Abstract
Purpose: Hyperthermia can modulate inflammation and the immune response. Based on the recruitment of mesenchymal stem cells (MSCs) to inflamed tissues and the immunomodulatory properties of these cells, the aim of this study was to examine the effects of hyperthermia on the immunomodulatory properties of MSCs in a mixed lymphocyte reaction (MLR).
Materials and methods: Passages 4–6 of human umbilical cord vein mesenchymal stem cells were co-cultured in a two-way MLR. Cells in the hyperthermia groups were incubated at 41 °C for 45 min. A colorimetric assay was employed to examine the effects of MSCs on cell proliferation. The levels of IL-4 and TNF-α proteins in the cell culture supernatant were measured, and non-adherent cells were used for RNA extraction, which was then used for cDNA synthesis. RT-PCR was utilised to assess levels of IL-10, IL-17A, IL-4, TNF-α, TGF-β1, FOX P3, IFN-γ, CXCL12 and β-actin mRNA expression.
Results: UCV-MSCs co-cultured in an MLR reduced lymphocyte proliferation at 37 °C, whereas hyperthermia attenuated this effect. Hyperthermia increased expression of IL-10, TGF-β1 and FOXP3 mRNAs in co-culture; however, no effects on IL-17A and IFN-γ were observed, and it reduced CXCL12 expression. In co-culture, IL-4 mRNA and protein increased at 37 °C, an effect that was reduced by hyperthermia. No considerable change in TNF-α mRNA expression was found in hyperthermia-treated cells.
Conclusion: Hyperthermia increases cell proliferation of the peripheral blood mononuclear cells and modifies the cytokine profile in the presence of UCV-MSCs.
Introduction
Hyperthermia can be applied as a therapeutic approach for cancer treatment, whereby the temperature of the tumour must reach at least 40–41 °C for a certain period of time. Hyperthermia changes the blood circulation and oxygen supply to tumours, reduces the ATP level, increases anaerobic metabolites and the activity of DNA repair proteins, and sensitises tumour cells to radiotherapy and chemotherapy. These effects all depend on the temperature and exposure time [Citation1]. In arthritis of the hip, increased local temperature combined with physiotherapy leads to pain relief, reduction in muscle stiffness and increased muscle flexibility [Citation2]. A temperature of 43–45 °C accelerates muscle metabolism, improves tissue healing and increases the flexibility of tendons [Citation3]. In addition, hyperthermia is effective in the treatment of head and neck tumours through an immune-dependent mechanism [Citation4].
Hyperthermia has various effects on the immune system, such as increased peripheral blood mononuclear cell proliferation, increased cytotoxic activity of CD8+ T cells and augmented secretion of IFN-γ by these cells. It also causes the secretion of inflammatory cytokines such as TNF-α and IL-1, alters the migration of Langerhans cells and provokes lymphocyte homing into secondary lymphoid tissues [Citation5–10].
Hyperthermia can affect a variety of cell types, including mesenchymal stem cells (MSCs). Heat-shocked MSCs (43 °C for 45 min) can enhance tumour cell death and exhibit an inhibitory effect on tumour growth [Citation11]. MCF7 cells co-cultured with hyperthermia-treated MSCs results in cell G2/M-phase cycle arrest in the former as well as increased expression of MHC-I, FAS ligand and TNF-α receptor while decreasing that of multi-drug resistance proteins [Citation12].
Mesenchymal stem cells are non-haematopoietic stem cells and fibroblast-like adherent cells, which can be isolated from various tissues, such as bone marrow, umbilical cord, adipose tissue, peripheral blood, spleen and skin [Citation13]. These cells can be cultured for consecutive passages and have the capacity to differentiate into different cell types, including osteocytes, adipocytes and chondrocytes [Citation14,Citation15]. They also exhibit immunomodulatory properties [Citation16].
Mesenchymal stem cells inhibit the proliferation of helper and cytotoxic T cells and B lymphocytes through cell cycle arrest in G0/G1 phase. MSCs inhibit the differentiation of Th1 and Th17 while increasing that of Th2 cells, and they also increase the number of regulatory T cells [Citation11]. MSCs can promote the phagocytic activity of neutrophils and macrophages and decrease the number of NK cells, reduce their cytotoxic activity and decrease the production of pro-inflammatory cytokines by these cells. In addition to inhibiting dendritic cell (DC) maturation, these stem cells decrease or inhibit antigen presentation and decrease expression of co-stimulatory molecules CD40, CD80 and CD86 on DCs [Citation14,Citation17].
