Abstract
Purpose: to determine the correlation of umbilical temperatures (Tumb) with simultaneously recorded chest wall temperature (Tchest) and rectal temperature (Trectal) in adults during rest, heat exposure and exercise.
Methods: A total of 28 healthy men, wearing different types of clothing (athletic garb, a spandex full body heating garment, firefighter bunker gear) had average and peak umbilical, chest wall and rectal temperature measurements taken during sedentary temperature stabilisation stages, heat exposure periods and active exercise phases.
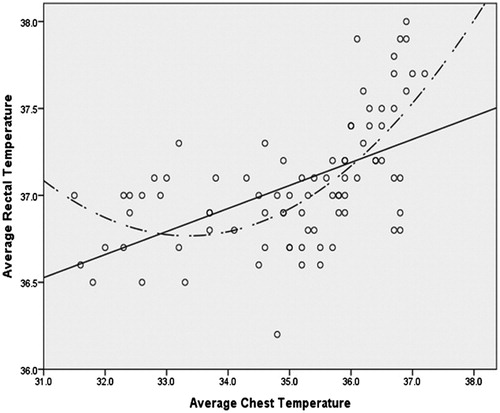
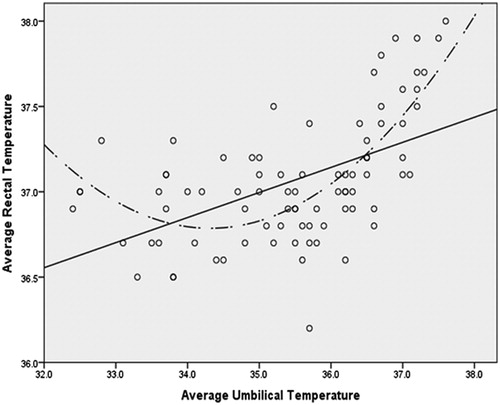
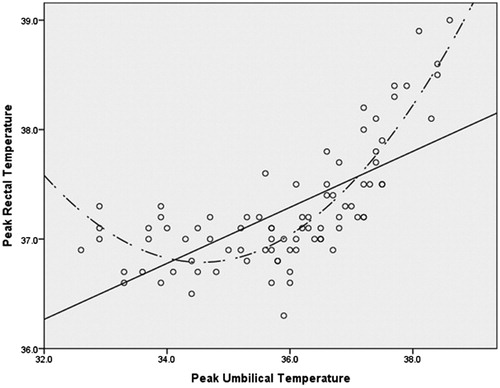
Results: Curvilinear relationships were noted between Tchest and Tumb compared with Trectal and their association became noticeably positive and linear at approximately 35.5 °C. Polynomial regression analysis of Trectal with linear and quadratic forms of Tchest and Tumb indicated an overall R2 of 0.657 and 0.767, respectively. Bivariate analysis of a restricted data set (where Tchest and Tumb ≥35.5°), indicated that Tumb was significantly associated with Trectal (raverage = 0.710, p <0.001; rpeak = 0.841, p <0.001) and Tchest was also significantly associated with Trectal, but less so (raverage = 0.570, p <0.001; rpeak = 0.699, p <0.001).
Conclusions: the umbilicus offers a non-invasive, peripheral site for measurement of temperature that more closely correlated with body core temperature than Tchest when core temperature was ≥35.5 °C.
