Abstract
Purpose: To retrospectively evaluate the added benefit of adding intraluminal radiofrequency ablation (RFA) to biliary metal stent placement for patients with malignant biliary obstruction (MBO).
Methods: From November 2013 to December 2015, 89 patients with MBO who had undergone percutaneous intraluminal RFA and stent placement (RFA-stent group, n = 50) or stent placement only (stent group, n = 39) were included. Outcomes were compared according to the type of tumour: cholangiocarcinoma or non-cholangiocarcinoma.
Results: Primary and secondary stent patency (PSP, SSP) were significantly higher for the RFA-stent group than the stent group (PSP: 7.0 months vs. 5.0 months, p = 0.006; SSP: 10.0 months vs. 5.6 months, p < 0.001), with overall survival being comparable (5.0 months vs. 4.7 months, p = 0.068). In subgroup analysis, RFA-stent showed significant PSP benefits compared to stent alone in patients with cholangiocarcinoma (7.4 months vs. 4.3 months; p = 0.009), but with comparable outcomes in patients with non-cholangiocarcinoma (6.3 months vs. 5.2 months; p = 0.266). The SSP was improved in both subgroups (cholangiocarcinoma, 12.6 months vs. 5.0 months, p < 0.001; non-cholangiocarcinoma, 10.3 months vs. 5.5 months, p = 0.013). Technical success and clinical success were not significantly different between the two groups. The rate of complication was higher for the RFA-stent group, but tolerable when compared to the stent group.
Conclusions: Although survival was comparable between the groups, RFA-stent confers therapeutic benefits to patients with MBO in terms of stent patency compared to stent placement alone, especially in those with cholangiocarcinoma.
Introduction
Malignant biliary obstruction (MBO) is usually caused by different types of tumours, principally cholangiocarcinoma, as well as pancreatic and hepatocellular carcinomas. Stent placement is a well-established and widely accepted treatment in patients with unresectable malignant biliary strictures [Citation1,Citation2], having a lower rate of complication and post-stenting occlusion than surgical decompression [Citation3]. The use of metal stents further reduces the need for re-intervention and the incidence of cholangitis compared to the use of plastic and polyethylene stents [Citation1,Citation4]. However, maintaining stent patency is a challenge with tumour overgrowth, epithelial hyperplasia, biofilm deposition and sludge formation limiting the median patency of a metal stent to merely 6 months [Citation5].
Radiofrequency ablation (RFA) is a well-known percutaneous approach that has widely been used in the treatment of primary and metastatic hepatic malignancy and lung tumours, with demonstrated effectiveness [Citation6–8]. The application of RF ablation in the treatment of extrahepatic cholangiocarcinoma [Citation9], colorectal cancer [Citation10] and oesophagus dysplasia [Citation11] has also been evaluated. Recently, a Percutaneous Endobiliary Radiofrequency catheter (HabibTM PERF; EMcision Ltd, London, UK) has been developed specifically for the treatment of MBO. Within the bile duct, RFA uses specific endobiliary probes that enable increased precision in the delivery of thermal energy in the biliary tree and pancreas, which appears to be safe and may result in decreased epithelial hyperplasia and tumour ingrowth [Citation12]. Several studies have confirmed the safety and feasibility of this radiofrequency technique for clinical use [Citation12–14], with promising results reported for the palliative treatment of malignant biliary strictures; to prevent stent occlusion [Citation15–20], clear blocked metal stents [Citation21] and prolong stent patency [Citation22]; as well as to improve patient survival [Citation23]. However, due to limited clinical experience using intraductal RFA and the small number of reports regarding this technique, the beneficial effect of a treatment combining intraluminal RFA and stent placement compared to stent placement alone remains to be evaluated [Citation22–24]. Therefore, the purpose of our study was to retrospectively compare the outcome of RFA and metal stent placement (RFA-stent) with metal stent placement alone (stent) in patients with unresectable MBO.
