Abstract
Background: During the Fifth International Workshop on Peritoneal Surface Malignancy in Milan in 2008, a consensus was reached that contrast-enhanced CT (ceCT) was the principal imaging modality for patients being evaluated for treatment of peritoneal metastases. This fact being accepted, the radiologic criteria for that may exclude patients from a high value cytoreductive surgery (CRS) plus hyperthermic perioperative chemotherapy (HIPEC) have not been reliably determined.
Methods: From a consensus of surgeons and radiologists, radiologic images were selected and their determinant radiologic characteristics described. The anatomic pathology causing the abnormal images were identified and characterised. The cytoreductive surgical procedures that may, in selected patients, result in a complete resection of the pathology identified were presented.
Results: Radiographs of 15 CT images that cause concern when a patient is being evaluated for CRS were listed. The anatomic pathology these images define and possible surgical resections they require were reviewed. The surgical implications of the absence or presence of a single, or of multiple concerning CT features was extracted from the surgical and radiologic literature.
Conclusions: There is a definite need to identify new pre-operative imaging parameters to define optimal indication of CRS with HIPEC. The presence of a single concerning radiologic feature is associated with the possibility of an adverse outcome or technically more complex resections associated with increased morbidity and mortality. If two or more of the concerning radiologic features are described from the CT, suboptimal cytoreduction will usually occur.
Introduction
Distortions of the normal abdominal and pelvic anatomy by peritoneal metastases cause the concerning radiologic features to occur. Identification of the anatomic pathology that results in these abnormal radiographs is essential to interpreting their clinical significance. Also, the identification of surgical resections that can clear peritoneal metastases at specific anatomic sites must be defined. A knowledgeable combination of anatomic pathology with surgical options for complete (optimal) cytoreduction is required to understand the implications of these concerning radiologic features for patient management. Institutional review board (IRB) approval was received for this study.
Optimal cytoreduction of parietal versus visceral peritoneal metastases
The surgical procedures that have been described for resection of peritoneal metastases are listed in . The surgical capabilities for resection of parietal peritoneal cancer nodules far exceed the possibility for clear resection of visceral peritoneal cancer nodules. The peritonectomy procedures are, with a single exception, parietal peritoneal resections. High-voltage ball tip electrosurgery allows, if necessary, a complete resection of all parietal peritoneum including that from beneath right and left hemidiaphragms, from right and left paracolic sulci, from retroperitoneum, from pelvis, from posterior aspect of the omental bursa [Citation1].
Table 1. Peritonectomy procedures and visceral resections required to achieve a complete cytoreduction.
In sharp contrast, peritoneal nodules on visceral peritoneum usually require the resection of the portion of bowel involved. There is a well-defined limit to possible resections of stomach, small bowel, and colorectum, so that resection of peritoneal metastases on visceral surfaces is seriously limited. In many patients, cancer nodules that limit the possibility of a complete cytoreduction are located on visceral peritoneum of the small bowel or its mesentery. Invasive peritoneal cancer nodules on small bowel or large bowel surface or mesentery routinely require resection of the involved structure. Sometimes if mesenteric invasion and retraction are limited, resections of peritoneum at the base of small or large bowel mesentery are possible [Citation2]. However, cancer nodules at the junction of small bowel with its mesentery require segmental small bowel resection. Also, cancer invading along the lesser curvature of the stomach and involving the vascular arcade of the right and left gastric arteries may not be resectable unless a near total gastrectomy can be tolerated by the patient. In contrast, visceral peritoneal cancer nodules on the liver surface are routinely completely resected using an electrosurgical Glisson’s capsulectomy. Also, peritoneal cancer nodules on the anterior or posterior aspect of the stomach can usually be safely removed because of the thick muscular wall of this structure.
Optimal cytoreduction with retroperitoneal or pelvic invasion by peritoneal metastases
A second anatomic site severely limited in cytoreductive surgical options is the retroperitoneum. The ureters, common, external and internal iliac arteries are usually considered unresectable anatomic sites for peritoneal metastases.
Necessity to consider the histopathologic diagnosis
The invasive versus non-invasive histopathology of the peritoneal metastases may have profound implications regarding the possibility of a complete resection of peritoneal metastases on bowel surfaces [Citation3]. Non-invasive peritoneal nodules may coat the visceral surface extensively but are completely resectable. Malignancies in this category include appendiceal mucinous neoplasms with histopathology of disseminated peritoneal adenomucinosis (DPAM), cystic and papillary mesothelioma, low malignant potential ovarian tumours and some low grade rare tumours resulting in peritoneal metastases such as urachal tumours, malignant mesenteric cysts, mucinous endocervical adenocarcinoma and perhaps others as yet to be defined [Citation4].
