Abstract
Purpose: Pneumothorax is the most common complication following a pulmonary percutaneous radiofrequency ablation (RFA), and thoracic drainages are the most frequent causes of an extended hospital stay. Our main objective was to show that the use of gelatin torpedoes may significantly decrease the number of chest tube placement.
Materials and methods: Seventy-three patients were prospectively included in this study and then randomised into two groups: 34 with embolisation and without 39 without embolisation. Each group was comparable for different pneumothorax risk factors.
Results: There were 16 (47%) pneumothorax in Group A (“with embolisation”), which was significantly lower (p < .0001) than the 35 pneumothorax (90%) in Group B (“without embolisation”). The pneumothorax volume (p = .02) was significantly lower in Group A (22.7% average, standard deviation 15.6%) than in Group B (average 34.1%, standard deviation 17.1%). The number of drainages was significantly smaller in those with embolisation (3 drainages or 8%) than those without embolisation (25 drainages or 64%) (p < .001).
Conclusion: When using absorbable gelatin torpedoes, pulmonary RFA pathways embolisation significantly decreased the number of pneumothorax and thoracic drainages to the advantage of therapeutic abstention and exsufflation, non-invasive and functional operational techniques.
Introduction
Pneumothorax is the most common complication following a percutaneous radiofrequency lung intervention and, according to much earlier studies, there is an estimated incidence between 6 and 29% [Citation1,Citation2].
A figure closer to a 70% incidence [Citation3] is based on more current and more pertinent research using a larger population for the treatment of over 1000 metastases.
Among all patients with a pneumothorax in this same study, 28% received no treatment; 14% received one exsufflation and 58% received a chest tube. Putting in a thoracic tube occurs approximately for 39% of those treated with pulmonary RFA and is the most common reason for an extended hospitalisation [Citation4].
Even if the post-RFA pulmonary mortality is very low (s < 1% [Citation3]), an estimated one in two deaths is due to a pneumothorax or morbidity induced by its occurrence [Citation3,Citation5]. The literature identifies the following pneumothorax risk factors [Citation6]:
Advanced patient age;
Male;
Absence of ipsilateral thoracic surgery;
High number of treated tumours; and
Increased length of the electrode pathway within the ventilated pulmonary parenchyma.
Some embolisation tools, such as biological adhesives, autologous blood patches, NaCl, negative pressure initiator systems and hydrogel plugs were tested in biopsies.
As with digestive oncology interventional radiology, some studies have shown that portal embolisation, percutaneous liver or kidney biopsies [Citation7] can benefit from an absorbable gelatin torpedoto prevent bleeding.
Our objective was to evaluate how this same technique would impact pneumothorax occurrences and its management. The main objective was to show that the use of gelatin torpedoes significantly decreased the number of chest tube placement.
Materials and methods
This study obtained Institutional Review Board (IRB) approval. The main objective was to show that the use of gelatin torpedoes significantly decreased the number of chest tube placement. Considering this primary outcome measure, we prospectively included 73 patients treated with pulmonary RFA for primary or secondary lesions in our centre between January and September 2016. Patients needing treatment for multiple lesions in the same lung were excluded since the possibility of a pneumothorax requiring drainage on the first tumour might distort any results observed when treating other tumours. Each patient was included into one of two groups by block randomisation: those with (Group A) or without (Group B) embolisation. Oral informed consents were obtained for all patients.
For each patient we identified:
the potential pneumothorax risk factors (advanced age, gender, history of ipsilateral thoracic surgery and distance between the pleura and the lesion along the RFA needle pathway),
the presence of emphysema on a CT (computed tomography) scan,
the number and volume of immediate pneumothorax,
the pneumothorax management (no treatment, exsufflation, drainage), and
A pneumothorax at 48 h and 1-month examination so as to avoid missing any delayed occurrence.
The abundance of pneumothorax was calculated using the Collins profile inter-pleural distance measurements [Citation8]. RFA procedures were performed with CT-scan guidance or Cone Beam CT 3 D acquisition on all patients in a standardised manner using a coaxial needle system 15 Gauge Boston Scientific (Boston Scientific Corporation, Natick, MA).
