Abstract
Background: The aim of this study was to evaluate the therapeutic outcome of percutaneous computed tomography (CT)-guided radiofrequency ablation (RFA) for extrahepatic oligometastases of hepatocellular carcinoma (HCC).
Methods: Institutional review board approval was obtained for this retrospective study, and all patients provided written informed consent. Between April 2004 and December 2015, 116 oligometastases (diameter, 5–50 mm; 20.3 ± 10.4) in 79 consecutive HCC patients (73 men and 6 women; average age, 50.3 years ±13.0) were treated with RFA. We focussed on patients with 1–3 extrahepatic metastases (EHM) confined to 1–2 organs (including the lung, adrenal gland, bone, lymph node and pleura/peritoneum) who were treated naïve with curative intent. Survival, technical success and safety were evaluated. The log-rank test and Cox proportional hazards regression models were used to analyse the survival data.
Results: No immediate technical failure occurred, and at 1 month, the technique effectiveness rate was determined to be 95.8%. After a median follow-up time of 28.0 months (range, 6–108 months), the 1-, 2- and 3-year overall survival (OS) rates were 91, 70 and 48%, respectively, with a median survival time of 33.5 months. Time to unoligometastatic progression (TTUP) of less than 6 months (p < 0.001) and a Child–Pugh score of more than 5 (p = 0.001) were significant indicators of shorter OS. The 1-, 2- and 3-year disease free survival (DFS) rates were 34, 21 and 8%, respectively, with a median DFS time of 6.8 months. DFS was better for those with lung metastases (p = 0.006). Major complication occurred in nine (9.5%, 9/95) RFA sessions without treatment-related mortality.
Conclusions: CT-guided RFA for oligometastatic HCC may provide favourable efficacy and technical success with a minimally invasive approach.
Introduction
Hepatocellular carcinoma (HCC) continues to be the most prevalent primary malignancy of the liver and is the third leading cause of cancer-related deaths worldwide [Citation1–3]. Approximately, 13.5–36.7% patients with HCC will develop extrahepatic metastases (EHM), which are indicative of advanced stage disease according to the Barcelona clinic liver cancer (BCLC) staging system [Citation4–7]. Even with stand-alone systematic therapy based on sorafenib, the median survival time of HCC patients with EHM is modest at only 9.6–10.3 months [Citation8,Citation9] and thus more effective therapeutic approaches are needed for patients with EHM.
A proportion of patients with restricted metastatic capacity (either 1–3 or 1–5, with/without well-controlled primary lesions) at the moment of diagnosis and clinical staging (so-called oligometastasis) are probably different from those with extensive metastatic disease [Citation10,Citation11]. Support for the hypothesis of oligometastasis was found within studies of surgical resection for liver metastases in case of colorectal cancer, stereotactic body radiotherapy (SBRT) for distant metastases in case of lung cancer and radiofrequency ablation (RFA) for metastases from melanoma [Citation12–15]. In regards to HCC patients with limited EHM and well-controlled primary tumours, several studies have shown that they can achieve 5-year survival rates of 30–67% following surgical resection [Citation16,Citation17].
RFA, which is similar to the surgical management of oligometastatic disease, has been used by our group in patients with HCC pulmonary and lymph node metastases, and benefits have been observed with respect to progression free survival and overall survival (OS) [Citation18,Citation19]. However, these studies included patients with incurable intrahepatic disease, which is always a key determinant of survival and may indicate that local therapy is futile [Citation6,Citation10,Citation20]. The purpose of our study was to evaluate OS, disease-free survival (DFS) and safety after RFA treatment for oligometastatic HCC in case of well-controlled intrahepatic disease.
Materials and methods
Patients
This retrospective study was conducted using a prospectively collected database on our cancer centre. It was approved by the Ethics Committee of Sun Yat-sen University Cancer Center and was in compliance with the Declaration of Helsinki and the Norton–Simon hypothesis [Citation21]. All patients provided written informed consent before RFA. Between April 2004 and December 2015, a total of 816 patients underwent RFA for extrahepatic metastatic HCC out of a pool of 14 231 HCC patients registered in our centre. Seventy-nine consecutive patients who underwent RFA as a first-line treatment for EHM were selected for this study. A multidisciplinary panel composed of hepatologists, liver surgeons, interventional radiologists and radiologists established the eligibility criteria. The inclusion criteria were as follows: (a) largest EHM less than 5 cm in diameter; (b) up to three EHM; (c) no more than two extrahepatic organs with metastasis (including the lungs, adrenal glands, bones, lymph nodes and pleura and peritoneum); (d) EHM located more than 1 cm from a major blood vessel, main bronchus, mediastinal organs or nerve tract and those in proximity to the gastrointestinal tract that could be protected with hydro-dissection or other protective methods; (e) intrahepatic disease: inactive or active but not exceeding the Milan criteria with well controlled; (f) Child–Pugh score of 5–8 (Child–Pugh score of 7 or 8 allowed only in the absence of ascites); (g) an Eastern Cooperative Oncology Group (ECOG) performance status of 0 or 1; (h) no history of other malignant disease; and (i) age 16 years or older. Patients with a coagulopathy (platelet count <50 000/mm3 or international normalised ratio of prothrombin >1.5) were excluded.