However, there are few or no studies about the effect of hyperthermia on MSC immunomodulation. Therefore, in this study, we aimed to investigate the effect of hyperthermia on the immunomodulatory properties of human umbilical cord vein mesenchymal stem cells (UCV-MSCs) in a mixed lymphocyte reaction (MLR). MLR was used to assess the T cell response to foreign MHC alleles by co-culturing peripheral blood mononuclear cells (PBMCs) from two individuals, as T cells from two individuals can react with each other, proliferate and secrete different types of cytokines [Citation18].
Materials and methods
UCV-MSC isolation and culture
Parents were provided with comprehensive information on the procedure and the aim of the study, and written consent was obtained from them. Briefly, 38–41 week healthy term umbilical cords were transported to the laboratory in Hanks Balanced Salt Solution (HBSS) buffer containing 300 μg/ml streptomycin and 300 U/ml penicillin (Labtech International Ltd, UK) at 2–8 °C. The isolation process was begun within an hour. The umbilical cord veins were filled with 0.1% collagenase IV (Gibco, Waltham, MA) and incubated for 20 min at 37 °C with 5% CO2. The isolated cells were cultured in Dulbecco’s Modified Eagle’s Medium-Low Glucose (DMEM-LG) containing 15% foetal bovine serum (FBS; Gibco, Waltham, MA), 10 ng/ml foetal growth factor (FGF; Royan Institute, Tehran, Iran), 100 μg/ml streptomycin, and 100 U/ml penicillin in a 6-well plate at 37 °C with 5% CO2. After 24 h, the medium was replaced; replacement was then repeated every 72 h, then the cells were passaged at 70–80% confluency [Citation19]. To assay colony-forming unit fibroblasts (CFU-Fs), 1500 cells of passage 1 were seeded into a 6-well plate and cultured in DMEM-LG containing 15% FBS, 10 ng/ml FGF, 100 μg/ml streptomycin, and 100 U/ml penicillin in 37 °C with 5% CO2. After 14 d, the cells were washed, fixed with 5% methanol, and stained with 0.5% crystal violet for 10 min. The plates were assessed for CFU-Fs of more than 20 cells.
Flow cytometry
Passages 4–6 of UCV-MSCs harvested by trypsin were pelleted by centrifugation at 1500 rpm for 5 min and then resuspended in DMEM-LG at a concentration of 1 × 106/ml. A 100-μl aliquot of the cells was labelled for 45 min at 4 °C with an appropriate dilution of one of the following anti-human monoclonal antibodies: PE-conjugated CD105, CD73 and CD34, FITC-conjugated CD45, and mouse isotypic antibodies PerCP-Cy5.5-IgG1κ, PE-IgG1κ, and FITC-IgG1κ (Biolegend, San Diego, CA). After staining, the cells were assessed using a BD FACSCalibur™ flow cytometer and analysed using FlowJo software (FlowJo LLC, Ashland, OR).
UCV-MSC differentiation
Adipogenic differentiation
UCV-MSCs of passages 4–6 were seeded into 24-well plates at a density of 4 × 104/well and cultured until reaching higher than 80% confluency. The medium was replaced with adipogenic differentiation medium (Bon Yakhteh-e-Royan, Tehran, Iran), and cells were incubated in a 37 °C incubator with 5% CO2; the medium was replaced every 3 d for 14 d. Accumulation of fat droplets was confirmed by Oil Red O staining; the cells were fixed with 4% paraformaldehyde for 30 min, washed, and stained with 0.15% Oil Red O (Bon Yakhteh-e-Royan, Tehran, Iran) for 25 min.
Osteogenic differentiation
For osteogenic differentiation, MSCs of passages 4–6 were seeded at a density of 3 × 103/cm2 in a 24-well plate and cultured in a 37 °C incubator with 5% CO2 for up to 4 d. The medium was replaced with osteogenic differentiation medium (StemPro, Gibco, Waltham, MA), and the cells were incubated in a 37 °C incubator with 5% CO2; the medium was replaced every 3 d for 21 d. Mineralisation of the cell layer was assessed using Alizarin Red S (ROTH, Karlsruhe, Germany). Briefly, the cells were fixed with 4% paraformaldehyde for 30 min, washed and stained with 2% Alizarin Red S for 3 min.