Another histopathologic consideration in radiologic interpretation is mucinous versus intestinal type disease. By the motion hypothesis, mucinous cells adhere less to surfaces in near continuous peristaltic motion such as the small bowel. In some patients large volumes of high-grade tumour may spare small bowel and its mesentery. With extensive parietal peritonectomy procedures and selected visceral resections, an optimal cytoreduction can be achieved. Much smaller amounts of cancer invasive into visceral peritoneum and subperitoneal lymphatics may cause incomplete cytoreduction.
Categories of concerning radiologic features
A list of 15 concerning radiologic features imaged by CT that may be associated with an increasing incidence of incomplete CRS is listed in . This list is divided into five categories that include images of small bowel and its mesentery, retroperitoneal images, pelvic images, gastrohepatic or hepatoduodenal ligaments images, and images associated with ascites.
Table 2. Concerning radiologic features observed on preoperative CT as a prognostic assessment for patients with peritoneal metastases.
Concerning features associated with images of small bowel and its mesentery
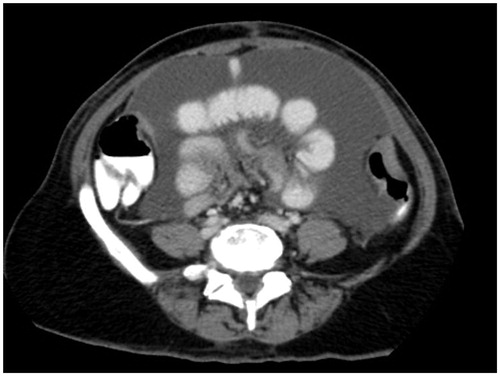
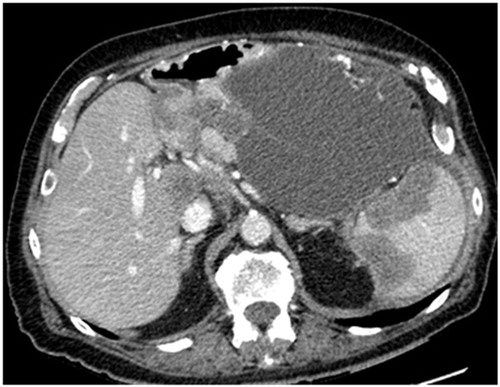
presents a CT slice through the mid-abdomen that shows loops of small bowel obstructed or partially obstructed at more than one site. The loops of bowel lack uniformity in their diameter and are irregular in their shape. The wall of the bowel loops are thickened suggesting a layering of cancer is present. There is a retractile thick fat stranding of the adjacent small bowel mesentery. Multiple loops are involved in the process suggesting multiple sites of partial obstruction as compared with a solitary site of obstruction. A single site of obstruction may suggest an adhesive process as an alternative cause of small bowel obstruction.
Figure 1. CT slice showing bowel obstruction or partial obstruction at more than one site (chaotic bowel pattern).
The anatomic pathology of multiple sites of small bowel resection is usually multiple invasive sites of peritoneal metastases on the bowel wall itself. In a patient with extensive prior surgery, intestinal adhesions, and fibrotic bands may be infiltrated by cancer.
The complete cytoreduction of cancerous small bowel obstruction is usually limited to small bowel resection. Sometimes segments of bowel with multiple cancerous nodules can be identified so that major portions of functional small bowel can be spared. Cancerous nodules distributed along the entire extent of the small bowel are a prominent cause of incomplete cytoreductive surgery.
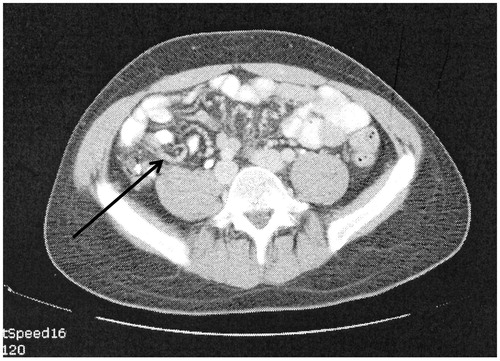
is a CT slice showing the mesentery drawn together by tumour. This is a strong predictor of diffuse involvement of visceral peritoneum by a tumour of high-grade histology. The small bowel itself may appear relatively normal with the mesentery foreshortened toward the retroperitoneum.
The anatomic pathology is a layering of fibrotic cancer upon the small bowel mesentery. Also, the leaves of the mesentery may be fused together adding to the immobility of the small bowel. A fibrous contraction of the mesentery and bowel toward the retroperitoneum causes the clumped appearance.