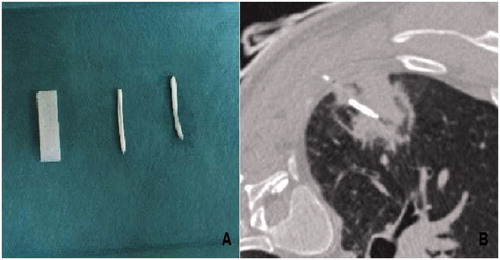
At the end of the procedure, we embolised the RFA pathway for that in Group A by using Curaspon® torpedoes (CuraMedical, B. V., Assendelft, Netherlands) () soaked in contrast iodine (Xenetix® 350 Guerbet, Roissy, France) to achieve a radiopaque and watertight state.
Figure 1. Macroscopic appearance of iodine soaked, waterproof torpedo Curaspon® (CuraMedical), (A) CT of radiofrequency pathway of lung Curaspon torpedoes and CT after implantation of Curaspon torpedoes along the radiofrequency lung pathway (B).
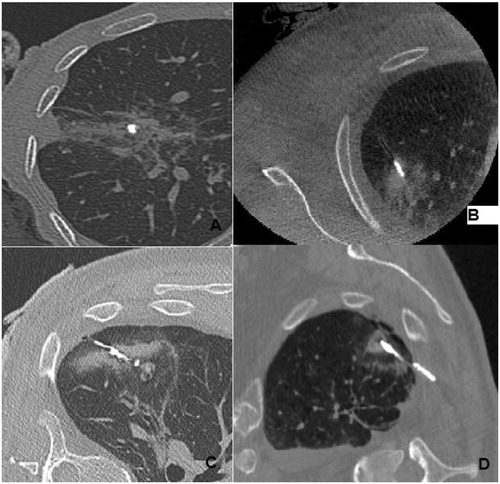
Three torpedoes were placed inside the coaxial needle and pushed to the end with the mandrin to “occlude” RFA scar, for the 3 last torpedoes, we placed them just before the end of the coaxial needle and then progressively withdrew it, the mandrin held fixedly, to embolise the entire intraparenchymal lung route till the pleura. The learning curve was rather short (). For patients in the Group B, the RFA needle and the coaxial were completely removed, and a CT scan was performed for each patient at the end of the percutaneous procedure.
Figure 2. A fast learning curve: initially torpedoes were in small fragments occupying the base of the RFA pathway (A), then occupying the intratumoral pathway portion (B), the entire pathway, (C) or even exceeding the RFA pathway within the chest wall (D).
The international recommendations for pneumothorax management were followed [Citation9,Citation10]. All patients with a low abundance pneumothorax (volume <10%) who were stable at 5 min received no treatment. For those with a mean abundance pneumothorax (volume between 11% and 30%), we performed exsufflation by using a short venous catheter (18 gauge). Finally, patients with an immediate and/or persistent abundant pneumothorax (volume >31%) despite exsufflation received an 8 French chest tube (Prodimed, Plastimed®, à Neuilly En Thelle, France).
All patients had a CT scan at 48 h and at 1 month. This study is based on the intent-to-treat principle; all patients in Group A were analysed even if it was technically impossible to implement a torpedo.
Continuous characteristics, such as age, the distance between the pleura and the lesion, the lesion size and the quantity of pneumothorax, were expressed as a mean [SD] and compared between the two groups using Mann–Whitney tests. The categorical characteristics such as gender, surgical history, emphysema, pneumothorax occurrence, and pneumothorax treatments were expressed as proportions and compared using χ2 or Fisher’s exact tests.
All statistical analyses were two-tailed and considered statistically significant when p values were <.05. They were performed using SPSS PAWS Statistics 20.0 (IBM Inc., New York, NY).
Results
We included 73 patients, randomly divided into two groups of 34 with embolisation (“group A”) and without 39 without embolisation (“group B”).