Chondrogenic differentiation
Passages 4–6 of UCV-MSCs were used for chondrogenesis in a micromass culture system by seeding 5-μl droplets of a cell solution of 1.6 × 107 cells/ml into the centre of the wells of 24-well plates. After cultivating the micromass for 2 h under high-humidity conditions, chondrogenesis medium (StemPro, Gibco) was added to the culture vessels, and the cells were incubated in a 37 °C incubator with 5% CO2. The differentiation medium was replaced every 2–3 d. After 14 d, the cells were fixed with 4% paraformaldehyde for 30 min and stained for 30 min with 1% Alcian Blue (ROTH) solution prepared in 0.1 N HCl.
MLR and co-culture
To isolate PBMCs, blood samples were added to Ficoll–Hypaque medium and centrifuged for 25 min at 600g. PBMCs were counted using a haemocytometer, and viability was assessed using trypan blue exclusion. For MLR, equal numbers of PBMCs from two healthy donors were seeded into 96-well plates (1 × 105 from each donor) and 24-well plates (1 × 106 from each donor) (SPL, Gyeonggi-do, Korea) in 200 and 600 μl, respectively, of DMEM-LG containing 10% FBS, 100 μg/ml streptomycin, and 100 U/ml penicillin; the cells were cultured for 5 d. For co-culture, 3 × 103 and 1 × 104 of UCV-MSCs (passages 4–6) were seeded into each well of 96-well and 24-well plates, respectively, on day one. On day two, 1 × 105 of PBMCs from two healthy donors in 200 μl was added to each well of the MSCs in the 96-well plates; 1 × 106 of the PBMCs from the same two healthy donors in 600 μl was added to each well containing MSCs in the 24-well plates; the medium for the PBMCs was DMEM-LG containing 10% FBS, 100 μg/ml streptomycin and 100 U/ml penicillin. After 5 d of incubation at 37 °C with 5% CO2 and high humidity, a CCK colorimetric assay (Promokines, Heidelberg, Germany) was used to examine cell proliferation in the 96-well plates according to the manufacturer’s protocol. On days 3 and 5, the supernatant from the 24-well plates was removed and stored at –80 °C, and the non-adherent cells were used for RNA extraction.
Temperature setup
The UCV-MSCs and MLR plates were both incubated at 41 °C for 0, 30, 45, 60, 90 and 120 min, after which all of the plates were cultured at 37 °C with 5% CO2 for 5 d. The viability of the cells was assessed every day using a CCK colorimetric assay. Minimum cytotoxicity in both the UCV-MSCs and MLR was observed at 45 min (data not shown).
RT-PCR
RNA was extracted using phenol chloroform based on the manufacturer’s protocol (Pars-Tous, Mashhad, Iran), and the concentration was calculated at 260 nm. cDNA was synthesised from 1 μg of the extracted RNA (Pars-Tous, Iran). To analyse levels of IL-10, IL-17A, IL-4, TNF-α, TGF-β1, FOXP3, IFN-γ, CXCL12 and β-actin mRNA expression, specific primers were designed for each gene (). PCR reactions were amplified using a thermocycler (Analytik Jena, Jena, Germany) (), and PCR products were visualised using electrophoresis through a 2% agarose gel. All experiments were repeated five times. GelQuant.NET software (biochemlabsolutions.com) was used for densitometry analysis of the bands.
Table 1. (A) Sequence of primers used for reverse transcriptase-polymerase chain reaction (RT-PCR); (B) Reaction conditions of PCR.
Cytokine assay
IL-4 is an important cytokine in tissue repair through inducing macrophage M2a [Citation20] and TNF-α is a key pro-inflammatory cytokine [Citation21]. The levels of IL-4 and TNF-α proteins were measured in the supernatant of the cell cultures on days 3 and 5 of the MLR and co-culture using sandwich enzyme-linked immunosorbent assay (ELISA; BioLegend, San Diego, CA) according to the manufacturer’s instructions. All experiments were repeated five times.
Statistical analysis
Data were compared using a one-way ANOVA test with IBM™ SPSS version 16 (IBM Corp., Armonk, NY). p Values less than .05 were considered statistically significant.
