Abstract
Objectives: The incidence of pneumothorax is 7 times higher after lung radiofrequency ablation (RFA) than after lung biopsy. The reasons for such a difference have never been objectified. The histopathologic changes in lung tissue are well-studied and established for RF in the ablation zone. However, it has not been previously described what the nature of thermal injury might be along the shaft of the RF electrode as it traverses through normal lung tissue to reach the ablation zone. The purpose of this study was to determine the changes occurring around the RF needle along the pathway between the ablated zone and the pleura.
Material and methods: In 3 anaesthetised and ventilated swine, 6 RFA procedures (right and left lungs) were performed using a 14-gauge unipolar multi-tined retractable 3 cm radiofrequency LeVeen probe with a coaxial introducer positioned under CT fluoroscopic guidance. In compliance with literature guidelines, we implemented a gradually increasing thermo-ablation protocol using a RF generator. Helical CT images were acquired pre- and post-RFA procedure to detect and evaluate pneumothorax. Four percutaneous 19-gauge lung biopsies were also performed on the fourth swine under CT guidance. Swine were sacrificed for lung ex vivo examinations, scanning electron microscopy (SEM) and pathological analysis.
Results: Three severe (over 50 ml) pneumothorax were detected after RFA. In each one of them, pathological examination revealed a fistulous tract between ablation zone and pleura. No fistulous tract was observed after biopsies.
In the 3 cases of severe pneumothorax, the tract was wide open and clearly visible on post procedure CT images and SEM examinations. The RFA tract differed from the needle biopsy tract. The histological changes that are usually found in the ablated zone were observed in the RFA tract’s wall and were related to thermal lesions. These modifications caused the creation of a coagulated pulmonary parenchyma rim between the thermo-ablation zone and the pleural space. The structural properties of the damage can explain why the RFA tract is remains patent after needle withdrawal.
Conclusion: Our study demonstrates for the first time that the changes around the RF needle are the same as in the ablated zone. The damage could create fistulous tracts along the needle path between thermo-ablation zone and pleural space. These fistulas could certainly be responsible for severe pneumothorax that occurs in many patients treated with lung RFA.
Introduction
Percutaneous radiofrequency ablation (RFA) has gained acceptance as a viable therapeutic option to surgery [Citation1]. Experimental studies in animal tumour models have confirmed the efficacy of RFA in the destruction of experimentally induced pulmonary tumours [Citation2]. Pneumothorax is the most common complication during lung RFA and the most frequent cause for extended hospital stays. The incidence of pneumothorax after lung RFA is estimated around 70% [Citation1]. This incidence is estimated around 8.7% after percutaneous transthoracic needle biopsy [Citation3]. The reasons for the difference in incidence of pneumothorax between lung RFA and transthoracic needle biopsy have never been identified. The histopathologic changes in lung tissue after RFA procedures are long known for the ablated zone but not for the RFA pathway. The purpose of this study was to determine the changes occurring around the RF needle along the pathway between the ablated zone and the pleura.
Material and methods
Animals and experimental protocol
Based upon the Guide for the Care and Use of Laboratory Animals (National Research Council, National Academic Press, Washington, DC, 1996) and FDA approved and EC approved good laboratory practice for medical devices, the animals were handled in accordance with the institutional Animal Care and Use Committee which granted approval of this experimental protocol. Six pigs (initial body weight: 65 kg) at 7 months of age (Blossin SA, 13-Aubagne, France) were bred free range with natural daylight and free access to water, for a two-week acclimatisation period. Animals were fed with the standard diet for pregnant sows, otherwise shown to prevent excessive increases in weight and size.
Biopsy and RF procedures, specimen collection and histopathologic examination
We performed 6 lung RFA procedures in 3 swine and 4 lung biopsies in 1 swine. All procedures were done on anaesthetised pigs in dorsal recumbency, with the lower limbs folded down onto the table, according to the standard radiological positioning. Pigs were anaesthetised with ketamine (15 mg/kg, IM) followed by perfusion with midazolam (Hypnovel, Roche, 0.5 mg/10 mL/min) in isotonic Ringer-Lactate solution. Anesthesia was maintained with gaseous isoflurane (1.5%, V/V). Aseptic technique was used throughout the procedure, and prophylactic antibiotics (amoxicillin–clavulanic acid, 1 g:200 mg in 20 ml, IV) were given both before and after radiologic procedures. A Helical CT scan (Tomoscan M Mobile, Philips, Netherlands) was used for pre-therapeutic, per-procedure, post-therapeutic and follow-up imaging. Helical chest CT was performed on all pigs at the beginning of the procedure to assess anatomic conditions. For the RFA procedure, each pig underwent two sessions of lung RFA, one for each lung, with a unipolar multi-tined retractable 3 cm, 14-gauge radiofrequency LeVeen coaxial probe (Boston Scientific, Natick, MA) with 10 deployable electrodes that open in a flower-like manner [Citation4], positioned under CT fluoroscopic guidance. We used a RF3000 generator (Boston Scientific, Natick, MA), monitoring capabilities for tissue impedance and wattage were incorporated into the generator circuitry. We implemented a conventional thermo-ablation protocol according to literature guidelines [Citation5], until a major increase in impedance occurred (the "roll off") [Citation6–8]. The initial power used for all procedures was 5 W; the power was then increased for 5 W every 2 min until 30 W, then 5 Ws every minute until 50 W, then 10 W every minute until the roll off.