Introduction
Core temperature (Tcore) measurement is utilised extensively in medical evaluations and research studies as it is the single best indicator of the body’s thermal status [Citation1]. Tcore (i.e. intracranial, deep thoracic, oesophageal, intraabdominal, rectal) reflects the temperature of the related anatomic region’s internal milieu. Drawbacks to the use of Tcore measurements are that they are invasive, oftentimes uncomfortable, difficult to safely maintain inserted and associated with hygiene issues [Citation2,Citation3]. The need for a simple, non-invasive method to measure Tcore is evident [Citation2], and the use of skin temperature as an index of Tcore is an attractive notion, but requires that the former be a reliable method of monitoring the latter [Citation4]. The splanchnic abdominal organs (excluding the kidneys) of a resting human produce 33% of the body’s heat, though accounting for only 3.8% of body mass [Citation5]. Intraabdominal Tcore is generally measured with an ingested telemetric sensing capsule [Citation6], but this method is hampered by the possibility of temperature gradients along the gastrointestinal tract, acute modifying effects of fluid and food ingestion on Tcore and the uncertainty of sensor transit time [Citation7]. Skin temperature is a function of measurement depth [Citation4] and body orifices and depressions (e.g. external auditory canal, medial canthus of the eye, nares) are consistently warmer than flat skin surfaces because of cross-radiation of heat from their opposing skin surfaces and reduced air current effects [Citation8,Citation9], as well as closer proximity to internal heat sources. The umbilicus (navel) is a skin depression that is regularly identified as the warmest area of the abdominal wall on infra-red imaging studies [Citation9–13], suggesting that it may offer a window into intraabdominal Tcore. This assumption is plausible because of its anatomic features and attachments to various intraabdominal organs that may serve as thermal conduits () [Citation8,Citation9,Citation13–22]. A study of afebrile infants and children, reporting insulated umbilical temperatures (Tumb) comparable with oral and rectal readings [Citation23], suggests that Tumb could possibly serve as a surrogate index [Citation4] for Tcore, but adult data is scarce. This study was undertaken by the National Personal Protective Technology Laboratory of the National Institute for Occupational Safety and Health (NIOSH) to determine the relationship of Tumb with other concurrent body temperature readings in adults at rest, during heat exposure and while involved in exercise. Such data could be important for researchers and those individuals who are engaged in activities where physical stress, environmental factors, or the use of encapsulating protective clothing can result in significant elevations of Tcore.
Table 1. Anatomical rationale for the relationship of umbilical temperature with intraabdominal temperature.
Materials and methods
The study data was collected from subjects in three ongoing, but unrelated, NIOSH studies conducted under different conditions of clothing, ambient environments and work rates. The three studies were approved by the NIOSH Institutional Review Board and written informed consent was obtained from subjects that allowed for the inclusion of material pertaining to themselves, acknowledgement that they cannot be identified via this article, and that they have been fully anonymised. All subjects were healthy, non-smokers who were medically screened by a licenced physician prior to study entry. Each individual’s testing was completed on one laboratory visit and a physician was present during subject testing for safety purposes. Subjects (n = 28) for the current report were categorised into three groups (A, B and C) based upon the clothing worn for the studies. Group anthropometrics (±SD) and clothing are described, as follows:
Group A: 11 men (age 21.6 ± 0.9 years, height 186 ± 6.9 cm, weight 79.2 ± 6.8 kg, body mass index [BMI] 23.0 ± 2.0 kg/m2) who wore athletic shoes, shorts and tee shirts.
Group B: 7 men (age 25.6 ± 5.4 years, height 179 ± 9.1 cm, weight 80.1 ± 8.5 kg, BMI 25.0 ± 1.7 kg/m2) wearing a hooded, full body, spandex garment with internal tubing that circulates warm water for heating () and athletic shoes.
Group C: 10 men (age 23.4 ± 2.7 years, height 181 ± 9.2 cm, weight 79.54 ± 6.1 kg, BMI 26 ± 2.7 kg/m2) wearing shorts and a tee shirt under firefighter full bunker gear consisting of pants, jacket, hood, gloves, boots and helmet (Morning Pride Manufacturing Co., Dayton, OH), and a self-contained breathing apparatus (SCBA) with full facepiece respirator (the SCBA was not activated during the study due to its 40 min usage capacity that would not have been sufficient to carry out the study tasks).
Baseline temperature measurements (Study phase I) – Prior to measurement of rectal (Trectal), anterior chest wall (Tchest) and Tumb baseline temperatures, stabilisation of body temperature occurred with subjects seated for 15 min in an environmental chamber at the following ambient conditions: 25 °C/50% relative humidity (RH) (Group A), 25 °C/50% RH (Group B), and 30 °C/70% RH (Group C). Group B’s heating garment was not activated during these baseline measurements. Group C subjects did not wear the SCBA tank during baseline measurements, but did wear the full facepiece respirator, without a regulator.