The complete cytoreduction of clumped small bowel mesentery is not unusually possible. This radiologic image is often considered a contraindication to an attempt at complete cytoreduction.
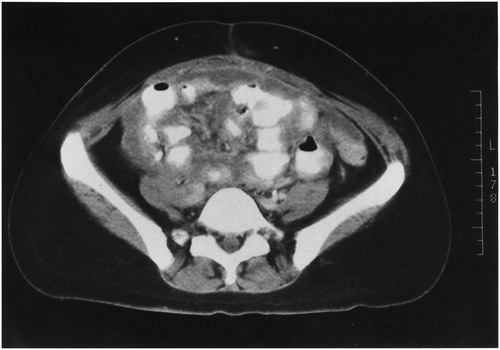
is a CT slice showing spaces between leaves of the mesentery separated from each other. The separation is not caused by ascites fluid. Although the tissue between the leaves of mesentery may appear as a layer, in some areas nodules are present. The bowel is usually not obstructed nor is the mesentery foreshortened (not clumped bowel as in ). If the malignancy is of large volume, mucinous, and of low-grade histology this separation of the leaves of the mesentery may not be meaningful.
Figure 3. CT slice showing tumour infiltrating between leaves of small bowel mesentery (string of pearls).
The anatomic pathology may be a continuing accumulation of cancerous nodules which eventually become confluent. If the tumour is high grade and has caused separation of the leaves of the mesentery, there is a layering of cancer present on and invading into the visceral peritoneum of the small bowel mesentery. Peritoneal mesothelioma may be a cause of this anatomic pathology.
In patients with separation of the leaves of the mesentery, concern for complete cytoreduction is high. In some patients, especially peritoneal mesothelioma patients, mesenteric peritonectomy may be effective [Citation2]. However, complete clearing of small bowel mesentery of invasive tumour is rarely possible.
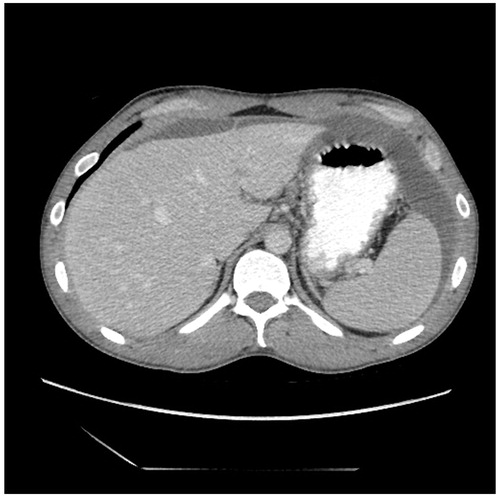
The CT slice in shows a tumour nodule greater than 5 cm in diameter in the jejunal and ileal regions of small bowel. The pathologic anatomy of this CT finding is a moderate to large size tumour nodule on the left side of the mid-abdomen associated with jejunal bowel or bowel mesentery.
Figure 4. CT slice showing tumour ≥5 cm in diameter in jejunal regions. There are also large tumour masses present in the ileal regions. The right sided tumour is likely to be resectable.
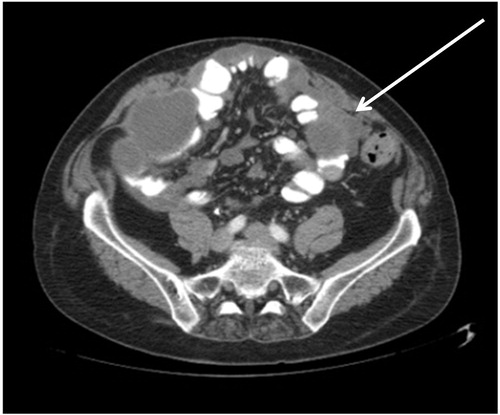
For optimal cytoreduction, extensive involvement of visceral peritoneum especially that of the small bowel and its mesentery is a common cause for suboptimal cytoreduction. There are portions of the small bowel that can be resected if there are tumour nodules present. The small bowel resections that are best tolerated are ileum, especially the terminal part of the ileum. However, from a technical perspective resections of proximal small bowel are more difficult. Also, they are poorly tolerated from a nutritional perspective by the patient. An assessment of small bowel involvement by small bowel regions may be helpful for preoperative radiologic predications of complete versus suboptimal cytoreduction. Involvement of peritoneal cancer index regions 9 and 10, the jejunal regions, has more ominous prognostic implications than involvement of regions 11 and 12.