Results
UCV-MSC characterisation
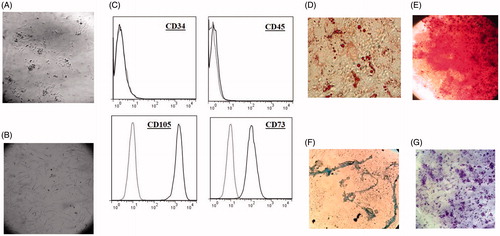
Based on inverted microscopy observation, the UCV-MSCs displayed a spindle-shaped appearance at 24 h after isolation (P0) and at passages 4–6 ()). Flow cytometer analysis (BD FACSCalibur™, Franklin Lakes, NJ) revealed that these cells were negative for the haematopoietic marker CD34 and the leukocyte marker CD45 but positive for CD73 and CD105 (). Cells at passages 4–6 were cultured under appropriate conditions to assess the differentiation potential of the MSCs into adipogenic, osteogenic and chondrogenic lineages. Adipogenesis of the MSCs was verified by Oil Red O-stained fat droplets, and cell layer mineralisation in osteogenic differentiation was demonstrated by Alizarin Red S staining. After culturing the cells in chondrogenesis medium, MSC micromasses were positive for Alcian Blue staining ()). MSCs at passage 1 showed the capacity to form CFU-Fs after 12 d ().
Figure 1. UCV-MSCs characterisation. (A–B) UCV-MSCs were spindle-shaped at passage 0 (24–48 h after isolation) and passage 4 (40× magnification). © Passages 4–6 of UCV-MSCs harvested and labelled with monoclonal antibodies CD34, CD45, CD73 and CD105 and analysed by flow cytometer. (D) Oil red O staining of differentiated cells into adipogenesis. (E) Alizarin Red S staining of differentiated cells into osteogenesis. (F) Alician blue staining of differentiated cells into chondrogenesis. (G) Formation of CFU-Fs stained with Crystal violet. UCV-MSCs (umbilical cord vein mesenchymal stem cells).
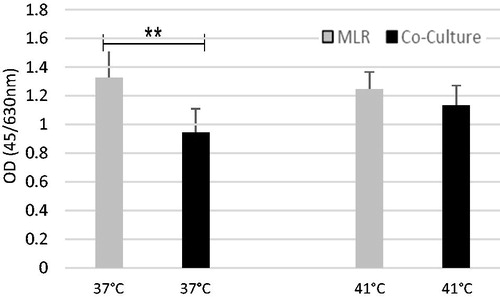
Hyperthermia attenuates the proliferation-inhibiting effect of UCV-MSCs
UCV-MSCs exhibited reduced cell proliferation in the two-way MLR at 37 °C, though this effect was attenuated at 41 °C (hyperthermia). At 37 °C, mean MLR proliferation was 1.325 ± 0.247 and 0.945 ± 0.165 in co-culture, a difference that was statistically significant (p < .001). In the hyperthermia groups, however, mean MLR proliferation was 1.244 ± 0.12 and 1.131 ± 0.139 in co-culture (p = .079) (). In other words, co-culture of the UCV-MSCs reduced the proliferation of the PBMCs by 28.7% at 37 °C and by 9.08% at 41 °C (p < .001).
Figure 2. At 37 °C, proliferation of the cells decreased to 0.945 ± 0.165 in the co-culture as compared with the MLR (1.325 ± 0.247), which is statistically significant (p < .001). In hyperthermia, however, cell proliferation decreased slightly in the co-culture as compared with the MLR (p = .079). The data are the mean of seven independent triplicate experiments and are normalised. UCV-MSCs (umbilical cord vein mesenchymal stem cells).
Gene expression
Expression of IL-10, IL-17A, IL-4, TNF-α, TGF-β1, FOX P3, IFN-γ, CXCL12 and β-actin mRNA in the non-adherent cells of the MLR and co-culture was assessed using RT-PCR on days 3 and 5. In the hyperthermia-treated co-culture groups, mRNA expression of IL-10 and FOXP3 increased by 16 and 43%, respectively, on day 3 and TGF-β1 by 49% on day 5 compared with their corresponding MLRs. In contrast, hyperthermia decreased the mRNA expression of IL-4 to 47% compared to its corresponding experiment at 37 °C on day 3. The mRNA level of TNF-α decreased by 20 and 18% on day 5 of co-culture at 37 and 41 °C, respectively, and expression of CXCL12 mRNA decreased by 22% on day 5 of co-culture in the hyperthermia-treated co-culture groups (p = .0032) compared with their corresponding MLR. No change in expression of IL-17A and IFN-γ mRNA in the hyperthermia-treated and non-hyperthermia-treated groups was observed ().