Heat exposure temperature measurements (Study phase II) – Subjects were seated for the same temperature measurements as obtained in the baseline studies. Group A subjects underwent passive heating seated in an environmental chamber (40 °C, 30% RH) for 30 min. Group B subjects sat in environmental chamber (40 °C, 50% RH) while wearing athletic pants and a sweatshirt over the full body heating garment that was being actively infused with 46 °C water until the subject reached a Trectal 0.5 °C above baseline (heating phase averaged 65.8 ± 5.7 min to complete). Group C subjects sat in an environmental chamber (30 °C, 70% RH) for 15 min (SCBA tank was not worn during this phase).
Exercise temperature measurements (Study phase III) – During exercise, subjects had the same temperature measurements as obtained in the baseline studies. Group A subjects pedalled a cycle ergometer, at an initial resistance of 75 watts, to volitional fatigue (average of 26.9 ± 5.1 min to termination) in environmental chamber conditions of 40 °C and 50% RH. Group B subjects pedalled a cycle ergometer, at an initial resistance of 75 watts increasing by 25 watts every 10 min, to volitional fatigue (average of 15.4 ± 3.2 min to termination, in environmental chamber conditions of 40 °C and 50% RH (the hooded sweatshirt and pants were removed and the heating garment was not activated during this phase). Group C subjects (wearing the SCBA, but breathing through the respirator without a regulator) treadmill exercised at 40% VO2 max for 40 min in environmental chamber conditions of 30 °C and 70% RH.
Measurement equipment – Trectal measurements were obtained using a Precision 401 rectal thermistor probe (YSI Temperature, Dayton, OH) with a 4.7 mm tip that was inserted 13 cm into the rectum. Tchest measurements used a Precision 409 b skin thermistor (Grant Industries, Surrey, UK) with a 9.5 mm tip applied to the right anterior chest wall at the second intercostal space region and insulated with a folded sterile cotton gauze pad covered by a moisture-and-air-permeable, transparent adhesive dressing (Tegaderm™, 3 M Company, St. Paul, MN). Tumb was measured with a Precision 401 rectal thermistor (Grant Industries), housed in a cone-shaped, pliable silicone shell that conformed to umbilical dimensions (). The thermistor was attached with a waterproof, transparent adhesive dressing (Tegaderm) and insulated with a self-adherent neoprene patch (5.5 cm diameter, 3 mm thickness) applied over the adhesive dressing.
Statistical analysis – Two different forms of Trectal, Tchest and Tumb were used in the analysis: (1) the average of the temperature taken throughout the study, and (2) the peak temperature reached throughout the course of the study. Through the varied experimental conditions and corresponding phases, a sufficient range in the variables was obtained.
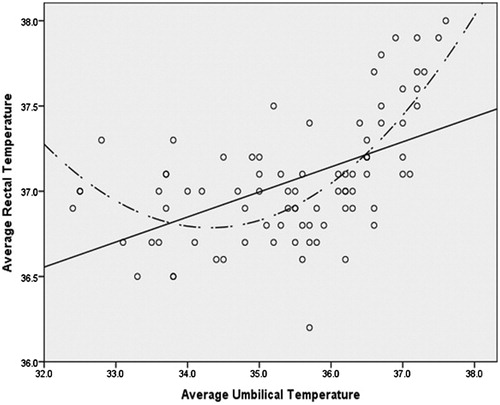
The associations were visually explored through scatter plots between Trectal, Tchest and Tumb. Visual inspection of the scatter plots revealed the appearance of a curvilinear relationship between each of the forms of the less invasive temperature measurements and Trectal (). The curvilinear relationships were verified through four distinct hierarchical polynomial regressions (R2) in which Trectal was regressed on both the linear and quadratic forms of Tchest and Tumb while controlling for age, height, BMI and weight. These hierarchical regressions took the form of:
Consistent with a hierarchical regression approach, the control variables were entered in model 1, model 2 includes the addition of the linear form of the independent variable, and model 3 includes the addition of the quadratic form of the independent variable.
Hierarchical polynomial regressions were repeated for average Tchest, average Tumb, peak Tchest and peak Tumb, and the total R2 (the coefficient of determination, a statistical measure of how close the data are to the fitted regression line), change in R2 (ΔR2), unstandardised regression coefficients (B), and significance levels were noted for each of the corresponding steps.