Methods
Study design and patient selection
We conducted a retrospective study of consecutive patients with unresectable MBO at the First Affiliated Hospital of Sun Yat-sen University, between November 2013 and December 2015. This study was approved by the institutional review board and the requirement for informed consent was waived. Treatment options included stent placement of uncovered self-expandable metallic stents (SEMSs) and biliary intraluminal RFA combined with the SEMSs. Before these patients underwent initial stent placement, the RFA-stent strategy was recommended by the attending physician (J.L. and Y.W.). Patients received information regarding the potential advantage in stent patency of the RFA-stent treatment compared to stent alone, but also of the extra cost and potential additional risks of intraductal RFA. The choice of treatment method was, ultimately, made by patients and their authorised representatives. If the patient agreed to the physician’s recommendation, intraluminal RFA was administered at the first stent session. Patients who refused RFA underwent stent only. Written informed consent was received from each patient.
The inclusion criteria were (1) age, 20–80 years; (2) MBO confirmed by computed tomography (CT), abdominal magnetic resonance imaging (MRI), with pathological confirmation whenever possible; (3) clinical jaundice, a serum bilirubin level >5 mg/dL and/or cholangitis and (4) unresectability of the tumour or refusal to be surgically treated. Nonresectability was established through either direct patient consultation with a hepatobiliary surgeon and/or the consensus opinion of a multidisciplinary tumour board. Identified patients were screened on the following exclusion criteria: (1) performance status score >2 [Citation25]; (2) identification of a secondary malignancy; (3) serious medical comorbidities; and (4) missing data. According to the aetiology and biological behaviour of the tumour [Citation22,Citation26,Citation27], we classified the types of MBO into two subgroups: (a) cholangiocarcinoma group, originating from the bile duct epithelium and mainly associated with intraluminal obstruction and (b) non-cholangiocarcinoma group, mainly including pancreatic carcinoma, hepatocellular carcinoma, and metastatic tumours, which involved the bile duct and was associated mainly with extraluminal obstruction.
In total, 137 consecutive patients with unresectable MBO were retrospectively observed during the study period, with 48 patients excluded from the analysis based on our exclusion criteria. Of the remaining patients, 50 had undergone RFA-stent (25 cholangiocarcinomas, 25 non-cholangiocarcinomas), and the other 39 patients underwent stent (14 cholangiocarcinomas, 25 non-cholangiocarcinomas). A flow diagram of patient selection is shown in .
Treatment
All patients had undergone previous percutaneous transhepatic cholangial external drainage (PTCED) at our hospital for initial management of their disease process. Two to four days after PTCED, SEMSs were inserted, with or without intraluminal RFA, by two interventional oncologists who were experienced in this procedure (J.L. and Y.W.).
In the RFA-stent group, percutaneous transhepatic cholangiography was performed under digital subtraction angiography (DSA) guidance to localise the site of biliary obstruction and to confirm its length and diameter. A guide wire was then passed through the stenosis via the PTCED catheter. After the catheter is retreated, the Habib Endo HPB probe was then advanced over the wire with the tip of the probe placed across the malignant stricture. The probe was attached to a standard high-frequency generator, with 10 W applied for 90 s. For patients with long segmental obstruction of the bile duct, RFA was performed section by section. For patients with a high level obstruction and tumours that involved the bilateral bile ducts, RFA of the intrahepatic bile ducts, bilaterally, was required. Immediately after RFA, an uncovered SEMS (Wallstent; Boston Scientific, Boston, MA), mounted on a delivery system, was placed. Generally, SEMSs were chosen according to the individual radiologist’s preference and the manufacturer’s protocol. Patients in the stent group underwent only stent insertion, using the same technique as for patients in the RFA-stent group. Cholangiography was used to confirm that the bile duct was clear. Follow-up assessment of drainage flow was performed, under DSA guidance, 3–4 days after the procedure, and the catheter removed if flow remained unobstructed.
Re-intervention
With recurrence of cholangitis or jaundice, an abdominal US and/or CT was performed to verify stent patency. If dilation of the drained bile duct was confirmed, repeat-ablation, RFA was repeated, with or without insertion of a new stent, in the RFA-stent group, with new stent insertion attempted in the stent group. If dilation of a drained bile duct was not confirmed, focal cholangitis of another undrained branch of the bile duct was suspected, and extra stenting in the stent group and stent with RFA in the RFA-stent group were attempted for that branch. If drainage failed, PTCED was performed.