Seventy-nine patients with 116 oligometastases were enrolled (73 men and 6 women); the mean age was 50.3 ± 13.0 years (range, 16–83). A single EHM was present in 53 subjects (67.1%), and in 67 patients (84.8%) there was solitary oligometastatic organ involved. The most common involved sites were lungs (n = 65), followed by lymph nodes (n = 24), pleura and peritoneum (n = 12), adrenal glands (n = 12) and bone (n = 3). The overall mean tumour size was 20.3 ± 10.4 mm (lung 14.1 ± 8.8 mm; lymph node 21.8 ± 8.8 mm; adrenal gland 26.9 ± 10.3 mm; pleura and peritoneal metastases 21.3 ± 11.0 mm; bone metastases 25.7 ± 3.8 mm).
A diagnosis of HCC was confirmed by postoperative histopathology, biopsy-based pathology or the practice guidelines of the American Association for the Study of Liver Diseases [Citation7]. Background liver cirrhosis is considered an influencing factor for the recurrence patterns of HCC [Citation22]. Fibrosis was confirmed by pathologic results in 66 patients and by typical computed tomography (CT)/magnetic resonance imaging (MRI) findings in the other 13 patients.
The diagnosis of EHM depended on the observed pathological or radiologic pattern. Thirty-two (40.5%) patients underwent percutaneous biopsy before RFA procedure. Forty-seven patients were diagnosed on the basis of contrast-enhanced multi-detector helical CT, contrast-enhanced MRI (n = 34, 43.0%) or F-18 fluorodeoxyglucose (FDG) positron emission tomography/computed tomography (PET/CT) (n = 13, 16.5%) findings by two independent experienced radiologists. Moreover, both following criteria were fulfilled: (a) new discovery or interval growth of enlarged lesions observed at dynamic follow-up; (b) a contrast-enhanced lesion was observed in the arterial or venous phases. If any case required further procedures to confirm the diagnosis of EHM, a PET/CT was performed. If both imaging tests could not lead to a clinical diagnosis, a CT-guided percutaneous biopsy was performed to confirm the presence of metastases.
RFA procedure
All RFA procedures were performed percutaneously with CT-guidance (Siemens, Munich, Germany) by one of two senior interventional radiologists (Z.M. and W.P.H, each of whom has at least 10 years of experience with RFA). The RFA method and therapeutic strategy have been reported in detail in our previous publications [Citation18,Citation23,Citation24]. The skin puncture point was selected that allowed the shortest and safest pathway that avoided great blood vessels, bronchovascular bundles, and the gastrointestinal tract among other structures. Most patients were administered moderate intravenous sedation and local anaesthesia. General anaesthesia (19 sessions and 15 patients) was administered when the patient requested it. A single straight electrode with a 2-, or 3-cm active tip (RITA UniBlate, AngioDynamics, Queensbury, NY; Cool-Tip, Valleylab, Boston, MA; or STARmed, Goyang-si, Gyeonggi-do, Korea) was applied depending on tumour size, location and equipment availability. In general, multiple overlapping ablations were typically applied to tumours 2.0 cm in diameter or larger. The energy deposition algorithm that was used followed the manufacturers recommended protocol. Ablation procedures were finished when the ablation zone was large enough to cover the entire focus and when ablative margins of at least 0.5 cm were achieved. After ablation, electrodes were retracted with cauterisation of the electrode path to prevent tumour seeding. Diagnostic postprocedural imaging was performed immediately to document technical success and intraoperative complications. If residual viable tumours were detected on CT images, another session of RFA was attempted during the same procedure. Technical success was defined as the ability to complete the RFA procedure as planned, while the technical effectiveness was defined as the rate of complete ablation observed on CT or MR imaging performed 1 month after the first RFA procedure. No patients included in our analysis received adjuvant radiation or intratumoural chemotherapy.