The CT-PCI is a global assessment of peritoneal metastases within the abdomen and pelvis [Citation5,Citation6]. Unfortunately, the failure of CT to image small tumour nodules interferes with the utility of CT-PCI for a prognostic assessment of peritoneal metastases (). A low CT-PCI does have some prognostic implication but it will often require revision when the PCI from exploratory laparotomy is determined. In contrast, a high CT-PCI is a reliable prognostic assessment. If the CT images tumour nodules, they are almost always present.
In summary, the implications for cytoreduction outcome from CT-PCI can be summarised as follows: because small high-grade tumour nodules of 2 cm or less are poorly detected, the PCI is much less accurate for patients selection for CRS and HIPEC. However, if the CT-PCI is 20 or greater the likelihood of optimal cytoreduction is extremely low. PCI from 0 to 10 may carry a favourable outcome although multiple small tumour nodules may be present and cause an unfavourable outcome. CT-PCI of 10–20 is suggestive but not confirmatory of an unfavourable outcome.
These general remarks about CT-PCI should have some qualification. CT-PCI accuracy with mucinous tumour is considerably more reliable that with intestinal type tumour. Also, in general, the more mucinous the malignancy, the more reliable the CT-PCI becomes. With mucinous appendiceal tumours, it becomes quite accurate and can be trusted to give an accurate radiologic assessment of the extent and anatomic location of pseudomyxoma peritonei [Citation7].
Concerning radiologic features associated with retroperitoneal images
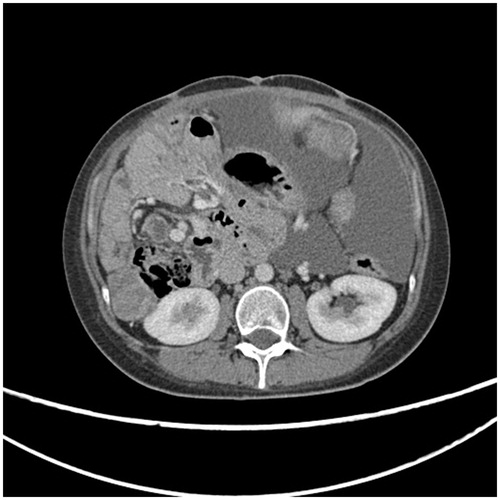
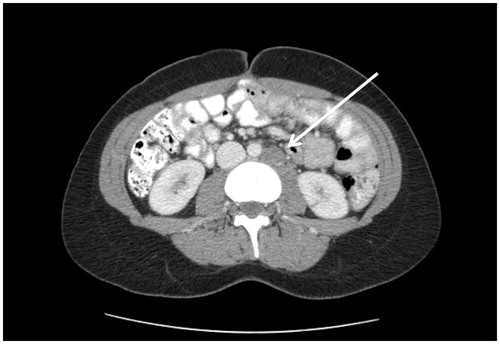
Three CT findings associated with retroperitoneal structures have been described in peritoneal metastases patients. These are mesenteric or para-aortic lymphadenopathy (), hydroureter (), and psoas muscle invasion ().
Figure 7. CT slice showing right pelvicaliectasis that may be caused by retroperitoneal cancer invasion of the right ureter.
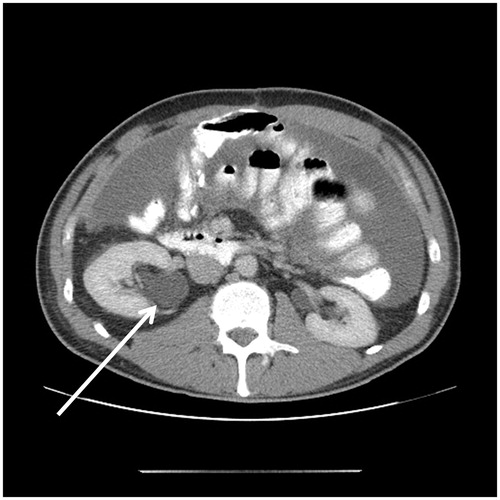
Figure 8. CT slice showing psoas muscle invasion. The mucinous appendiceal adenocarcinoma invaded extensively along the psoas muscle.