Assessment and follow-up
Technical success was defined as passage of the stent across the stricture, with good radiographic positioning, along with flow of contrast and/or bile through the stent [Citation28]. Clinical success was defined as improvement of symptoms, such as jaundice and pruritus, and total bilirubin levels to less than half or less than the normal upper limit, within 14 days post-intervention. Overall survival (OS) was calculated from the date of the first procedure until the date of death. Primary stent patency (PSP) was defined as the interval between the first stent insertion procedure and the recurrence of symptoms of restructure without repeated ablation or stent insertion. Secondary stent patency (SSP) was defined as the interval between the day of the initial procedure and recurrence of symptoms of obstruction with subsequent ablation or stent insertion. If there was no evidence of obstruction during the patient’s life, the patency period was considered to be equal to the survival period, but censored. Stent patency was confirmed by the absence of jaundice, normal level of direct bilirubin and the absence of expansion of the bile duct on US, CT or MR imaging during the follow-up [Citation22].
The incidence of complications associated with the procedures was investigated. Early complications were classified as major or minor according to the clinical practice guidelines of the Society of Interventional Radiology Standards of Practice Committee [Citation29]. A diagnosis of cholangitis was made in the presence of fever associated with leucocytosis and positive blood culture, without any other infectious focus outside the hepatobiliary system identified, or recurrence of jaundice and cholestasis persisting for >48 h post-intervention [Citation28]. Mild bleeding was defined by no requirement for transfusion within 48 h of the intervention. Moderate bleeding was defined by a need for a blood transfusion of more than 2 units or a haemostatic procedure, including both pharmaceutical and surgical intervention, after a drainage procedure [Citation30]. Late complications of biliary stenting mostly included biliary obstruction recurrence. The definitions of causes of recurrent biliary obstruction were based on the 2014 Tokyo criteria for transpapillary biliary stenting [Citation31].
Outcomes were stent patency, OS, technical success, clinical success and complication rate. After adequate palliation of the biliary obstruction, patients were discharged with follow-up arranged through the outpatient clinic at intervals from 2 weeks up to 3 months. Patients’ continuing medical history was included in the medical record. For patients unable to attend medical appointments because of clinical deterioration, their relatives were interviewed by telephone at the scheduled intervals of 2 weeks up to 3 months, or until the patient’s death, and the information was included in the medical record. Patients who died were excluded at the date of their last follow-up. Follow-up continued from the stent insertion to the death of the patient or the end of the study.
Statistical analysis
Descriptive statistics were calculated using the mean ± standard deviation (SD) or median and range, as appropriate for the data type. OS and stent patency were evaluated using Kaplan–Meier curves, with between-group differences compared using the log-rank test. All analyses were performed using SPSS statistics (IBM, New York, NY). All p values were two sided, with a level of 0.05 considered to be significant.
Results
Detailed baseline characteristics for patients in the RFA-stent and stent groups are summarised in . The mean age was older in the stent group than in the RFA-stent group (p = 0.021). No significant differences were identified between the two groups in terms of sex, aetiology, level of biliary obstruction, performance status score, co-therapy for primary disease and previous cholangitis before the procedure. In the subgroups of patients with cholangiocarcinoma and non-cholangiocarcinoma, with the exception of age, the other baseline characteristics between the two treatment groups were also comparable.
Table 1. Demographic and baseline clinical characteristics of patients in the two groups.
The details of the procedures are shown in . The baseline of length of initial stricture, the rate of technical success, and clinical success were comparable between the groups. In the RFA-stent group, unilateral stent placement was performed in 39 (78.0%) patients, with 11 (22.0%) patients requiring bilateral stents at the initial procedure. In this group, 44 (88.0%) underwent one ablation and stent placement session, while five (10.0%) underwent two ablation sessions, with no new stent placement in two patients and one (2.0%) underwent three ablations without stent placement due to recurrence of biliary obstruction. In the stent group, unilateral stent placement was performed in 35 (89.7%) patients, with four (10.3%) requiring bilateral stents. In this group, 34 (87.2%) underwent one stent session, while five (12.8%) underwent two sessions due to recurrence of biliary obstruction.