Results
Subject anthropometrics and descriptive statistics for variables by study and phase are presented in . For the first set of regressions, in which the average Trectal was modelled as the dependent variable, age was the only significant control variable (p = 0.006) (). There was a significant increase in R2 when the linear form of the average Tumb was added in model 2 (ΔR2 = 0.242, p <0.001). There was also a significant increase in R2 when the quadratic form of average Tumb was added (ΔR2 = 0.224, p <0.001). The overall R2 for the final model in which the average Tumb was used as the predictor was 0.583 (; ).
Table 2. Subject characteristics and descriptive statistics by study and phase.
Table 3. Results for hierarchical polynomial regressions.
When the linear form of average Tchest was added to the control variables, there was a significant increase in R2 (ΔR2 = 0.236, p <0.001). There was an additional significant increase in R2 when the quadratic form of average Tchest (ΔR2 = 0.124, p <0.001). The overall R2 for the final model in which the average Tchest was used as the predictor was 0.477 (; ).
For the second set of regressions, in which the peak Trectal was modelled as the dependent variable, none of the control variables significantly predicted the outcome. There was a significant increase in R2 when the linear form of peak Tumb was added to the model (ΔR2 = 0.425, p <0.001). There was an additional significant increase in R2 when the quadratic form of peak Tumb was added in model 3 (ΔR2 = 0.264, p <0.001). The overall R2 for the final model in which peak Tumb was used as the predictor was 0.767 (; ).
The linear form of peak Tchest also significantly increased the R2 when added to the model (ΔR2 = 0.402, p <0.001). There was an additional significant increase in R2 when the quadratic form of peak Tchest was added to the model (ΔR2 = 0.177, p = 0.014). The overall R2 for the final model in which peak Tchest was used as the predictor was 0.657 (; ).
Discussion
As is indicated by the overall model R2, Tumb explains more of the variance in Trectal when both the average and peak temperatures are considered. These analyses suggest that Tumb shares a stronger association with Trectal than the Tchest counterpart. It seems prudent, however, to further consider the application and to explore the contexts that optimise the practical utility of the use of the Tumb as a less invasive surrogate method of temperature measurement.
As can be observed from , the association between each of the predictors and the forms of Trectal noticeably becomes positive and linear at the approximate point of 35.5 °C. In order to explore the strength of the linear association at this point, bivariate correlations were computed between each of the predictors and the appropriate form of Trectal in the restricted dataset (where Tumb and Tchest ≥35.5 °C [thereby excluding most of phase I trials]). These correlations are reported in .
Table 4. Correlations between rectal temperature with umbilical and chest temperature in restricted dataset.
Consistent with the results of the polynomial regressions, Tumb displayed higher bivariate correlations with Trectal when compared to Tchest across the types of average and peak variables. Also consistent with the results of the polynomial regressions, the associations are higher in the peak variables when compared to the average temperatures. Tumb was significantly associated with Trectal (raverage = 0.710, p <0.001; rpeak = 0.841, p <0.001). Tchest was also significantly associated with Trectal, but less so (raverage = 0.570, p <0.001; rpeak = 0.699, p <0.001) ().
It has been stated that the temperature of an organ depends partially upon its depth below the skin and its anatomical relationship to the abdominal cavity [Citation4,Citation24]. The umbilicus serves as an appropriate example that, due to its thinness relative to the abdominal wall [Citation21] and its attachment to the peritoneum [Citation17], likely functions as a conduit for intraperitoneal heat. Although Tchest and Tumb are technically both skin temperature measurements, the generally higher Tumb values in this study indicate that additional heat was being dissipated across the umbilicus, the additional source being heat from the peritoneal cavity. Further, the umbilicus itself is relatively avascular and thus retains heat better than more vascular structures [Citation25]. The amount of heat flowing to the surface from the peritoneal cavity is a function of the temperature gradient between the surface of the body and the heat-producing intraperitoneal organs, as well as the overall conductivity of the human body [Citation26]. Principal sources of body heat in the resting individual are intraperitoneal, chiefly from the liver and, to a lesser extent, the intestines [Citation24]. One study (75 healthy subjects), utilising inserted thermocouples, reported a morning mean liver temperature of 36.68 ± 0.03 °C and corresponding Trectal of 36.89 ± 0.04 °C in a temperate ambient environment [Citation27]. This data aligns with findings from another human study (11 healthy subjects) that noted a liver temperature averaging 0.44 °C below Trectal under similar ambient conditions [Citation28]. However, liver temperature does not equate precisely with intraperitoneal temperature in the basal state because the latter will be lower due to a peritoneal cavity temperature gradient across the abdominal wall (in normal