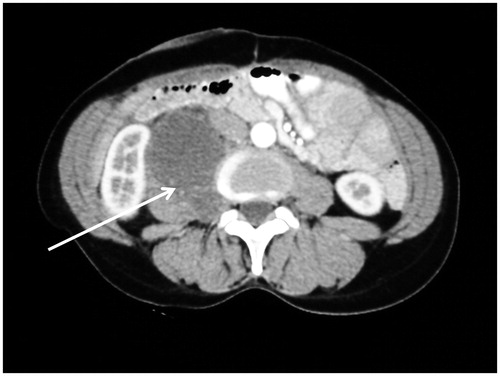
If peritoneal metastases are associated with infiltration of the retroperitoneal structures, the likelihood of an optimal CRS is low. Mesenteric and/or para-aortic lymph node infiltration indicates a poor outcome not only because complete cancer resection may be difficult, but because this dissemination indicates high-grade invasive disease that is progressing outside the peritoneal space ().
A caveat is needed regarding nodular images associated with the retroperitoneal structures. It is possible that these images are of peritoneal metastatic nodules that can be resected with a margin rather than metastatic disease progressive into lymph nodes. The prognostic implications of these two different causes of retroperitoneal nodules are very real.
Another CT finding indicating a guarded outcome is an obstructed ureter. This is caused by high-grade cancer progressing along the ureter, which is a retroperitoneal structure. The likelihood of a complete resection with margins on the ureter and surrounding cancer is small. Ureteral resection or uretero-nephrectomy may be needed if other sites of disease can be cleared ().
In the resection of some colon and appendiceal cancers, the psoas muscle is the deep margin of resection. Appendiceal, caecal, ascending colon, descending colon, and rectosigmoid colon resections can require a stripping of tissue from the anterior aspect of the psoas muscle. Reseeding of minimal residual disease on the psoas muscle can progress as cancer infiltration of this muscle. This CT finding indicates established disease within the retroperitoneal structures. Deep infiltration along the fascicles of the psoas muscle may occur and is difficult to eradicate surgically (). Complete resection of the psoas muscle may be required.
Concerning radiologic features associated with of peritoneal metastases within the pelvis
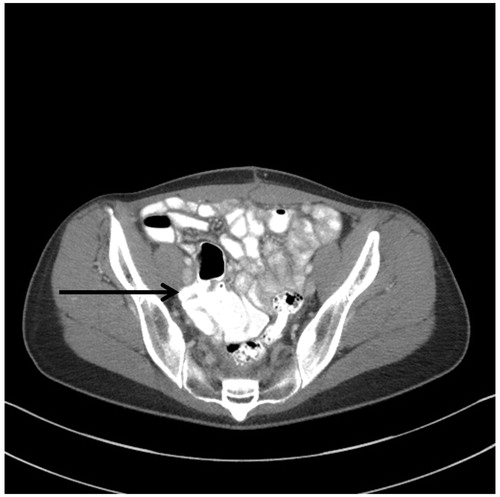
Surgical management of ovarian and rectal cancer usually requires cancer resections within the pelvis and generous removal of pelvic peritoneum. Also, resections of the pelvic sidewall lymph nodes are a standard part of primary ovarian cancer resections. Reseeding of these pelvic dissections can result in a disease progression deep within the pelvic sidewall (). This is difficult or usually impossible to resect even with a minimal margin. Also, encasement of common or external iliac vessels may preclude complete surgery.
Figure 9. CT slice showing pelvic sidewall invasion. A patient with peritoneal mucinous carcinoma of appendiceal origin underwent pelvic lymphadenectomy and tumour reseeded on the right pelvic sidewall. At her first surgery she was thought to have ovarian cancer.
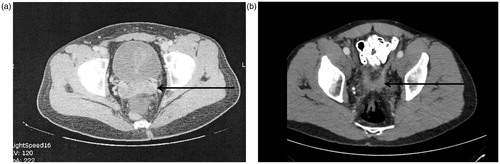
The rectouterine space in the female and rectovesical space in the male are sites of special concern for progression of peritoneal metastases. Although unusual to appear on CT, stenosis of the mid-rectum can occur most commonly observed in the female with peritoneal metastases deep in the cul-de-sac. In a male, the peritoneal metastases can invade into the seminal vesicles (). The seminal vesicles are identified by the cross sectional CT slice but nodules of tumour from the rectovesical peritoneum invade into and involve the seminal vesicles in the absence of a free flat plane. On a CT cut just above the seminal vesicles is the “rabbit ears” appearance. Tumour is lodged medially between the right and left seminal vesicles and is progressing as a layer of cancer within the peritoneum above the pararectal fossa ().
Figure 10. (A) CT slice showing peritoneal metastases between right and left seminal vesicle. A fat plane is absent. (B) CT slice a few millimetres above seminal vesicles with tumour layered out on peritoneum of pararectal fossa (rabbit ears appearance).