Both groups had similar risk factors ():
Age: (average 63.82 years [15.5] in Group B vs. 66.79 years in the Group A [9.1], p = .73).
Gender: (16 women and 23 men in the Group B compared with 15 women and 19 men in Group A; p = .81),
Previous surgery: (19 patients with history in the Group B compared with 10 in Group A; p = .23),
Presence of emphysema: (22 patients in Group B as compared with 24 in the Group A; p = .23),
Lesion size: (16 mm [7.9] in Group B vs. 13.3 mm [6.1] in the Group A; p = .11)
Distance of the lesion from the pleura: (average of 26.7 mm [15.02] in group B vs. 24.6 mm [11.8] in group A; p = .53).
Table 1. Comparability of groups “without” and “with” RFA path embolisation.
In the group A, we had a single failure of gelatin torpedo placement, the patient presented a pneumothorax treated by exsufflation. The number of pneumothorax was significantly less (p < .0001) in Group A with 16 pneumothorax (47%) than in Group B with 35 pneumothorax (90%). The volume of pneumothorax was significantly less abundant (p = .02) in Group A (34.1% on average, standard deviation 15.6%) than in Group B (22.7%, on average, standard deviation 17.1%).
We decided not to treat pneumothorax for four patients (11.4%) in Group B (without embolisation) and seven patients (43.7%) in Group A (with embolisation) (p = .063). () The most significant result concerned the installation of a drain. In fact, the number of drains was significantly lower (3 drainages, 8.8%) in the group with embolisation than in the group without embolisation (25 drainages, 73.5%) (p < .0001) ().
Figure 3. Example of an effective exsufflation after RFA pathway embolisation by gelatin torpedo (A, B and C) interventional appearance with installation and deployment of the RFA needle. (D) Appearance of a pneumothorax (D) requiring exsufflation (E). No pneumothorax.
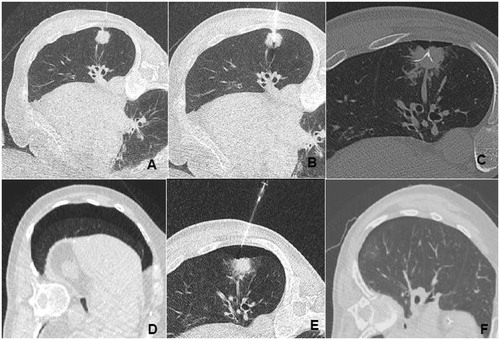
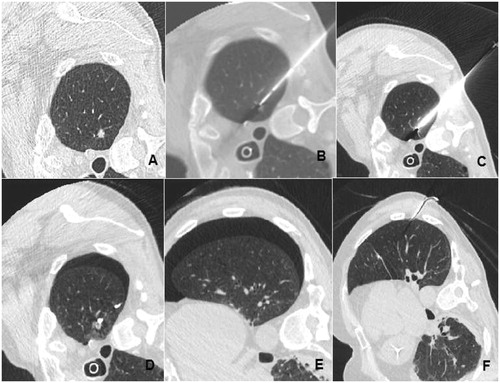
Figure 4. Example of one of two patients requiring drainage despite embolisation of the RFA pathway: (A) lesion before RFA, (B, C) interventional appearance, (D) gelatine torpedo in place, (E) pneumothorax residual despite exsufflation, (F) chest tube in place.
There were 15 patients without torpedoes who presented with a persistent pneumothorax after 48 h.
All the 54 patients had a CT scanner examination at one month, no one had a pneumothorax.
We did not see any residual material at CT scan follow-up after one month CT.
During the first month CT scan follow-up, we did not see the material.
Discussion
The genesis of post RFA pneumothorax is the result of complex and specific phenomena not found during biopsies.
While these phenomena do not exist in the biopsies, the literature is replete with studies of embolised pulmonary pathways. Several embolisation agents and tools, including biological adhesives were tested in biopsies [Citation11], most notably autologous blood patches [Citation12]; NaCl [Citation13]; negative pressure initiator systems [Citation14]; and finally hydrogel plugs [Citation15].
