdIONP-mediated hyperthermia therapy. In order to better characterise the effect of these two working mechanisms, the tissues were counterstained by PIMO for hypoxia and CD31 for vessels (). The CD31 positive cells of the control group were randomly distributed all over the tumour, suggesting the vessels were intervened throughout the hypoxia and necrosis regions (). This is consistent with a general opinion that the hypoxia and necrosis of growing tumours mainly due to the malfunction of tumour vessels [Citation26].
Figure 3. H & E and PIMO staining of tumour of control (A and C) and thermotherapy (B and D) one day (A and B) and one week (C and D) after treatment. The percentage of necrosis (E) and hypoxia (F) of the control and thermotherapy (TT)-treated TRAMP-C1 tumours at day 1 and week 1 after TT. The largest cross section of the TRAMP-C1 tumour was used to normalise the necrosis and hypoxia areas. Necrosis region were delineated by the yellow outlines. The pimonidazole (PIMO) was administrated i.v. injection for detecting hypoxia. Bars indicate the SE of 3–5 tumours at each time point. The hypoxia fraction was defined as the area positive of pimonidazole divided by the total tumour area (necrosis excluded). *p < .05.
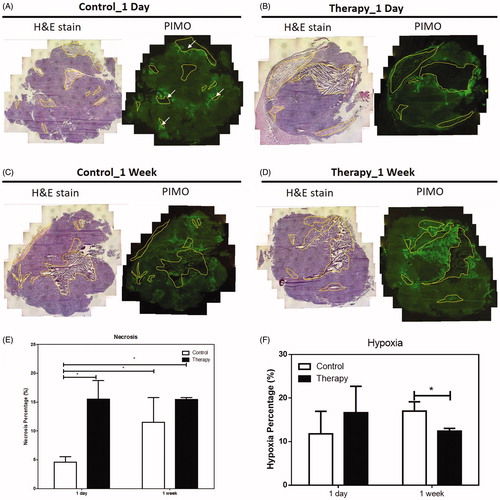
Figure 4. (A) The distribution of necrosis denoted by N, hypoxia stained by PIMO (green colour), and vasculature stained by CD31 (red colour) in control and TT treated TRAMP-C1 tumours at one day and one week after treatment. The scale bar equals 100 μm (B) The change of MVD in hypoxic and nonhypoxic regions of the control and TT-treated TRAMP-C1 tumours at day 1 and week 1 after TT is shown as a bar graph. MVD was defined by the percentage of CD31 + area to the total tumour in field of view. The Bars indicate the SE of measurements from five fields (non-necrotic areas) of 3–5 tumours for each time point.
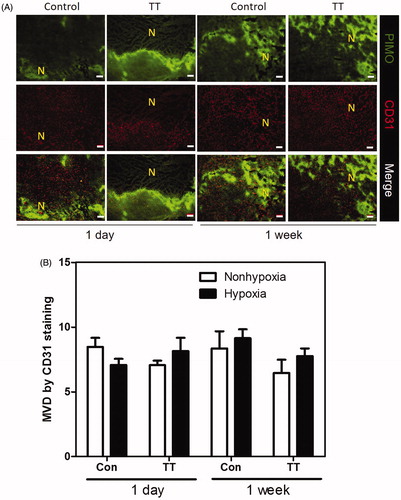
In contract, almost no vessels were observed in the necrotic regions at day 1 post TT. The MVD in the non-necrotic regions () did not significantly change at day 1 and week 1 post TT. This again supports the theory that thermal ablation effect took place at regions subject to high temperature and resulted in massive vessel disruption and necrosis, while the MTH on the contrary did not cause significant impact on vessel numbers. We further evaluated the changes in necrosis, hypoxia, and MVD of the tumour specimens for the four experimental groups, (control, RT, TT and RT + TT), at 15 days post treatment despite that the control tumour was removed at day 10 due to ethical issue (). As the inference from the previous experiments, the necrosis ratio in tumour of the control group was further increased to 44.3% () while the ratio of hypoxia was maintained at a 15.4% level (). This might be owing to the relative constant MVD at untreated tumours (). It should be noted that the necrotic region was excluded from the calculation of hypoxia ratio and MVD.
Figure 5. (A) hypoxia (green), vasculature (red), and nuclei (blue) areas revealed by immunofluorescence staining for the control (10 days), RT (15 days), TT (15 days), and combined therapy (15 days) treated TRAMP-C1 tumours. The change of (B) necrosis, (C) hypoxia and (D) MVD in hypoxic and nonhypoxic regions of control and TT-treated tumours were represented using a bar graph. There was almost no vasculature in the hypoxia regions of irradiated tumours (RT and combined therapy). Bar: SE of five fields (non-necrotic areas) of 3–5 tumours for each time point.