The anatomic pathology of pelvic involvement by peritoneal metastases is predictable from the data regarding peritoneal fluid flow and prior publications on the distribution of peritoneal metastases [Citation8,Citation9]. By gravity and flow of peritoneal fluid, peritoneal metastases circulate to and then lodge within the pelvis as progressive disease.
From a cytoreductive perspective, invasive disease within the pelvis has profound implications for an optimal resection. Pelvic peritoneum, female internal genitalia, and rectosigmoid colon may require resection. Also, if the seminal vesicles are invaded, these must be resected with definite implication for sexual function postoperatively. In some patients the rectovesical invasion is so deep that the superior aspect of the prostate requires resection. Larger or deeper lesion with involvement of the trigone of the bladder may preclude complete cytoreduction.
Concerning radiologic features associated with gastrohepatic or hepatoduodenal ligaments
The gastrohepatic ligament, subpyloric space, and hepatoduodenal ligaments are prominent sites for progression of peritoneal metastases. Tumour implantation and progression within fissures associated with the liver, gallbladder, and portal structures are clinically important. The subpyloric space and omental bursa should be carefully assessed.
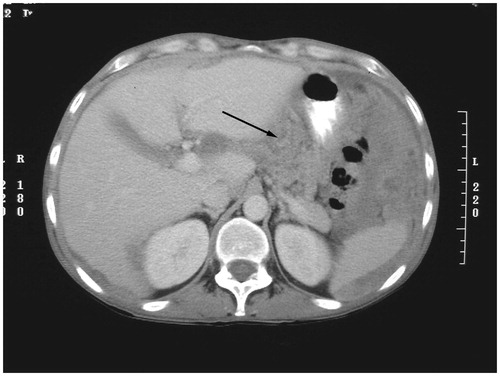
High-grade nodules with sclerotic capabilities may narrow or even occlude the common hepatic duct. Cancer nodules on the posterior aspect of the hepatoduodenal ligament may involve the portal vein. Tumour within the hepatic bridge (pont hepatique) that surrounds the round ligament of the liver is clinically imperative to identify if obstruction of the left bile duct is to be averted [Citation10]. Tumour in large volume in the gastrohepatic ligament may involve the left gastric artery ().
The distribution and progression of peritoneal metastases in the gastrohepatic ligament may occur because of the phagocytic capabilities of the lesser omentum to accumulate tumour at this anatomic site. Tumour cells coming in through the Foramen of Winslow can lodge and progress within the subhepatic space and other parts of the lesser sac, especially the superior recess. In patients with gastric outlet obstruction or partial obstruction, tumour accumulates on the fixed (immobile), gastric antrum, pylorus, and first portion of the duodenum. Tumour accumulations anterior and posterior to the antrum of the stomach may result in gastric outlet obstruction ().
In some patients, CRS can clear tumour from the porta hepatis region. A cholecystectomy followed by peritonectomy of the anterior hepatoduodenal ligament may be effective. For extensive disease within the Foramen of Winslow and adherent/invasive into the posterior hepatoduodenal ligament, the Kocher manoeuvre can make tumour removal possible and safe.
To resect peritoneal metastases along the round ligament and covered by the hepatic bridge, the liver anterior to this tunnel must be divided. Then under clear vision, peritoneal metastases can be resected along with a high ligation of the round ligament as it enters the liver.
Cytoreductive surgery utilising partial or near total gastrectomy may be required to clear tumour from the distal stomach. This increases the extent of the peritonectomy. Also, the reconstruction required will add to the incidence of postoperative complications. Usually, the gastroepiploic arcade must be resected with a complete greater omentectomy. Also, if the right gastric artery is embedded within tumour at the porta hepatis, the sole blood supply to the stomach is the left gastric artery. Damage to this vessel in the resection of extensive disease in the lesser omentum may devascularize the body of the stomach and necessitate near total gastrectomy.
Concerning radiologic features associated with ascites
Bloody ascites in a cancer patient often indicates spontaneous necrosis of a high-grade tumour and this may indicate diffuse peritoneal nodules throughout the abdomen. Indeed, most of haemorrhagic ascites due to peritoneal metastases has attenuation of less than 30 Hounsfield Unit (HU) with a similar appearance to serous ascites. Serous ascites (non-mucinous ascites) is often coming from the surface of tumour nodules (). The ascites accumulates because lymphatic stomata and lymphoid aggregates are obstructed by tumour and ascites fluid that would normally resorbed continues to accumulate. Mucinous ascites with compartmentalised small bowel is not usually a concerning radiologic feature. Also, extensive ascites with malignant peritoneal mesothelioma is expected. However, serous ascites or haemorrhagic ascites is often associated with diffuse and invasive nodules on bowel and mesenteric surfaces. Other causes of serous ascites such as cirrhosis should be ruled out.