No studies were found in the literature concerning pulmonary embolisation pathways after percutaneous RFA. In hepatic interventional radiology, RFA pathways are usually not embolised but tract ablation technique can be performed to avoid potential seeding.
This technique can be used for the lung, but can contribute to perpetuating a gaping fistula by increasing the thermal lesions related to an “oven effect” [Citation16]. The incidence of intractable pneumothorax due to bronchopleural fistula is about 0.6%.
The detection of lung lesions accompanying the RFA coaxial needle’s intra-parenchymal pathway between the visceral pleura and the tumour, and the CT finding of this visible “open” pathway pursuant to lung ablations, led us to propose an occlusion method. Since resorbable gelatins are easily available and inexpensive, and radiologists are accustomed to using them for embolisation interventions, we therefore imagined using them to obtain an occlusion of the intra-pulmonary parenchymal pathway after RFA.
As expected, this technique has a very brief “learning curve” and implementation posed no problem for our team. The drainage remedy is very low in Group A with a significant difference vis-à-vis Group B. This drainage rate is comparable to the most recent literature series [3 Japanese series in 1000 ablations].
Low volume pneumothorax management by therapeutic abstention or single exsufflation was not significantly different between the two groups, nor surprising when considering the small number of drainages done in Group A. Because of the torpedo’s effectiveness, fewer drains with a lower number of well-tolerated residual pneumothorax were proportionately greater in Group A than in Group B, whereas more than half of patients, drained, had no residual pneumothorax. Additionally, the reduced number of pneumothorax in Group A also explains the lack of significance in comparing these two groups of “minimally” treated pneumothorax.
Apart from the number of drains placed in our patients, the significant reduction of post-RFA pneumothorax observed and the lower, although not significant, number of pneumothorax visible on the scanner after 48 h seems to confirm the gelatin torpedoes’ watertight efficiency in the RFA needle pathway’s occlusion.
The study’s main limitation is its modest number of patients. The embolisation technique limitation for a pulmonary radiofrequency pathway concerns only the sub-pleural lesions for which the RFA electrode withdrawal displaces the coaxial out of the pathway and prevents the torpedo’s appropriate implementation. Unfortunately, we did not quantify the emphysema, we just determine the presence or not of emphysema on the scanner performed before the procedure.
Indeed, there seems to be no migration risk of vascular material as optical and electron microscopy images have shown () [Citation16,Citation17], the induced alterations in the RFA pathway cause a zone of parenchyma condensation, and clot and intravascular thrombosis destruction without residual healthy vessels.
Figure 5. Scanning electron microscopy. Axial slice, showing a widely open tract (arrow). Condensation of the lung parenchyma around the tract (arrowheads).
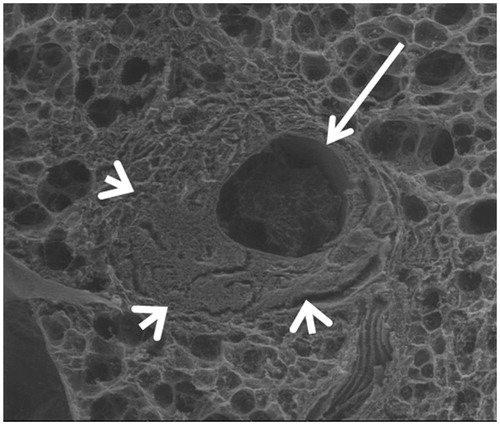
In the case of massive necrosis along the pathway with a possible extension of the necrosis (as with microwaves), the torpedo could be insufficient to occlude correctly the trajectory given their absence of enlargement along the time (in comparison with hydrogel systems which are enlarging progressively under the condition of being hydrated).
This technique seems to be recommended particularly for patients with emphysema, since the presence of emphysema is detected as a risk factor of severe pneumothorax in several studies [Citation17].