Table 2. Procedure details of patients in the two groups.
Among patients in the RFA-stent group, two died within 30 days of the procedure of septic shock. In comparison, two died in the stent group, one from septic shock, with the other death being unrelated to biliary stent placement (upper gastrointestinal haemorrhage). The rates of complications tended to be slightly higher in the RFA-stent group than in the stent group (). No severe complications related to RFA, such as bile duct perforation, bile leak were identified post-procedure. Two patients in the RFA-stent group and one in the stent group required blood transfusion and pharmaceutical treatment for post-procedure bleeding. In the RFA-stent group, one patient with a history of coronary heart disease, percutaneous coronary intervention, atrial fibrillation, hypertension and hyperthyroidism, developed an acute state of chronic heart failure caused by atrial fibrillation and rapid ventricular rate. Conservative treatment was successful for this patient. Of note is the comparable incidence rate of cholangitis for the two groups, with an overall rate of 17.9% (16 of 89 patients). Patients presented symptoms of bacterial cholangitis, with antibiotic treatment successfully resolving fever and normalising white blood cell count.
Table 3. Complications of procedures in the two groups.
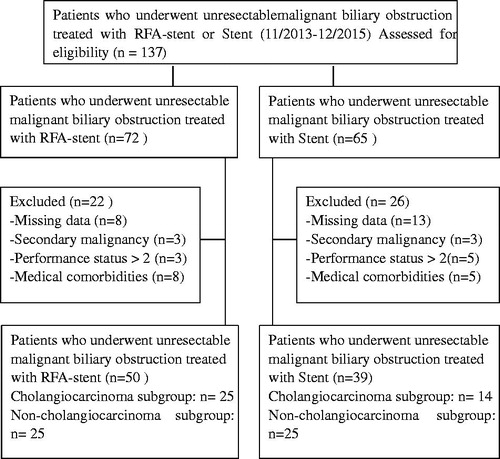
Median follow-up at the time of data analysis was 6 months. At the end-point of the study, 10 (20%) patients in the RFA-stent group and seven (17.9%) patients in the stent group were still alive. Overall, survival ranged between 0.25 and 19.2 months, with a median survival of 5.0 (95%CI: 4.6, 5.4) months, respectively. Kaplan–Meier survival analysis () identified no between-group difference in survival (p = 0.068), with a median survival of 5.0 (95%CI: 4.0, 6.0) months for the RFA-stent group compared to 4.7 (95%CI: 4.1, 5.3) months for the stent group. On subgroup analyses, OS in the RFA-stent group and the stent group, respectively, was as follows: In patients with cholangiocarcinoma, the median OS was 6.7 months versus 4.5 months, respectively (p = 0.307); ); in patients with non-cholangiocarcinoma, the median OS was 7.3 months vs. 5.3 months, respectively (p = 0.137; ).
Figure 2. Overall survival curves in patients with unresectable MBO who underwent RFA-stent and stent. Data were obtained with Kaplan–Meier method. (A) Whole study population (RFA-stent group: n = 50, median OS = 5.0 months; stent group: n = 39, median OS = 4.7 months; p = 0.068). (B) Patients in cholangiocarcinoma subgroup (RFA-stent group: n = 25, median OS = 6.7 months; stent group: n = 14, median OS = 4.5 months; p = 0.307). (C) Patients in non-cholangiocarcinoma subgroup (RFA-stent group: n = 25, median OS = 7.3 months; stent group: n = 25, median OS = 5.3 months; p = 0.137).