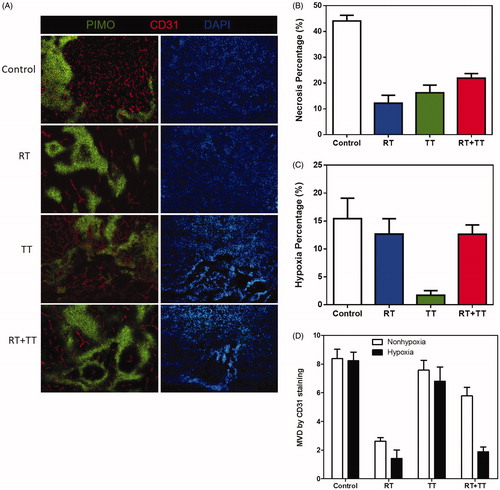
On the other hand, although the 25 Gy of irradiation (RT group) and the two-dose 40-min GdIONP-mediated TT group produced similar tumour growth delay (), they might contribute differently to the changes of necrosis, hypoxia, and MVD. The tissues from TT-treated tumours had a higher percentage of necrosis, lower percentage of hypoxia, and higher MVD than those in RT-treated tissues. This indicates different anti-tumour mechanisms might be involved in RT vs. TT. The combined radio-thermotherapy had the greatest effect on reducing tumour size and brought about higher percentage of necrosis (21.87%, ) and hypoxia (∼13%, ), but mixed results in MVD (). This indicates a complex interactive mechanism between RT and TT.
Discussion
This study describes the anti-tumour effect and microenvironmental changes after the GdIONP-mediated TT, radiation therapy or combined radio-thermotherapy. The results suggest that the combined radio-thermotherapy can significantly inhibit tumour growth better than RT or TT alone. The enhancement of radiation therapy by GdIONP-mediated TT resulted from a mixed thermotherapeutic effect, high temperature-mediated thermal ablation and mild-temperature hyperthermia (MTH)-mediated tumour reoxygenation. It has been shown that thermal ablation could lead to tumour destruction by direct cell necrosis, coagulation, and protein denaturation [Citation29]. The significant increase of necrosis percentage one day after TT and the spatial correlation of necrosis pattern and GdIONP distribution tracked by MR imaging support the ablation effect of the GdIONP induced hyperthermia (temperature >45 °C). The dysfunction of the vessel collapsed at GdIONP-concentrated regions was due to the thermo-sensitive response of vessels in tumours. The tumour vasculature system is less capable in heat dissipation owing to blood flow reduction and therefore is more likely to be damaged by hyperthermia [Citation30–32]. Owing to inefficient blood flow and oxygen transported by the immature blood vessels the tumour cells reside in an acidic and nutrient-deprived environment that confers them greater thermosensitivity. This is the main effect contributed by high temperature TT. The effect of TT on vessel networks was not so evident when the MVD of non-necrotic area only was used for evaluation. However, decrease in the number of vessels and enlargement in vessel diameter were observed. It has been suggested that blood flow and perfusion could be increased by mild hyperthermia (heating to 39–42 °C) [Citation14,Citation31,Citation33]. Due to the temperature gradients induced by the magnetic nanoparticle in hyperthermia (), a mild hyperthermia effects could occur on the periphery of the GdIONP concentrated region. The temperature gradient of tumour is −0.75 °C/mm from the centre of MNPs for multi-layer structures such as skin, fat and muscle according to the numerical analysis results of MNPs-mediated hyperthermia [Citation27]. MR imaging and LA–ICP–MS mapping showed that GdIONP were localised in the injected site throughout the hyperthermia and remained within an area of 3–5 mm in diameter inside the tumour for two weeks after TT [Citation28]. The temperature at tumour core was more than 45 °C and decreased from 43 to 40 °C at 2.7 to 6.7 mm away from the GdIONPs-deposited region (). Therefore, the periphery of GdIONP concentrated-tumour core might be subject to mild-hyperthermia. This is possibly the reason that the hypoxia ratio in the TT-treated group was the lowest among all four groups after two weeks (). It needs to remind that the TT was given on day 0. The histological data that we saw from the samples of week 2 were a re-growing tumour, of which the microenvironment was affected by the tumour microenvironment altered by the TT therapy at day 0. In opposite to the TT, RT resulted in small and dispersed necrosis areas [Citation26]. The combined radio-thermotherapy produced both effects thus resulting in large necrotic areas in GdIONP-concentrated tumour core and small necrosis in the surroundings. This partially explains the increased effect on the tumour growth delay (). It also indicated that GdIONP-mediated hyperthermia and RT might have complementary role in tumour therapy. In addition to the reduction in MVD, RT, as TT, it also resulted in increase in vessel diameter, which is in consistent with the published results [Citation26,Citation34]. These results demonstrate that TT and RT not only have complementary anti-tumour effect, but also result in vessel dilation.