Discussion
The relative importance of these 15 concerning radiologic features to identify patients who will have suboptimal cytoreductive surgery has not as yet been determined. These images indicate unresectability and some are signs indicating complex resections that may be technically feasible, but are associated with increased morbidity and mortality. The optimal CT acquisition techniques to be able to detect these findings (thin slices thickness, positive contrast, coronal reconstruction, etc.) are the subject of another manuscript. The information in this study should be regarded as a first step to describe the CT images associated with peritoneal metastases, understand the pathologic anatomy which causes them, and then to associate the implications that these images have for planning a cytoreductive surgical intervention. Gathering data regarding their importance is difficult in a prospective manner. Experience has associated these concerning radiologic features with a guarded surgical outcome, so that surgeries do not often occur in patients with these CT findings. Only retrospective data would be available to us.
The major clinical implication of a concerning radiologic feature is to alert the multidisciplinary team to the possibility of an incomplete cytoreduction. However, another important application may be to suggest the need for laparoscopy prior to planning a surgical intervention. In other words, patients with no concerning radiologic features would, with the appropriate clinical information, move towards recommendation of cytoreductive surgery plus perioperative chemotherapy. Those patients who on radiologic evaluation had a single concerning radiologic feature would become candidates for a laparoscopic exploration. If at the time of laparoscopy the extent of disease suggested complete cytoreduction, the management would move towards surgery. Alternatively, knowing that this group of patients would be at high risk for incomplete cytoreduction, the combination of a single concerning radiologic feature with a concerning laparoscopic examination would suggest that cytoreductive surgery is not indicated. Palliative surgical interventions might be considered if the patient is symptomatic.
Implications of one versus two or more concerning radiologic features
Jacquet et al. in 1995 reviewed CT parameters in 45 patients with known mucinous peritoneal metastases and described the CT findings. The 16 parameters they identified were statistically analysed regarding their association with complete versus incomplete cytoreductive surgery. Both a single concerning CT finding and two CT findings were statistically analysed regarding complete cytoreduction [Citation11]. In a tree-structured analysis, patients who had obstruction of bowel segments by tumour and also tumour volume greater than 5 cm on proximal bowel surfaces had an incomplete cytoreduction 88% of the time. Those patients who had no obstruction of bowel segments and no tumour nodules greater than 0.5 cm on proximal small bowel had a 92% incidence of complete cytoreduction. In this study, the combination of two concerning radiologic features was strongly predictive of the outcome of cytoreductive surgery.
Yan et al. in 2004 studied 30 patients with malignant peritoneal mesothelioma [Citation12]. They assessed the tumour extent by CT in 13 abdominopelvic regions and from 10 interpretative CT parameters. There was a 100% suboptimal cytoreduction if patients showed both tumour greater than 5 cm in the epigastric region and the interpretative assessment of small bowel and small bowel regions was class 3. In contrast, if the tumour size in the epigastric region was less than 5 cm and there were no class 3 interpretative findings in small bowel or small bowel mesentery, adequate cytoreduction was achieved in 94% of patients.
Rivard et al. in 2014 critically evaluated preoperative CT and its implications for patient selection for treatment of peritoneal metastases. This was a retrospective study of 15 patients. Although patients with a single concerning radiologic feature had the same percentage (87% versus 93%) of complete versus incomplete cytoreduction, those seven patients with two concerning radiologic features only had a single complete cytoreduction. This was significant with a p value of 0.04 [Citation13]. Finally, Yong et al. in 2016 evaluated unresectability of 172 consecutive patients who underwent CRS and HIPEC. They reported that abdominal distension from ascites, omental thickening, CT evidence of lymphadenopathy, and CT evidence for small bowel disease were all associated with a statistically significant incidence of incomplete cytoreduction. This group did not assess the implications of the presence of 1 and 2, versus multiple concerning radiologic features [Citation14].