Figure 3. (A) The mRNA expressions of IL-10, IL-17 A, IL-4, TNF-α, TGF-β1, FOXP3, IFN-γ, CXCL12 and β actin were analysed by RT-PCR. Total RNA was extracted using phenol chloroform in the non-adherent cells of the co-culture and the MLR on days 3 and 5. After reverse transcription to cDNA, PCR was performed with the designed primers. PCR products were visualised on 2% agarose gel. (B) Densitometry analysis of bands were used to compare the data. The data are the mean of five independent experiments. *p Value <.05.
IL-4 and TNF-α protein levels in the culture supernatant
The level of IL-4 protein increased in the co-culture supernatant of the non-hyperthermia-treated groups on day 3 (21.7 pg/ml ± 14) and day 5 (6.4 pg/ml ± 4.1) compared with their corresponding MLRs (15.4 pg/ml ± 7.8 and 4.2 pg/ml ±2.3, respectively). However, the results for in the hyperthermia-treated groups were the opposite: IL-4 decreased in the co-culture supernatant on day 3 (5.8 pg/ml ± 3.8) and day 5 (6.4 pg/ml ± 3) compared with their corresponding MLRs (6.9 pg/ml ± 3.9 and 9.5 pg/ml ± 7.7, respectively) (). These changes were not statistically significant.
Figure 4. The effect of hyperthermia on the protein levels of IL-4 (A, B) and TNF-α (C, D) in the MLR and the co-culture. The protein level of these cytokines were measured in the culture supernatant by sandwich ELISA on days 3 and 5. The data are the mean of five independent experiments.
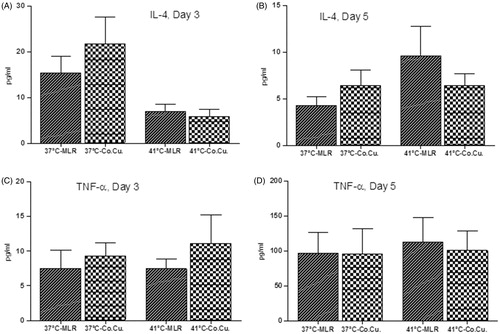
The amount of TNF-α protein increased in the co-culture hyperthermia-treated (11.10 pg/ml ± 8.2) and non-hyperthermia-treated (9.23 pg/ml ± 4.9) groups on day 3 compared with their corresponding MLRs (7.45 pg/ml ± 3.4 and 7.45 pg/ml ± 5.5, respectively). Five days after hyperthermia treatment, however, the level of TNF-α was decreased in the co-culture supernatant (112 pg/ml ± 86) compared with its corresponding MLR (100 pg/ml ± 68). No considerable difference was observed in TNF-α between the co-culture and MLR at 37 °C on day 5, though an overall increase in the level of TNF-α in all hyperthermia-treated groups compared with the non-treated groups was observed (). Nonetheless, these changes were not statistically significant.
Discussion
This study demonstrated that MLR co-culture with UCV-MSCs decreases cell proliferation at 37 °C but that hyperthermia treatment attenuates this effect. Human whole-body hyperthermia (41.8 °C) was found to increase apoptosis in T and B lymphocytes [Citation22]. In addition, B lymphocytes co-cultured with human bone marrow MSCs exhibited decreased cell proliferation via G0/G1-phase cell cycle arrest of B cells, though the viability of these cells increased [Citation23]. An MLR co-culture with human lung allograft-isolated MSCs reduced the proliferation of the recipients’ lymphocytes [Citation24], and in the presence of IFN-γ, indoleamine 2,3-dioxygenase-producing MSCs increased apoptosis in activated T lymphocytes [Citation25]. Overall, human bone marrow MSCs were found to equally inhibit the proliferation of activated CD8 and CD4 lymphocytes [Citation26]. In this study, adding MSCs to an MLR led to inhibition of proliferation at 37 °C, whereas hyperthermia attenuated this inhibitory effect. Considering that this temperature did not kill the MSCs or lymphocytes, it appears that hyperthermia modifies the effect of MSCs on lymphocyte proliferation.