Helical chest CT was performed at the end of each ablation on all pigs to detect and quantify pneumothorax and post-RF fistulous tracts. For the last pig, we also performed 4 lung biopsies (2 for each lung) at the end of the procedure using the same coaxial needle introducer and semi-automatic 18-gauge 20 cm biopsy set (Temno, Cardinal Health, Waukegan Road, Waukegan, IL). After the procedures, animals were euthanized (midazolam 15 mg and chlorpromazine 25 mg in KCl 15% (p/v), 20 ml, i.v. bolus). Both lungs were surgically removed. We first performed ventilated lung ex vivo examination to detect any air leak, then analysed both gross and microscopic pathologic findings by scanning electron microscopy (SEM) and pathological examinations. For pathological examinations, samples were fixed in 4% formalin and paraffin embedded. Four μm thick slides were stained with Hematoxylin Eosin (H&E). For immunohistochemical analysis, TTF1 antibody was used as pneumocyte marker using automated procedures on a Ventana ULTRA device, using E-view detection Ventana. Surfaces have been quantified on virtual slides.
Results
Procedure
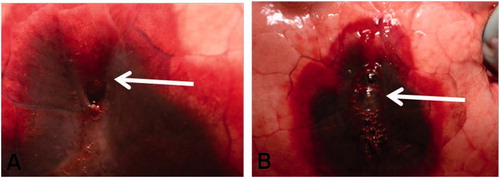
The maximum average power output used for ablation was 39.17 W (standard deviation 10.68); the average duration of ablation was 8.03 min (standard deviation 2.7). Among the 6 lung RFA, we were able to identify 6 pneumothoraces. Three of them were small pneumothoraces (volume <50 ml) that occurred during the lung puncture and the needle placement. The three other were bigger pneumothoraces (volume ≥50 ml) and appeared or increased on needle withdrawal. In our series, the prevalence of pneumothorax ≥50 ml was 50%. One of them caused a sub-total lung collapse and required fine needle aspiration because of poor tolerance. The prevalence of clinically significant pneumothorax was 16.7%. In two cases of severe pneumothoraces after RFA, a gaping hole was observed in the pleura, and an air leakage was visible during the ex-vivo examination in ventilated lungs (). No air leakage was visible during the ex-vivo examination in ventilated lungs after biopsies. Among the 4 biopsies, we were able to identify one small pneumothorax, without any visible air leakage.
Specimen collection and histopathologic examination
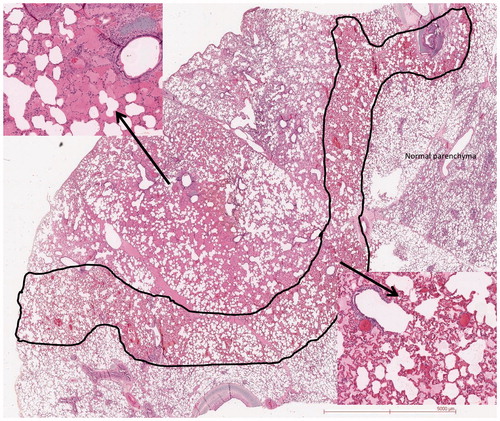
Pathological examination depicted fistulous tracts between ablation zone and pleura; these tracts were wide open and clearly visible (in CT, Optical Microscopy and SEM) in the 3 cases of severe pneumothorax after RF (). The RFA pathways were surrounded by a 1–2 mm rim of coagulated pulmonary parenchyma. The histological analysis of the 16-gauge lung biopsy tract revealed low haemorrhagic modifications (). No open transfissural needle tract was observed by microscopy in the animal undergoing lung biopsy. Considering the RFA, all cases of pneumothorax after lung RFA exhibited specific concentric histological changes all along the tract. A large haemorrhagic area was present around the RFA needle with two distinct lesions: the centre with alveolar spaces filled by haemorrhagic fluid. This area represents 69 mm2 on a slide. The periphery with an intense congestion of the capillaries forming a ring of about 42 mm2. There was no evidence of epithelial cell necrosis (). TTF1 expression was lost in both areas meaning pneumocytes injury at the proteic level (not shown).
Figure 2. Scanning electron microscopy. Axial slice, showing a widely open tract (arrow). Condensation of the lung parenchyma around the tract (arrowheads).