From a financial point of view, thanks to this method, we are considering to reduce the hospitalisation time of all patients who underwent a lung RFA without chest tube placement.
Conclusion
Radiofrequency lung embolisation pathways using absorbable gelatin significantly decrease the number and abundance of pneumothorax. This technique reduces the number of thoracic drainages in favour of functional non-invasive techniques with no treatment and exsufflation.
Supplemental File
Download PDF (143.4 KB)Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Okuma T, Matsuoka T, Yamamoto A, et al. (2008). Frequency and risk factors of various complications after computed tomography-guided radiofrequency ablation of lung tumors. Cardiovasc Intervent Radiol 31:122–30.
- Clasen S, Kettenbach J, Kosan B, et al. (2009). Delayed development of pneumothorax after pulmonary radiofrequency ablation. Cardiovasc Intervent Radiol 32:484–90.
- de Baère T, Aupérin A, Deschamps F, et al. (2015). Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 26:987–91.
- Lencioni R, Crocetti L, Cioni R, et al. (2008). Response to radiofrequency ablation of pulmonary tumours: a prospective, intention-to-treat, multicentre clinical trial (the RAPTURE study). Lancet Oncol 9:621–8.
- Palussiere J, Lagarde P, Aupérin A, et al. (2015). Percutaneous lung thermal ablation of non-surgical clinical N0 non-small cell lung cancer: results of eight years’ experience in 87 patients from two centers. Cardiovasc Intervent Radiol 38:160–6.
- Kennedy SA, Milovanovic L, Dao D, et al. (2014). Risk factors for pneumothorax complicating radiofrequency ablation for lung malignancy: a systematic review and meta-analysis. J Vasc Interv Radiol 25:1671–81.e1.
- Kessel DO, Ray CE. (2010). Transcatheter embolization and therapy, 305 techniques in interventional radiology. Springer-Verlag London Limited. doi:10.1007/978-1-84800-897-7_31
- Collins CD, Lopez A, Mathie A, et al. (1995). Quantification of pneumothorax size on chest radiographs using interpleural distances: regression analysis based on volume measurements from helical CT. AJR Am J Roentgenol 165:1127–30.
- Pereira PL, Masala S. (2012). Standards of practice: guidelines for thermal ablation of primary and secondary lung tumors CVIR. Cardiovasc Intervent Radiol 35:247–54.
- Yamagami T, Kato T, Iida S, et al. (2005). Efficacy of manual aspiration immediately after complicated pneumothorax in CT-guided lung biopsy. J Vasc Interv Radiol 16:477–83.
- Petsas T, Siamblis D, Giannakenas C, et al. (1995). Fibrin glue for sealing the needle track in fine-needle percutaneous lung biopsy using a coaxial system: part II–clinical study. Cardiovasc Intervent Radiol 18:373–7.
- Lang K, Ghavami R, Schreiner V, et al. (2000). Autologous blood clot seal to prevent pneumothorax at CT-guided lung biopsy. Radiology 216:93–6.
- Billich C, Muche R, Brenner G, et al. (2008). CT-guided lung biopsy: incidence of pneumothorax after instillation of NaCl into the biopsy track. Eur Radiol 18:1146–52.
- Morello F, Wright K, Lembo T. (2005). New suction guide needle designed to reduce the incidence of biopsy-related pneumothorax: experimental evaluation in canine model. Radiology 235:1045–9.
- Zaetta J, Licht M, Fisher J, Avelar R. (2010). Lung biopsy tract plug for reduction of postbiopsy pneumothorax and other complications: results of a prospective, multicenter, randomized, controlled clinical study. J Vasc Interv Radiol 21:1235–43.e3.
- Hiraki T, Gobara H, Fujiwara H, et al. (2013). Lung cancer ablation: complications. Semin Intervent Radiol 30:169–75.
- Kashima M, Yamakado K, Takaki H, et al. (2011). Complications after 1000 lung radiofrequency ablation sessions in 420 patients: a single center’s experiences. Am J Roentgenol 197:W576–80.