We found the mRNA levels of IL-10, FOXP3 and TGF-β1 to be increased in co-culture at 41 °C compared with MLR, but no change was observed in the mRNA level of these cytokines at 37 °C. We also found that MLR co-culture with MSCs raised the levels IL-4 mRNA and protein at 37 °C, whereas this effect was reduced at 41 °C. Similarly, Matsui and Kakeda [Citation27] demonstrated that mild hyperthermia (39 °C) increased the level of IL-10 in MLR. In vitro hyperthermia (2 h at 41.5 °C) also increased the mRNA level of TGF-β in the culture supernatant of cardiac-isolated fibroblasts, and hearts isolated from hyperthermia-treated rats (15 min at 41.5 °C) showed an increase in TGF-β1 mRNA. Therefore, hyperthermia might have cardio-protective effects by increasing TGF-β1 [Citation28].
MSCs derived from human adipose tissue reduced lymphocyte proliferation in MLR in a dose-dependent manner while expanding the number of FOXP3+ T cells [Citation29], and FOXP3 expression is increased in the peripheral blood of patients with multiple sclerosis (MS) who had intrathecally received autologous bone marrow MSCs [Citation30]. In our study, hyperthermia increased expression of FOXP3 mRNA too.
In our study, hyperthermia had no effect on the mRNA levels of TNF-α and IFN-γ, and the results of ELISA showed decreases in TNF-α protein at 41 °C on day 5. Although raising the body temperature of mice to 39.8 °C had no effect on the serum levels of TNF-α and acute-phase proteins, hyperthermia with lipopolysaccharide (LPS) led to an increase in the serum levels of TNF-α compared to LPS alone [Citation31]. Moreover, myeloid dendritic cells co-cultured with LPS-stimulated MSCs showed reduced secretion of TNF-α [Citation32].
In this study, expression of CXCL12 was decreased in co-culture on day 5 at 41 °C. Therefore, hyperthermia can reduce MSC migration to inflammatory sites by reducing CXCL12 expression. It has been observed that tumour-associated MSCs might be a treatment resistance factor for ovarian cancer in thermal therapy, and inhibition of the CXCL12-CXCR4 axis in these cells reduces this resistance [Citation33].
Our study demonstrated that hyperthermia attenuates the proliferation-inhibiting effect of UCV-MSCs. Considering the increases in the modulatory molecules FOXP3, TGF-β1 and IL-10 along with the decrease in IL-4, as well as the fact that there was no change in IFN-γ and IL-17A and no considerable change in TNF-α, it appears that in MLR, concomitant exposure to hyperthermia and MSCs led to polarisation of the inflammation towards a non-destructive response. From these data, it could be speculated that hyperthermia treatment can promote tissue repair rather than aggravating inflammation.
Acknowledgements
This paper is the result of a thesis for a master’s degree in immunology funded by the Kurdistan University of Medical Sciences. The authors gratefully appreciate the blood donors who participated in this study.
Disclosure statement
The authors have declared that no conflict of interest exists.
References
- Frey B, Weiss E-M, Rubner Y, et al. (2012). Old and new facts about hyperthermia-induced modulations of the immune system. Int J Hyperthermia 28:528–42.
- Castagna A, Rinaldi S, Fontani V, et al. (2011). Comparison of two treatments for coxarthrosis: local hyperthermia versus radio electric asymmetrical brain stimulation. Clin Interv Aging 6:201.
- Giombini A, Giovannini V, Di Cesare A, et al. (2007). Hyperthermia induced by microwave diathermy in the management of muscle and tendon injuries. Brit Med Bull 83:379–96.
- Vertrees RA, Leeth A, Girouard M, et al. (2002). Whole-body hyperthermia: a review of theory, design and application. Perfusion 17:279–90.
- Wang W-C, Goldman LM, Schleider DM, et al. (1998). Fever-range hyperthermia enhances L-selectin-dependent adhesion of lymphocytes to vascular endothelium. J Immunol 160:961–9.
- Evans SS, Wang W-C, Bain MD, et al. (2001). Fever-range hyperthermia dynamically regulates lymphocyte delivery to high endothelial venules. Blood 97:2727–33.
- Ostberg J, Patel R, Repasky E. (2000). Regulation of immune activity by mild (fever-range) whole body hyperthermia: effects on epidermal Langerhans cells. Cell Stress Chaperones 5:458.
- Huang Y, Haegerstrand A, Frostegård J. (1996). Effects of in vitro hyperthermia on proliferative responses and lymphocyte activity. Clin Exp Immunol 103:61–6.
- Mace TA, Zhong L, Kokolus KM, Repasky EA. (2012). Effector CD8+ T cell IFN-γ production and cytotoxicity are enhanced by mild hyperthermia. Int J Hyperthermia 28:9–18.