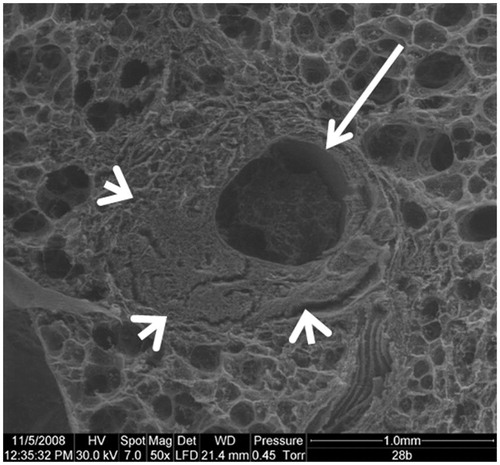
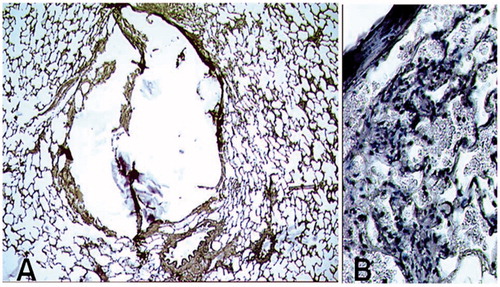
Figure 3. Optical Microscopy with orcein staining. Lung damages related to the needle positioning alone, without any thermo-ablation. A: Biopsy path without haemorrhagic modifications, peripheral parenchyma is preserved (mag ×50). B: Hematoxylin staining showing haemorrhagic modifications, with red blood cells in the alveoli (mag ×200).
Discussion
Tracts after lung RFA differed from simple biopsy needle tract. The specific histological changes within the RF tract’s wall could be related with thermal lesion. The same histological changes were seen in the ablated zone. The RF pathways were surrounded by a rim of coagulated pulmonary parenchyma, with structural properties that could explain why the RFA tracts were still open after needle withdrawal, as shown in Optical Microscopy and scanning electron microscopy (SEM). Several studies have examined the histological changes of lung RFA scars but no study treated the alterations along the RFA tract. There is no description of this condition in the literature. This tract seems to be specific to the lung parenchyma, because of the “oven effect” [Citation5,Citation9]. Due to the low impedance of the lung parenchyma, caused by the presence of air, heat conductivity may happen along the needle. Taking these data in consideration may lead to improve the thermo-ablation protocol, modify the RFA needle or to develop a device that can occlude this specific tract. Other studies have shown that microwave ablation produces larger tumour ablation volumes than radiofrequency [Citation10]. The nature of microwave propagation in tissue is quite different from RFA and the temperatures generated are higher. Pathological effects of MW along the needle tracks should be more important and it would be useful to carry out a specific study on this subject. Finally, although clinically severe post-RFA pneumothoraces are not very common, they induce a major increase of the overall procedure cost. The occurrence of pneumothorax increases the numbers of CXR and CT scan, the need of pain relief treatment and the duration of hospital stays [Citation5].
Conclusion
After lung RFA, we identified pathological damage around the needle track. This damage created a patent iatrogenic fistula extending from the thermo-ablation zone and the pleural space. This type of fistula is likely responsible for pneumothorax after RFA procedures.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- de Baère T, Aupérin A, Deschamps F, et al. (2015). Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol. 26:987–91.
- Miao Y, Ni Y, Bosmans H, et al. (2001). Radiofrequency ablation for eradication of pulmonary tumor in rabbits. J Surg Res 99:265–71.
- Wang Y, Jiang F, Tan X, Tian P. (2016). CT-guided percutaneous transthoracic needle biopsy for paramediastinal and nonparamediastinal lung lesions: diagnostic yield and complications in 1484 patients. Medicine (Baltimore) 95:e4460.
- Goldberg SN, Grassi C, Cardella J, et al. (2005). Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 235:728–39.
- Lencioni R, Crocetti L, Cioni R, et al. (2008). Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 9:621–8.
- Zhu JC, Yan T-D, Morris DL. (2008). A systematic review of radiofrequency ablation for lung tumors. Ann Surg Oncol 15:1765–74.
- Simon CJ, Dupuy D, DiPetrillo T, et al. (2007). Pulmonary radiofrequency ablation: long-term safety and efficacy in 153 patients. Radiology 243:268–75.
- Steinke K, Sewell P, Dupuy D, et al. (2004). Pulmonary radiofrequency ablation – an international study survey. Anticancer Res 24:339–43.
- Pawel M, Goldberg W, Yang W, Goldberg S. (2008). Radiofrequency ablation: the effect of distance and baseline temperature on thermal dose required for coagulation. Int J Hyperthermia 24:550–9.
- Sidoff L, Dupuy DE. (2017). Clinical experiences with microwave thermal ablation of lung malignancies. Int J Hyperthermia 33:25–33.