Follow-up
The follow-up period was defined as the duration from the date of enrolment in the RFA procedure until death or the last visit. Patients were followed-up at clinic visits every 4–6 weeks during the first year after RFA and then every 3–6 months thereafter. Follow-up ended on June 30, 2016. Each follow-up appointment included a physical examination, serum alpha-fetoprotein (AFP) assay and liver function tests; moreover, tumour recurrence was monitored by CT or MRI scan. A bone radionuclide study or PET/CT was conducted if metastasis was suspected. The effectiveness of RFA was assessed by the change in contrast enhancement and the size of the target tumours according to mRECIST criteria [Citation25]. Detailed local evaluation methods were described in our previous studies [Citation18,Citation19,Citation26]. For those who also received contrast-enhanced CT or MRI examinations, enlargement of the treated lesion or new lesions with enhanced nodularity in or around the ablation zone was deemed positive for local recurrence. In 17 patients, PET/CT was performed in the first follow-up to assess response and local control in comparison with the baseline. The duration from RFA therapy time to PET/CT was at least 1 month, given that post-RFA inflammation can result in a false positive PET/CT [Citation27]. For patients who received PET/CT, a new focus or increasing FDG uptake in the ablation region was considered evidence for progression. The assessment of follow-up imaging was completed independently by two senior radiologists, each of whom has more than 5 years of clinical experience. None of the radiologists was involved in any of the RFA procedures. In cases of disagreement, a third radiologist was consulted. Potential complications, such as pleural or peritoneal effusion, false aneurysm and bleeding were monitored and reported if present.
The primary efficacy objective was OS, which was measured from the time of RFA until death, the last follow-up or the time the patient was lost to follow-up. The secondary efficacy objective was DFS, which was defined as the time from ablation to the time evidence was found of intra/extrahepatic recurrence on CT/MRI scans, the last follow-up or the time the patient was lost to follow-up. Unoligometastatic progression was defined based on one or more of following criteria: (a) EHM were distributed in more than 2 organs, or if they numbered more than 3; (b) The maximum diameter of the EHM was >5 cm; (c) the development of contraindications for RFA, including uncontrolled intrahepatic lesions, ECOG performance status >2, portal vein tumour thrombus, sustained ascites, or Child–Pugh class C liver function. Time to unoligometastatic progression (TTUP) refers to the interval from the time of enrolment to the time point of progression to an unoligometastatic state. We further classified patients into the following two subgroups: the long oligometastatic state group was defined as continuous TTUP for >6 months, and the short oligometastatic state group was defined as TTUP for ≤6 months. Oligoprogression refers to those still in an oligometastatic state when new lesions emerge. The definition of oligorecurrence is local recurrence near or within the target lesions, which keeps patients in an oligometastatic state. The definition of minor and major complications was assigned according to the Society of Interventional Radiology Classification System for Complications by Outcome [Citation28].
Statistical analysis
Patients survival was calculated by the Kaplan–Meier method and was compared by the log-rank test. The independent variables were tested in a univariate analysis. All variables that showed a possible association with survival in the univariate analysis were entered in a multivariate analysis (Cox proportional hazards model) to identify independent prognostic factors. A backward elimination approach was used to obtain hazard ratios (HRs) with 95% confidence intervals. The studied factors were gender, age, disease-free interval (DFI), hepatitis subtype, initial tumour stage, use of sorafenib, context of onset of EHM, number and size of targeted metastasis, involved organ (i.e. lung, adrenal gland or bone) and TTUP. We used a median DFS period of 6 months to separate patients into groups defined by short and long TTUP. DFI was defined as the time interval between the date of primary resection and the date of EHM detection. The size of the tumour was calculated in accordance with the maximum diameter, and the size of the lymph nodes were calculated according to the shortest diameter. For all analyses, p < 0.05 was considered statistically significant different. SPSS 19.0 software for Windows (SPSS, Inc., Chicago, IL) was used to perform the statistical analysis.
Results
Study population
The baseline characteristics of the patients and oligometastases are summarised in and . Four patients had synchronous EHM identified at the time of their hepatic resection or on preoperative investigations. The EHM of fifteen patients were ablated within 2 months of hepatectomy of primary liver cancer. The time intervals between the two treatments were 1.1, 1.7, 0.5 and 0.7 months. The median DFI was 14.0 months (range, 1.3–144 months) for the remaining 75 patients (metachronous oligometastases). The intrahepatic tumours in these patients were controlled based on CT or MRI without the presence of intrahepatic enhanced lesions. The absence of active intrahepatic disease continued for more than two follow-up periods after the multidisciplinary treatments, which consisted of hepatectomy (n = 37), RFA (n = 2), transcatheter hepatic arterial chemoembolisation (TACE) + RFA (n = 11), hepatectomy + TACE + RFA (n = 17) or hepatectomy + TACE (n = 12).