weight individuals) and diaphragm [Citation28,Citation29]. Data on direct intraperitoneal temperature measurements are limited, but a study of gasless laparoscopy (thereby negating the effects of pressurised CO2 gas on intraabdominal temperatures) reported a mean intraabdominal temperature of 36.46 ± 0.56 °C [Citation30]. Based on available data, it appears that intraperitoneal temperature may be ∼0.2 °C less than liver temperature that is itself 0.2–0.6 °C subrectal temperature [Citation31]. Further, although the umbilicus itself is collagenous scar tissue and relatively avascular, arterial blood flow to the umbilical skin from subdermal blood vessels and perforating vessels of the deep inferior epigastric artery [Citation22] may attenuate some of the heat transfer to the umbilicus from the peritoneal cavity in the basal state. Also, insulated skin sites have been shown to be less effective in the thermoneutral range than in detecting the onset of heat strain [Citation32]. An exception to this would be in persons with umbilical hernias wherein the Tumb would more directly reflect intraintestinal temperature [Citation9]. Thus, the temperature offset between Trectal and intraperitoneal temperature must be taken into consideration when evaluating Tumb.
Tumb values generally exceeded Tchest in this study due to the minimum air current effects and minimal radiant heat exchange on the umbilicus (dependent on umbilical depth to some degree) [Citation9] and the umbilicus’ aforementioned intimate anatomic relationships with the peritoneal cavity and its contents (). Additionally, sensor insulation been shown to be important to prevent heat loss from the skin [Citation33,Citation34], even when wearing protective clothing as in study Group C [Citation3]. However, this was not entirely the case as noted during phase II (heating) studies, when use of the heating garment resulted in Tchest that actually exceeded Trectal (), a recognised phenomenon that occurs when the skin is warmed by an outside heat source that directly contacts the temperature sensors [Citation34]. Of added note, during the exercise phase of study Group C, the peak and mean Tumb values were within 0.5 °C of Trectal, a level of accuracy required between skin site and Trectal to be considered clinically utilitarian (though this value has not been firmly established) [Citation1,Citation35]. This result was clearly impacted by the relatively impermeable, encapsulating firefighter ensemble worn that, combined with heat and exercise, resulted in a hot, humid microclimate conducive to the convergence of skin and core temperatures [Citation4,Citation36].
Fox and Solman [Citation37] first introduced the concept of zero heat flux, wherein an insulated, heated skin surface sensor equilibrates skin temperature with deep body temperature creating a region of zero heat flow that allows for measurement of core temperature at the skin surface. An early study [Citation38] at various ambient conditions, noted that there was good agreement between a heated sensor at the sternum with tympanic membrane and ingested sensor temperatures in warmer ambient temperatures at decreased walking speeds. Ball et al. [Citation39] noted a mean difference of 0.1 ± 0.5 °C between a sternal zero heat flux sensor and Trectal. Studies comparing a forehead zero heat flux sensor with Trectal reported a mean difference of 0.9 ± 0.4 °C [Citation40] and 0.12 ± 0.24 °C [Citation41]. One recent study, examining the relationship between core temperature measured by ingested core temperature sensor, skin temperature measured by ceramic heat flow sensor and heat flux of subjects who treadmill walked in different ambient environments reported R2 values of 0.40 for the forehead and 0.75 for the sternum [Citation42]. A similar study by the same authors reported an R2 of 0.70 for the sternum compared with Trectal [Citation43]. Thus, in some studies [Citation42,Citation43], the sternum is associated with the highest R2 values that compared to Trectal and align with the reported values for Tumb data from this study. The use of a “double sensor” (one probe adjacent to the skin and separated by a standard insulator from a second probe facing the environment) has been investigated in several studies [Citation44–46]. Kimberger et al. [Citation44] compared a forehead “double sensor” with oesophageal temperatures in perioperative and intensive care patients; 98% of measurements were within 0.5 °C of oesophageal values with a mean bias of 0.08 °C and limits of agreement of −0.66–0.50 °C between methods [Citation44]. Gunga et al. [Citation45] compared forehead “double sensor” readings with Trectal over 36 h of bedrest and reported r = 0.704 with 0.08 ± 0.32 °C difference between methods. Another study [Citation46], comparing Trectal and a helmet-mounted forehead “double sensor” at 25–55% VO2Max for 2 + h each at ambient temperatures of 10, 25 and 40 °C reported correlations of 0.49, 0.69 and 0.75, respectively. This study’s finding, that use of the umbilicus as a temperature measurement site correlates best with Trectal when Tumb ≥35.5 °C, implies that this parameter might be most useful in monitoring situations that are associated with heat stress and hyperthermia. Tumb might be especially useful in situations where moderate-to-high physical activity is associated with elevated ambient heat and the use of restrictive clothing (e.g. firefighters, military personnel, foundry workers, etc.).