- Calderwood SK, Theriault JR, Gong J. (2005). How is the immune response affected by hyperthermia and heat shock proteins? Int J Hyperthermia 21:713–16.
- Cho JA, Park H, Kim HK, et al. (2009). Hyperthermia-treated mesenchymal stem cells exert antitumor effects on human carcinoma cell line. Cancer 115:311–23.
- Park H, Cho J-A, Kim S-K, et al. (2008). Hyperthermia on mesenchymal stem cells (MSCs) can sensitize tumor cells to undergo cell death. Int J Hyperthermia 24:638–48.
- Srivatanakul P. (2014). Mesenchymal stem cells. Bangkok Med J 6:71–9.
- Scheibe F, Ladhoff J, Huck J, et al. (2012). Immune effects of mesenchymal stromal cells in experimental stroke. J Cereb Blood Flow Metab 32:1578–88.
- Zuk PA, Zhu M, Ashjian P, et al. (2002). Human adipose tissue is a source of multipotent stem cells. Molec Biol Cell 13:4279–95.
- Uccelli A, Moretta L, Pistoia V. (2006). Immunoregulatory function of mesenchymal stem cells. Eur J Immunol 36:2566–73.
- Caplan AI. (2007). Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol 213:341–7.
- Abbas AK. (2015). Cellular and molecular immunology. Canada: Elsevier.
- Kadivar M, Khatami S, Mortazavi Y, et al. (2005). Isolation, culture and characterization of postnatal human umbilical vein-derived mesenchymal stem cells. DARU J Pharmaceut Sci 13:170–6.
- Rőszer T. (2015). Understanding the mysterious M2 macrophage through activation markers and effector mechanisms. Mediators Inflamm 2015:Article ID 816460. http://dx.doi.org/10.1155/2015/816460
- Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. (1996). Immune and inflammatory responses in TNF alpha-deficient mice: a critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med 184:1397.
- Dieing A, Ahlers O, Kerner T, et al. (2003). Whole body hyperthermia induces apoptosis in subpopulations of blood lymphocytes. Immunobiology 207:265–73.
- Tabera S, Pérez-Simón JA, Díez-Campelo M, et al. (2008). The effect of mesenchymal stem cells on the viability, proliferation and differentiation of B-lymphocytes. Haematologica 93:1301–9.
- Jarvinen L, Badri L, Wettlaufer S, et al. (2008). Lung resident mesenchymal stem cells isolated from human lung allografts inhibit T cell proliferation via a soluble mediator. J Immunol 181:4389–96.
- Plumas J, Chaperot L, Richard M-J, et al. (2005). Mesenchymal stem cells induce apoptosis of activated T cells. Leukemia 19:1597–604.
- Ramasamy R, Tong CK, Seow HF, et al. (2008). The immunosuppressive effects of human bone marrow-derived mesenchymal stem cells target T cell proliferation but not its effector function. Cell Immunol 251:131–6.
- Matsui T, Kakeda T. (2008). IL-10 production is reduced by hypothermia but augmented by hyperthermia in rat microglia. J Neurotrauma 25:709–15.
- Flanders KC, Winokur TS, Holder MG, Sporn M. (1993). Hyperthermia induces expression of transforming growth factor-beta s in rat cardiac cells in vitro and in vivo. J Clin Invest 92:404.
- Engela AU, Hoogduijn MJ, Boer K, et al. (2013). Human adipose-tissue derived mesenchymal stem cells induce functional de-novo regulatory T cells with methylated FOXP3 gene DNA. Clin Exp Immunol 173:343–54.
- Mohajeri M, Farazmand A, Bonab MM, et al. (2011). FOXP3 gene expression in multiple sclerosis patients pre- and post mesenchymal stem cell therapy. Iran J Allergy Asthma Immunol 10:155–61.
- Ostberg JR, Taylor SL, Baumann H, Repasky EA. (2000). Regulatory effects of fever-range whole-body hyperthermia on the LPS-induced acute inflammatory response. J Leukocyte Biol 68:815–20.
- Aggarwal S, Pittenger MF. (2005). Human mesenchymal stem cells modulate allogeneic immune cell responses. Blood 105:1815–22.
- Lis R, Touboul C, Mirshahi P, et al. (2011). Tumor associated mesenchymal stem cells protects ovarian cancer cells from hyperthermia through CXCL12. Int J Cancer 128:715–25.