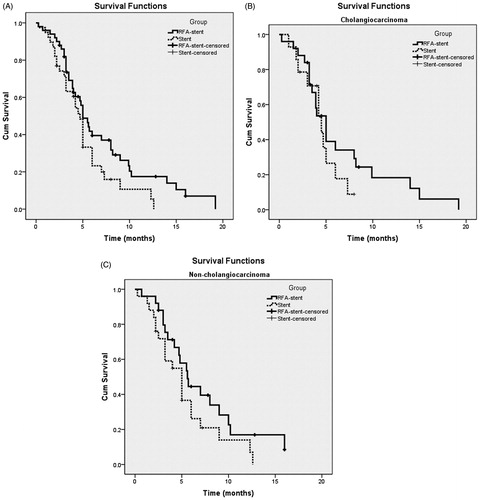
Overall, the median PSP and SSP were 6.0 (95%CI: 5.0, 7.0) and 8.2 (95%CI: 6.3, 10.1) months, respectively. Kaplan–Meier analysis () identified a significant group difference (p = 0.006), with a median duration of PSP of 7.0 (95%CI: 5.3, 8.7) months for the RFA-stent group and 5.0 (95%CI: 4.1, 5.9) months for the stent group. Kaplan–Meier survival analysis () identified a significant between-group different in survival (p < 0.001), with a median SSP of 10.0 (95%CI: 5.9, 14.1) months for the RFA-stent group compared to 5.6 (95%CI: 4.6, 6.6) months for the stent group. Subgroup analyses of PSP in patients with different types of tumours showed that the median PSP of patients with cholangiocarcinoma and non-cholangiocarcinoma was 7.6 months (95%CI: 6.8, 9.2) and 6.3 months (95%CI: 5.4, 6.6), respectively, in the RFA-stent group, but 4.3 months (95%CI: 1.7, 8.5) and 5.2 months (95%CI: 3.8, 6.2), respectively, in the stent group (). Subgroup analyses of SSP in patients with different types of tumours showed that the median SSP of patients with cholangiocarcinoma and non-cholangiocarcinoma was 12.6 months (95%CI: 8.9, 16.3) and 10.3 months (95%CI: 9.5, 10.5), respectively, in the RFA-stent group but 5.0 months (95%CI: 2.3, 7.9) and 5.5 months (95%CI: 3.9, 7.3), respectively, in the stent group. The median PSP of patients with cholangiocarcinoma was significantly improved in the RFA-stent group compared with that in the stent group (p = 0.009; ), but with no significant difference in patients with non-cholangiocarcinoma between two treatment groups (p = 0.266; ). The median SSP of patients with cholangiocarcinoma or non-cholangiocarcinoma was significantly improved in the RFA-stent group compared with that in the stent group (p < 0.001 and p = 0.013, respectively; ).
Figure 3. Graph shows PSP for patients with unresectable MBO who underwent RFA-stent and stent. Data were obtained with Kaplan–Meier method. (A) Whole study population (RFA-stent group: n = 50, median PSP = 7.0 months; stent group: n = 39, median PSP = 5.0 months; p = 0.006). (B) Patients in cholangiocarcinoma subgroup (RFA-stent group: n = 25, median PSP =7.6 months; stent group: n = 14, median PSP = 4.3 months; p = 0.009). (C) Patients in non-cholangiocarcinoma subgroup (RFA-stent group: n = 25, median PSP = 6.3 months; stent group: n = 25, median PSP = 5.2 months; p = 0.266).
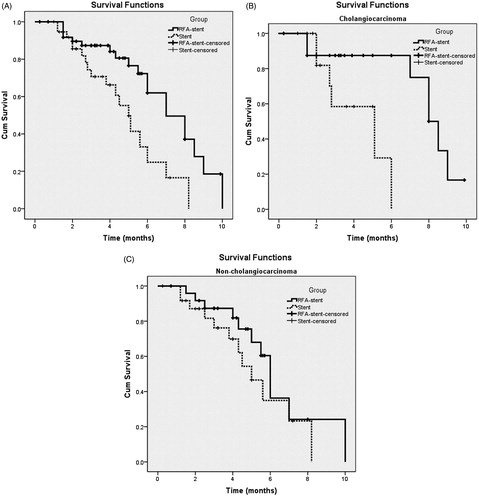
Figure 4. Graph shows SSP for patients with unresectable MBO who underwent RFA-stent and stent. Data were obtained with Kaplan–Meier method. (A) Whole study population (RFA-stent group: n = 58, median SSP = 10.0 months; stent group: n = 39, median SSP = 5.6 months; p < 0.001). (B) Patients in cholangiocarcinoma subgroup (RFA-stent group: n = 25, median SSP =12.6 months; stent group: n = 14, median SSP = 5.0 months; p < 0.001). (C) Patients in non-cholangiocarcinoma subgroup (RFA-stent group: n = 25, median SSP = 10.3 months; stent group: n = 25, median SSP = 5.5 months; p = 0.013).