Limitations of this study include the relatively low number of subjects tested in each group and the fact that no women subjects were tested so that we cannot comment on the possible effect of gender on Tumb. We did not analyse Tumb in relation to umbilicus depth, so there exists the possibility of deeper umbilici transmitting higher temperatures [Citation9]. Further, we did not test obese individuals whose panniculus could have served as an abdominal wall insulator [Citation14]. Skin thickness at measurement sites (e.g. forehead skin 1.81 mm) impacts temperature measurements and, although the anterior chest wall skin thickness (1.37 mm) is less than that of the abdominal skin covering the umbilicus (1.91 mm), the underlying chest muscle layer likely would increase the functional thickness of the chest wall [Citation47,Citation48]. An equipment limitation observed occasionally during testing was that, due to the relatively small contact area of the Tumb sensor (4.7 mm), motion-related displacement during exercise activities, with resultant loss of contact between the umbilicus and the sensor, necessitated repeat testing. This can occur with even minor movement of the sensor [Citation34]. In an attempt to circumvent this limitation, pilot testing using a wireless semiconductor temperature sensor with a larger surface area (I-Button, Dallas, TX) was carried out, but unsuccessful due to the sensor’s diameter (16.5 mm) exceeding the internal dimensions of the umbilicus. A similar wireless sensor, with smaller dimensions, might offer a reasonable solution to minimising Tumb sensor displacements.
Finding a skin site that correlates closely with core temperature has been the subject of much research interest for a number of years because of the varied issues associated with core temperature measurements (invasiveness, discomfort, etc.). Use of insulated skin temperature sensors, as in this study, results in closer correlations between skin and core temperatures than uninsulated sensors during exercise and heat exposure states and can allow for the development of prediction equations [Citation3,Citation31]. The umbilicus offers a temperature measurement site that, with the use of an appropriately-sized wireless sensor, could offer an unobtrusive site for temperature measurements that would not interfere with an individual’s ongoing activities. Further, in studies that might employ an externally applied heat source (as with this study’s heating garment), a small sensor housed in the recesses of the umbilical depression might be shielded from the effects of direct contact with a heating source. An appropriately-sized, wireless Tumb sensor could be programmed to deliver a vibratory or audible alarm to the wearer that, upon reaching a pre-programmed temperature setting, would indicate the need for heat remediation strategies to be undertaken. The umbilicus offers the possibility of a skin measurement site that correlates reasonably well with core temperature when Trectal is ≥35.5 °C. Future studies are needed to address the overall utility of Tumb, as well as for validation of this study’s findings.
Conclusions
This study has demonstrated that, when taking average and peak temperatures into consideration, Tumb correlated well with Trectal compared with Tchest. The umbilicus, due to its anatomical features and relationships (), offers a non-invasive, peripheral site for measurement of temperature that correlates reasonably well with core temperature when Trectal is ≥35.5 °C. Development of an insulated, wireless skin temperature sensor, that fits snuggly within the recesses of the umbilicus, may offer a non-invasive temperature measurement capability that supplements current methods of temperature measurement.
Acknowledgements
The authors thank W. Jon Williams, PhD, Lewis Radonovich Jr., MD, and Michael Bergman, MS, of NIOSH for their manuscript reviews and comments.
Disclosure statement
The authors report no declarations of interest.
The findings and conclusions in this report are those of the author(s) and do not necessarily represent the views of the National institute for Occupational Safety and Health. Mention of a product or use of a photo does not constitute NIOSH endorsement.