Mohkam et al. from Lyon Sud University Hospital in 2016 reported a low impact of imaging modalities on patient selection for CRS [Citation15]. This Mohkam study is in marked contrast to our current manuscript in that all patients had previously undergone a complete imaging work-up (CT and MRI, and/or an FDG-PET/CT) depending on the aetiology of peritoneal metastases. A staging laparoscopy was also performed in patients with a high tumour load for whom the risk of unresectability was high. As a result of this first step of selection, a large number of patients were excluded for CRS because of the evidence of unresectability, preoperative pathology, or progression under systemic chemotherapy. A total of 533 patients were treated by CRS after tumour board approval. In this highly selected group of patients, no clear unresectable feature was identified in the imaging reports of the operated patients. All the three imaging modalities thus failed to definitely depict unresectable lesions and despite this radiologic scrutiny one in six patients were brought to the operating room for CRS and HIPEC underwent exploratory laparotomy without achieving a complete cytoreductive surgery. In 86 patients with incomplete cytoreduction, they found that 31 (36%) were not resectable because of multiple involvements of small bowel, 15 patients (17%) because of associated invasion of different digestive segments such as combined colonic and gastric invasion and 12 patients (14%) were due to invasion of the root of the small bowel mesentery.
Summary
Although the relative importance of these concerning radiologic features has not yet been determined, clinically we have identified selected concerning features accepted to have a high association with incomplete cytoreduction. These are concerning features associated with images of small bowel and its mesentery, features associated with the retroperitoneum, features associated with pelvic images, features associated with gastrohepatic or hepatoduodenal ligaments, and features associated with ascites.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
References
- Sugarbaker PH. (2012). An overview of peritonectomy, visceral resections, and perioperative chemotherapy for peritoneal surface malignancy. In: Sugarbaker PH, ed. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy. Textbook and video atlas. Woodbury (CT): Cine-Med Publishing, 1–30.
- Deraco M, Baratti D, Kusamura S, et al. (2009). Surgical technique of parietal and visceral peritonectomy for peritoneal surface malignancies. J Surg Oncol 100:321–8.
- Glehen O, Gilly FN, Boutitie F, et al. (2010). Toward curative treatment of peritoneal carcinomatosis from nonovarian origin by cytoreductive surgery combined with reoperative intraperitoneal chemotherapy. Cancer 116:5608–18.
- Sugarbaker PH. (2012). Management of unusual abdominal and pelvic neoplasms with peritoneal dissemination: case reports and literature review. In: Sugarbaker PH, ed. Cytoreductive surgery & perioperative chemotherapy for peritoneal surface malignancy. Textbook and video atlas. Woodbury, CT: Cine-Med Publishing, 137–58.
- Esquivel J, Chua TC, Stojadinovic A, et al. (2010). Accuracy and clinical relevance of computed tomography scan interpretation of peritoneal cancer index in colorectal cancer peritoneal carcinomatosis: a multi-institutional study. J Surg Oncol 102:565–70.
- Koh JL, Yan TD, Glenn D, Morris DL. (2009). Evaluation of preoperative computed tomography in estimating peritoneal cancer index in colorectal peritoneal carcinomatosis. Ann Surg Oncol 16:327–33.
- Archer AG, Sugarbaker PH, Jelinek JS. (1996). Radiology of peritoneal carcinomatosis. In: Sugarbaker PH, ed. Peritoneal carcinomatosis: principles of management. Boston: Kluwer, 263–88.
- Sugarbaker PH. (2015). Peritoneum and peritoneal cavity. In: Standring S, ed. Gray’s anatomy. 41st ed. Chennai, India: Churchill Livingston/Elsevier, 1098–110 (Chapter 63).
- Carmignani P, Sugarbaker TA, Bromley CM, Sugarbaker PH. (2003). Intraperitoneal cancer dissemination: mechanisms of the patterns of spread. Cancer Metastasis Rev 22:465–72.
- Sugarbaker PH. (2010). Pont Hepatique (Hepatic Bridge), an important anatomic structure in cytoreductive surgery. J Surg Oncol 101:251–2.
- Jacquet P, Jelinek JS, Chang D, et al. (1995). Abdominal computed tomographic scan in the selection of patients with mucinous peritoneal carcinomatosis for cytoreductive surgery. J Am Coll Surg 181:530–8.
- Yan TD, Haveric N, Carmignani P, et al. (2005). Abdominal computed tomography scans in the selection of patients with malignant peritoneal mesothelioma for comprehensive treatment with cytoreductive surgery and perioperative intraperitoneal chemotherapy. Cancer 15:839–49.
- Rivard JD, Temple WJ, McConnell YJ, et al. (2014). Preoperative computed tomography does not predict resectability in peritoneal carcinomatosis. Am J Surg 207:760–5.
- Yong ZZ, Tan GH, Wong JF, et al. (2016). Unresectability during open surgical exploration in planned cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia 32:889–94.
- Mohkam K, Passot G, Cotte E, et al. (2016). Resectability of peritoneal carcinomatosis: learnings from a prospective cohort of 533 consecutive patients selected for cytoreductive surgery. Ann Surg Oncol 23:1261–70.