Discussion
Our study showed that treatment with RFA-stent conferred a significant stent patency advantage when compared to stent alone in patients with cholangiocarcinoma. Although the PSP was comparable in the non-cholangiocarcinoma subgroup for the two treatments, the SSP in the RFA-stent group was also improved. These results indicate that biliary RFA-stent may have a better response profile on cholangiocarcinoma given its biliary ductal origin. Our data are important because it indicates that use of biliary endoluminal RFA, which has not been adequately studied to date for unresectable MBO, should be explored prospectively in comparison with stenting alone, which is recommended by therapy guidelines, at least for the subgroup of patients with cholangiocarcinoma.
The potential therapeutic effect of RFA-stent has been evaluated with mixed findings reported [Citation22–24]. Yiannis et al. [Citation23] reported a significantly longer median survival for patients with advanced pancreatic malignancy and biliary obstruction treated with RFA-stent compared to stent alone, although the SEMS patency rate was comparable for both the treatments. In contrast, Sharaiha et al. [Citation24] reported similar OS rates for patients with end-stage cholangiocarcinoma and pancreatic cancer treated with either RFA-stent or stent alone. Li et al. [Citation22] reported a comparable stent patency rate for both RFA-stent and stent treatments at 3 months, although the patency rate was comparatively higher at 6 months in the RFA-stent group. In our study, treatment with combined RFA and stent placement improved PSP and SSP, compared to treatment by stent placement only. Differences in outcomes among studies could not be explained by differences in the baseline characteristics of the study cohorts but was differentiated by subgroup analysis. In the subgroup analysis of our study, the PSP was improved only in patients with cholangiocarcinoma, comparable to that in patients with non-cholangiocarcinoma, while the SSP was improved in both subgroups. These differences in outcomes on subanalysis could be explained as follows. Firstly, the local thermal effect of RFA was mainly associated with the difference of PSP due to different aetiology and biological behaviour of the tumour [Citation32]. Compared to non-cholangiocarcinomas, which involve the bile duct, cholangiocarcinoma may have a better response profile to endoluminal RFA given it originates from the bile duct epithelium. Regardless of which treatment was used, stent or RFA-stent, patients with non-cholangiocarcinomas had a worse PSP outcome. Secondly, due to the different pathologies in the non-cholangiocarcinoma group, the selected patients are relatively inhomogeneous. Future studies for different aetiologies in the non-cholangiocarcinoma group to confirm these results are needed. Moreover, RFA can be also used to clear occlusion of a previously deployed metal stent and restore the biliary flow without the need to insert a new stent inside the obstructed stent [Citation21]. Repeated RFA treatments and multiple sessions can be performed to increase tumour destruction, which was mainly associated with the difference in SSP. However, the OS of two groups was not significantly different, which can largely be explained by that our patient groups have a comparatively short OS and poor performance status. Due to local tumour progression, many patients in our RFA-stent group died of disease progression but not recurrent stent obstruction, with only a few patients receiving repeated ablation procedures.
We also did not identify a difference in complication rate between the RFA-stent and stent groups, a finding that has been previously reported [Citation17,Citation23,Citation24]. However, two patients in the RFA-stent group and one in the stent group died of septic shock within 30 days of the procedure. We underline that the incidence of cholangitis was very high in both groups. Accordingly, we further support the prophylactic administration of antibiotics before stent placement to lower the risk of cholangitis [Citation22,Citation33].