Additional information
Funding
References
- Sessler DI. (2008). Temperature monitoring and perioperative thermoregulation. Anesthesiol 109:318–38.
- Moran DS, Mendal L. (2002). Core temperature measurement: methods and current insights. Sports Med 32:879–85.
- Richmond VL, Wilkinson DM, Blacker SD, et al. (2013). Insulated skin temperature as a measure of core body temperature for individuals wearing CBRN protective clothing. Phys Meas 34:1531–43.
- Taylor NAS, Amos D. (1997). Insulated skin temperature and cardiac frequency as indices of thermal strain during work in hot environments. Commonwealth of Australia, Department of Defence, Defence Science and Technology Organisation: (Report DSTO-TR-0590).
- Schmidt-Nielsen K, editor. (1997). Animal physiology: adaptation and environment. 5th ed. Cambridge (UK): Cambridge University Press.
- Parsons K, ed. (2002). Human thermal environments: the effects of hot, moderate, and cold environments on human health, comfort and performance. 3rd ed. Boca Raton (FL): CRC Press.
- Byrne C, Lim CH. (2007). The ingestible telemetric body core temperature sensor: a review of validity and exercise applications. Br J Sports Med 41:126–33.
- Barnes RB. (1963). Thermography of the human body. Science 140:870–7.
- Barnes RB. (1967). Determination of body temperature by infrared emission. J Appl Physiol 22:1143–6.
- Millar KG. (1966). Placental localization by thermography. Br Med J 1:1571–4.
- Willman MK. (1978). Pitfalls of abdominal thermography. J Am Osteopath Assoc 72:913–20.
- Goodlin RV, Brooks PG. (1987). Abdominal wall hot spots in pregnant women. J Reprod Med 32:177–80.
- Siah CJR, Childs C. (2015). Thermographic mapping of the abdomen in healthy subjects and patients after enterostoma. J Wound Care 24:112–20.
- Savastano DM, Gorbach AM, Eden HS, et al. (2009). Adiposity and human regional body temperature. Am J Clin Nutr 90:1124–31.
- Rhodes RA. (2012). Laparoscopic trocar complications. In: Wetter PA, ed. Prevention and management of laparoendoscopic surgical complications. 3rd ed. Miami (FL): Society of Laparoscopic Surgeons. Available from: http://laparoscopy.blogs.com/prevention_management_3/2010/11/laparoscopic-trocar-complications.html.
- Cullen TS, ed. (1916). Embryology, anatomy and diseases of the umbilicus: together with disease of the urachus. Philadelphia (PA): WB Saunders.
- Neidhardt JPH. (1987). Surgical anatomy of the anterolateral and posterior abdominal walls and points of weakness. In: Chevrel JP, ed. Surgery of the abdominal wall. Berlin (GmbH): Springer-Verlag, 4–25.
- Braastad FW, Condon RE, Gyorkey F. (1967). The umbilical vein. Surgical anatomy in the normal adult. Arch Surg 95:948–55.
- Ying DJ, Ho GT, Cai JX. (1997). Anatomic bases of the vascularized hepatic teres ligament flap. Surg Radiol Anat 19:293–4.
- Begg RC. (1930). The urachus: its anatomy, histology and development. J Anat 64:170–83.
- Kim M, Oh ST. (2015). A computed tomographic study of umbilical anatomy: the relationship between umbilical thickness and diameter. Int J Morphol 33:1060–4.
- Stokes RB, Whetzel TP, Sommerhaug E, et al. (1998). Arterial vascular anatomy of the umbilicus. Plast Reconstr Surg 102:761–4.
- Kravitz H. (1966). Temperature of the umbilicus. J Pediatr 68:418–22.
- Grayson J. (1951). Observations on the temperature of the human rectum. Br Med J 2:1379–82.
- Behrens BJ, Nussbaum EL, Panus PC. (2014). Ultrasound and phonophoresis. In: Behrens BJ, Beinert H, eds. Physical agents: theory and practice. Philadelphia (PA): F.A. Davis, 90–118.
- Dollberg S, Xi Y, Donnelly MM. (1993). A noninvasive transcutaneous alternative to rectal thermometry for continuous measurement of core temperature in the piglet. Pediatr Res 34:512–17.