In this study, the TT was applied after RT since it has been reported that such schedule yielded the best enhancement for RT through heat [Citation23]. Hyperthermia acts as a potent and selective radiosensitizer by three major possible mechanisms: (1) inhibiting or impairing DNA repair, (2) devastating the radioresistant and hypoxic tumour cells, (3) altering the vascular microenvironment [Citation14]. The combination of heat and radiation in a clinical regimen will probably be conducted in a fractionated schedule. For clinical practice a pronounced treatment could be realised by adjusting the sequence, frequency, dosage, duration and the intermittent time for conjointly administering the two modalities [Citation35–37]. The combined radio-thermotherapy protocol used in this study contributed to the reduction in hypoxia and generated similar vascular patterns as the RT alone, indicating the involvement of mild hyperthermia effect implied by the observed enhanced effects from the tumour growth curve.
Conclusions
The GdIONP in this study were demonstrated to have a high SAR value and superparamagnetic characteristic [Citation15]. The improved SAR value of this Gd-doped system can be translated into improved tumour therapy [Citation16]. The MR imaging and LA–ICP–MS mapping verified the stability of the nanoparticles with no decomposition evident in the tissue and their potential usage as T2 contrast agents [Citation30]. In this study, we further demonstrate that GdIONP-mediated TT delivered mixed thermotherapeutic effects, showing thermal ablation in Gd-IONP-deposited region and mild hyperthermia away from the thermal ablation region. The approach enhances the efficacy of radiation therapy by reducing the fraction of hypoxic cells that contribute to radiation resistance and by inducing tumour-specific localised vascular disruption and necrosis. This study also presents a simple means to combine the advantages of these two radiation-modifying effects using a single strategy, GdIONP-mediated hyperthermia. The multiple modalities of the GdIONP highlight the potential for biomedical application.
Supplementary File
Download PDF (183.7 KB)Acknowledgements
This work is supported by MOST 104–2627-M-007–008 from the Ministry of Science and Technology, 104N2741E1 from National Tsing Hua University, and 104-EC-17-A-22–0777 from Ministry of Economic Affairs, Taiwan.
Disclosure statement
The author reports no conflicts of interest. The author alone is responsible for the content and writing of the paper.
Additional information
Funding
References
- Hiergeist R, Andra W, Buske N, et al. (1999). Application of magnetite ferrofluids for hyperthermia. J Magn Magn Mater 201:420–2.
- Pankhurst QA, Connolly J, Jones SK, Dobson J. (2003). Applications of magnetic nanoparticles in biomedicine. J Phys D Appl Phys 36:R167–81.
- Duguet E, Vasseur S, Mornet S, Devoisselle J-M. (2006). Magnetic nanoparticles and their applications in medicine. Nanomedicine 1:157–68.
- Yallapu MM, Othman SF, Curtis ET, et al. (2011). Multi-functional magnetic nanoparticles for magnetic resonance imaging and cancer therapy. Biomaterials 32:1890–905.
- Jordan A, Scholz R, Wust P, et al. (1999). Magnetic fluid hyperthermia (MFH): cancer treatment with AC magnetic field induced excitation of biocompatible superparamagnetic nanoparticles. J Magn Magn Mater 201:413–19.
- Moroz P, Jones SK, Gray BN. (2002). Magnetically mediated hyperthermia: current status and future directions. Int J Hypertherm 18:267–84.
- Salunkhe AB, Khot VM, Pawar SH. (2014). Magnetic hyperthermia with magnetic nanoparticles: a status review. Curr Top Med Chem 14:572–94.
- Rosensweig RE. (2002). Heating magnetic fluid with alternating magnetic field. J Magn Magn Mater 252:370–4.
- Babincova M, Altanerova V, Altaner C, et al. (2004). In vivo heating of magnetic nanoparticles in alternating magnetic field. Med Phys 31:2219–21.
- Wang XM, Gu HC, Yang ZQ. (2005). The heating effect of magnetic fluids in an alternating magnetic field. J Magn Magn Mater 293:334–40.
- Ma M, Wu Y, Zhou H, et al. (2004). Size dependence of specific power absorption of Fe3O4 particles in AC magnetic field. J Magn Magn Mater 268:33–9.
- Drake P, Cho H-J, Shih P-S, et al. (2007). Gd-doped iron-oxide nanoparticles for tumour therapy via magnetic field hyperthermia. J Mater Chem 17:4914–18.
- Jiang P-S, Drake P, Cho H-J, et al. (2012). Tailored nanoparticles for tumour therapy. J Nanosci Nanotechnol 12:5076–81.
- Horsman MR, Overgaard J. (2007). Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 19:418–26.