The limitations of our study should be noted. Foremost, this is a single-centre study, using a retrospective methods and a non-randomised design. Therefore, selection bias was unavoidable and the results may not be fully generalisable, in particular to patients of other ethnicities. In this study, there was a higher proportion of cholangiocarcinoma in the RFA-stent group and a higher proportion of pancreatic carcinoma and metastatic disease in the stent group. Cholangiocarcinoma may have a better response profile to endoluminal RFA given its biliary ductal origin. Lastly, further research on the technique itself is required. Specifically, it is possible that an endoscopic procedure could be more suitable than a percutaneous approach for patients with a low level obstruction, with endoscopy being more easily managed and less invasive than a percutaneous approach [Citation30].
Conclusions
We conclude that the efficacy of percutaneous RFA-stent treatment is better than that of stent alone for unresectable MBO. Although these findings should be confirmed by multi-centre prospective randomised controlled studies, our study does provide evidence of the therapeutic benefits of RFA-stent treatment on stent patency, as well the fact that it is well-tolerated by patients and associated with few complications. We therefore propose RFA-stent as an alternative and palliative treatment for patients with unresectable MBO, at least for the subgroup of patients with cholangiocarcinoma.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Sawas T, Al Halabi S, Parsi MA, Vargo JJ. (2015). Self-expandable metal stents versus plastic stents for malignant biliary obstruction: a meta-analysis. Gastrointest Endosc 82:256–67.
- Kahaleh M, Tokar J, Conaway MR, et al. (2005). Efficacy and complications of covered Wallstents in malignant distal biliary obstruction. Gastrointest Endosc 61:528–33.
- Moss AC, Morris E, Leyden J, MacMathuna P. (2007). Malignant distal biliary obstruction: a systematic review and meta-analysis of endoscopic and surgical bypass results. Cancer Treat Rev 33:213–21.
- Sangchan A, Kongkasame W, Pugkhem A, et al. (2012). Efficacy of metal and plastic stents in unresectable complex hilar cholangiocarcinoma: a randomized controlled trial. Gastrointest Endosc 76:93–9.
- Loew BJ, Howell DA, Sanders MK, et al. (2009). Comparative performance of uncoated, self-expanding metal biliary stents of different designs in 2 diameters: final results of an international multicenter, randomized, controlled trial. Gastrointest Endosc 70:445–53.
- Li Z, Zhang K, Lin SM, et al. (2016). Radiofrequency ablation combined with percutaneous ethanol injection for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia. [Epub ahead of print]. doi: 10.1080/02656736.2016.1237681.
- Cheng M, Fay M, Steinke K. (2016). Percutaneous CT-guided thermal ablation as salvage therapy for recurrent non-small cell lung cancer after external beam radiotherapy: a retrospective study. Int J Hyperthermia 32:316–23.
- Liu M, Huang GL, Xu M, et al. (2017). Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia. [Epub ahead of print]. doi: 10.1080/02656736.2017.1278622.
- Zoepf T, Jakobs R, Arnold JC, et al. (2005). Palliation of nonresectable bile duct cancer: improved survival after photodynamic therapy. Am J Gastroenterol 100:2426–30.
- Vavra P, Dostalik J, Zacharoulis D, et al. (2009). Endoscopic radiofrequency ablation in colorectal cancer: initial clinical results of a new bipolar radiofrequency ablation device. Dis Colon Rectum 52:355–8.
- Tuttle R, Nurkin SJ, Hochwald SN. (2014). Ablative therapy for esophageal dysplasia and early malignancy: focus on RFA. Biomed Res Int 2014:642063.
- Steel AW, Postgate AJ, Khorsandi S, et al. (2011). Endoscopically applied radiofrequency ablation appears to be safe in the treatment of malignant biliary obstruction. Gastrointest Endosc 73:149–53.
- Wu T, Li H, Li W, et al. (2015). Percutaneous intraluminal radiofrequency ablation for malignant extrahepatic biliary obstruction: a safe and feasible method. Dig Dis Sci 60:2158–63.