- Graf W. (1959). Patterns of human liver temperature. Acta Physiol Scand Suppl 46:1–135.
- Grayson J, Kinnear T. (1962). Observations on temperature, blood flow and heat production in the human liver in relation to environment and to glucose and insulin administration. Clin Sci 22:125–40.
- Brundin T, Thorne A, Wahren J. (1992). Heat leakage across the abdominal wall and meal-induced thermogenesis in normal-weight and obese subjects. Metabolism 41:49–55.
- Shinohara K, Hashimoto D, Hoshino T, et al. (1997). [Measurement of intra-abdominal temperature and humidity during the gas-less laparoscopic surgery by abdominal wall lifting]. Jap J Gastroenterol Surg 30:1065.
- Grayson J, Kinnear T. (1963). Temperature of human liver. Fed Proc Biol 22:775–6.
- Taylor NAS, Wilsmore BR, Amos D, et al. (1998). Indirect measurement of core temperature during work clothing and environmental influences. In: Hogdon JA, Heaney JH, Buono MJ, eds. Environmental Ergonomics VIII. Proceedings of the 8th International Conference on Environmental Ergonomics; Oct 18–23, 1998; San Diego (CA): San Diego State University, 325–328.
- Buono MJ, Ulrich RL. (1998). Comparison of mean skin temperature using “covered” versus “uncovered” contact thermistors. Physiol Meas 19:297–300.
- Thomas KA. (2003). Comparability of infant abdominal skin and axillary temperatures. Newborn Infant Nurs Rev 3:173–8.
- Bach AJE, Stewart IB, Disher AE, et al. (2015). A comparison between conductive and infrared devices for measuring mean skin temperature at rest, during exercise in the heat, and recovery. PLoS One 10:e0117907.
- Pandolf KB, Goldman RF. (1978). Convergence of skin and rectal temperatures as a criterion for heat tolerance. Aviat Space Environ Med 49:1095–101.
- Fox RH, Solman AJ. (1971). A new technique for monitoring the deep body temperature in man from the intact skin surface. J Physiol 212:8–10.
- Fox RH, Solman AJ, Isaacs R, et al. (1973). A new method for monitoring deep body temperature from the skin surface. Clin Sci 44:81–6.
- Ball SG, Chalmers DM, Morgan AG, et al. (1973). A clinical apraisal of transcutaneous deep body temperature. Biomed 18:190–4.
- Tsuji T, Nakajima K, Takeuchi T, et al. (1976). Dynamic thermometry by deep body thermometer in man. Brain Nerve 13:220–6.
- Teunissen LPJ, Klewer J, de Haan A, et al. (2011). Non-invasive continuous core temperature measurement by zero heat flux. Physiol Meas 32:559–70.
- Xu X, Karis AJ, Buller MJ, Santee WR. (2013). Relationship between core temperature, skin temperature, and heat flux during exercise in heat. Eur J Appl Physiol 113:2381–9.
- Santee WR, Xu X, Yokota M, et al. (2015). Core temperature and surface heat flux during exercise in heat while wearing body armor. Natick (MA): U.S. Army Research Institute of Environmental Medicine. Technical Report No T16-1, October, 2015. Available from: http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA622653.
- Kimberger O, Thell R, Schuh M, et al. (2009). Accuracy and precision of a novel non-invasive core thermometer. Br J Anaesthesia 103:226–31.
- Gunga HC, Werner A, Stahn A, et al. (2009). The double sensor – a non-invasive device to continuously monitor core temperature in humans on earth and in space. Resp Physiol Neurobiol 169:S63–8.
- Gunga HC, Sandsun M, Reinertsen RE, et al. (2008). A non-invasive device to continuously determine heat strain in humans. J Therm Biol 33:297–307.
- Moore TL, Lunt M, McManus B, et al. (2003). Seventeen-point dermal untrasound scoring system – a reliable measure of skin thickness in patients with systemic sclerosis. Rheumatology 42:1559–63.
- Laurent A, Mistretta F, Bottigioli D, et al. (2007). Echographic measurement of skin thickness in adults by high frequency ultrasound to assess the appropriate microneedle length for intradermal delivery of vaccines. Vaccine 25:6423–30.