- Moeller BJ, Richardson RA, Dewhirst MW. (2007). Hypoxia and radiotherapy: opportunities for improved outcomes in cancer treatment. Cancer Metastasis Rev 26:241–8.
- Tarnawski R, Kummermehr J, Trott KR. (1998). The radiosensitivity of recurrent clones of an irradiated murine squamous cell carcinoma in the in vitro megacolony system. Radiother Oncol 46:209–14.
- Zhang Y, Li M, Yao Q, Chen C. (2007). Recent advances in tumor hypoxia: tumor progression, molecular mechanisms, and therapeutic implications. Med Sci Monitor 13:RA175–80.
- Iversen AB, Busk M, Horsman MR. (2013). Induction of hypoxia by vascular disrupting agents and the significance for their combination with radiation therapy. Acta Oncol 52:1320–6.
- Kampinga HH, Dikomey E. (2001). Hyperthermic radiosensitization: mode of action and clinical relevance. Int J Radiat Biol 77:399–408.
- Wust P, Hildebrandt B, Sreenivasa G, et al. (2002). Hyperthermia in combined treatment of cancer. Lancet Oncol 3:487–97.
- Diagaradjane P, Shetty A, Wang JC, et al. (2008). Modulation of in vivo tumor radiation response via gold nanoshell-mediated vascular-focused hyperthermia: characterizing an integrated antihypoxic and localized vascular disrupting targeting strategy. Nano Lett 8:1492–500.
- Griffin RJ, Dings RPM, Jamshidi-Parsian A, Song CW. (2010). Mild temperature hyperthermia and radiation therapy: role of tumour vascular thermotolerance and relevant physiological factors. Int J Hyperthermia 26:256–63.
- Johannsen M, Thiesen B, Gneveckow U, et al. (2006). Thermotherapy using magnetic nanoparticles combined with external radiation in an orthotopic rat model of prostate cancer. Prostate 66:97–104.
- Attaluri A, Kandala SK, Wabler M, et al. (2015). Magnetic nanoparticle hyperthermia enhances radiation therapy: a study in mouse models of human prostate cancer. Int J Hyperthermia 31:359–74.
- Foster BAGJ, Kwon ED, Madias C, Greenberg NM. (1997). Characterization of prostatic epithelial cell lines derived from transgenic adenocarcinoma of the mouse prostate (TRAMP) model. Cancer Res 57:5.
- Chen F-H, Chiang C-S, Wang C-C, et al. (2009). Radiotherapy decreases vascular density and causes hypoxia with macrophage aggregation in TRAMP-C1 prostate tumors. Clin Cancer Res 15:1721–9.
- Wang Q, Deng ZS, Liu J. (2012). Theoretical evaluations of magnetic nanoparticle-enhanced heating on tumor embedded with large blood vessels during hyperthermia. J Nanopart Res 14:974–983.
- Hsieh Y-K, Jiang P-S, Yang B-S, et al. (2011). Using laser ablation/inductively coupled plasma mass spectrometry to bioimage multiple elements in mouse tumors after hyperthermia. Anal Bioanal Chem 401:909–15.
- Diederich CJ. (2005). Thermal ablation and high-temperature thermal therapy: overview of technology and clinical implementation. Int J Hyperthermia 21:745–53.
- Song CW. (1984). Effect of local hyperthermia on blood flow and microenvironment: a review. Cancer Res 44:10.
- Song CW, Shakil A, Osborn JL, Iwata K. (2009). Tumour oxygenation is increased by hyperthermia at mild temperatures. Int J Hyperthermia 25:91–5.
- Hokland SL, Nielsen T, Busk M, Horsman MR. (2010). Imaging tumour physiology and vasculature to predict and assess response to heat. Int J Hyperthermia 26:264–72.
- Song CW, Park HJ, Lee CK, Griffin R. (2005). Implications of increased tumor blood flow and oxygenation caused by mild temperature hyperthermia in tumor treatment. Int J Hyperthermia 21:761–7.
- Jain RK. (2005). Normalization of tumor vasculature: an emerging concept in antiangiogenic therapy. Science 307:58–62.
- Johannsen M, Jordan A, Scholz R, et al. (2004). Evaluation of magnetic fluid hyperthermia in a standard rat model of prostate cancer. J Endourol 18:495–500.
- Johannsen M, Gneveckow U, Eckelt L, et al. (2005). Clinical hyperthermia of prostate cancer using magnetic nanoparticles: presentation of a new interstitial technique. Int J Hypertherm 21:637–47.
- Johannsen M, Gneueckow U, Thiesen B, et al. (2007). Thermotherapy of prostate cancer using magnetic nanoparticles: Feasibility, imaging, and three-dimensional temperature distribution. Eur Urol 52:1653–62.