- Dolak W, Schreiber F, Schwaighofer H, et al. (2014). Endoscopic radiofrequency ablation for malignant biliary obstruction: a nationwide retrospective study of 84 consecutive applications. Surg Endosc 28:854–60.
- Monga A, Gupta R, Ramchandani M, et al. (2011). Endoscopic radiofrequency ablation of cholangiocarcinoma: new palliative treatment modality (with videos). Gastrointest Endosc 74:935–7.
- Liberato MJ, Canena JM. (2012). Endoscopic stenting for hilar cholangiocarcinoma: efficacy of unilateral and bilateral placement of plastic and metal stents in a retrospective review of 480 patients. BMC Gastroenterol 12:103.
- Figueroa-Barojas P, Bakhru MR, Habib NA, et al. (2013). Safety and efficacy of radiofrequency ablation in the management of unresectable bile duct and pancreatic cancer: a novel palliation technique. J Oncol 2013:1–5.
- Mizandari M, Pai M, Xi F, et al. (2013). Percutaneous intraductal radiofrequency ablation is a safe treatment for malignant biliary obstruction: feasibility and early results. Cardiovasc Inter Rad 36:814–19.
- Wadsworth CA, Westaby D, Khan SA. (2013). Endoscopic radiofrequency ablation for cholangiocarcinoma. Curr. Opin. Gastroenterol 29:305–11.
- Tal AO. (2014). Intraductal endoscopic radiofrequency ablation for the treatment of hilar non-resectable malignant bile duct obstruction. World J Gastrointest Endosc 6:13.
- Pai M, Valek V, Tomas A, et al. (2014). Percutaneous intraductal radiofrequency ablation for clearance of occluded metal stent in malignant biliary obstruction: feasibility and early results. Cardiovasc Inter Rad 37:235–40.
- Li TF, Huang GH, Li Z, et al. (2015). Percutaneous transhepatic cholangiography and intraductal radiofrequency ablation combined with biliary stent placement for malignant biliary obstruction. J Vasc Interv Radiol 26:715–21.
- Kallis Y, Phillips N, Steel A, et al. (2015). Analysis of endoscopic radiofrequency ablation of biliary malignant strictures in pancreatic cancer suggests potential survival benefit. Dig Dis Sci 60:3449–55.
- Sharaiha RZ, Natov N, Glockenberg KS, et al. (2014). Comparison of metal stenting with radiofrequency ablation versus stenting alone for treating malignant biliary strictures: is there an added benefit? Dig Dis Sci 59:3099–102.
- Oken MM, Creech RH, Tormey DC, et al. (1982). Toxicity and response criteria of the Eastern Cooperative Oncology Group. Am J Clin Oncol 5:649–55.
- Mensah ET, Martin J, Topazian M. (2016). Radiofrequency ablation for biliary malignancies. Curr Opin Gastroenterol 32:238–43.
- Sarkisian AM, Andalib I, Kumta NA, Sharaiha RZ. (2016). Radiofrequency ablation for pancreatobiliary disease. Curr Opin Gastroenterol. [Epub ahead of print]. doi: 10.1097/MOG.0000000000000300.
- De Palma GD, Pezzullo A, Rega M, et al. (2003). Unilateral placement of metallic stents for malignant hilar obstruction: a prospective study. Gastrointest Endosc 58:50–3.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Paik WH, Park YS, Hwang JH, et al. (2009). Palliative treatment with self-expandable metallic stents in patients with advanced type III or IV hilar cholangiocarcinoma: a percutaneous versus endoscopic approach. Gastrointest Endosc 69:55–62.
- Isayama H, Hamada T, Yasuda I, et al. (2015). TOKYO criteria 2014 for transpapillary biliary stenting. Dig Endosc 27:259–64.
- Singh S, Repaka R. (2016). Temperature-controlled radiofrequency ablation of different tissues using two-compartment models. Int J Hyperthermia. [Epub ahead of print]. doi: 10.1080/02656736.2016.1223890.
- Gerges C, Schumacher B, Terheggen G, Neuhaus H. (2011). Expandable metal stents for malignant hilar biliary obstruction. Gastrointest Endosc Clin N Am 21:481–97.