Table 1. Characteristics of the 79 patients.
Table 2. Characteristics of the 116 oligometastatic HCCs.
Technical success and TTUP
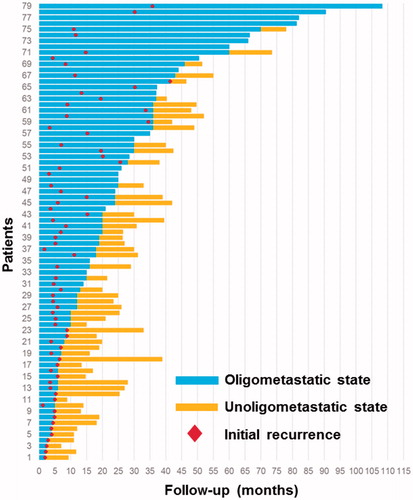
Immediate technical success was achieved in all patients who underwent RFA (100%). The technique effectiveness rate was calculated 1 month after RFA and was determined to be 95.8% due to four cases of incomplete ablation. According to the treatment protocol, eleven patients (13.9%) with a median follow-up of 44 months (range, 15–108 months) did not show disease progression. Sixty-eight patients (86.1%) experienced intrahepatic or extrahepatic disease progression within a median of 6.8 months. Most patients experienced progression in a limited number of organs including the liver and extrahepatic organs; 47 demonstrated progression at one site, five demonstrated progression at two sites, three demonstrated progression at three sites and 13 (16.5%) demonstrated progression at more than three sites (unoligometastatic state). Progression in 55 (55/68, 80.9%) patients was amenable to additional local therapy. This included 44 patients with oligoprogression, eight patients with oligorecurrence and three patients with both. The disease course revealed a 25.3-month median to unoligometastatic progression with an extremely wide range (2–108 months, ).
Survival and prognostic factors
The median duration of follow-up was 28 months (range, 6.6–108.0 months). At the time of the last follow-up (∼June 30, 2016), 51 patients (51/79; 64.6%) were deceased, 7 (7/79; 8.9%) were alive with disease and 21 (21/79; 26.6%) patients were still alive without evidence of intrahepatic and extrahepatic recurrence. Among the 51 fatal cases, a total of 36 (70.6%) patients died of hepatic functional deterioration caused by progressive intrahepatic HCC, which accounted for the majority of deaths. Other deaths were attributed to the progression of metastatic disease from HCC (8 patients, 15.7%) including brain metastasis (three patients, 5.9%), bleeding (four patients, 7.8%), infection of the biliary tract (one patient, 1.96%) and combined causes (two patients, 3.9%).
The actuarial OS rates at 1, 2 and 3 years were 91, 70 and 48%, respectively. The median OS was 33.5 months (). The median DFS was 6.8 months, while the 1-year, 2-year and 3–5-year DFS rates were 34, 21 and 8%, respectively (). Cox regression analyses were performed for two end points: OS and DFS (). The Child–Pugh score of six or higher (p < 0.001), ECOG score of 1 (p = 0.001), AFP level >400 μg/ml (p = 0.014), GGT more than 200 U/L (p < 0.001), incomplete ablation (p < 0.001), oligometastasis in a site other than the lung (p = 0.005) and TTUP ≤6 months (p < 0.001) were significant indicators of a worse prognosis according to the univariate analysis. A Child–Pugh score of six or higher (p = 0.001) () and TTUP ≤6 months (p < 0.001) () remained significant in the Cox proportional hazards model. Moreover, the maximum diameter of oligometastases >2 cm (p = 0.238), the number of oligometastases >1 (p = 0.961) and the locations of oligometastases >1 (p = 0.735) were not favourable prognostic factors for OS (–). The factors found to be associated with DFS in the univariate analysis were as follows: GGT more than 200 U/L (p = 0.006) and oligometastases located in the lung (p = 0.035). In the multivariate analysis, only oligometastasis in the lung was an independent prognostic factor related to DFS (p = 0.006) (). According to a subgroup analysis, patients with oligometastasis in the lung had a longer median DFS of 7.0 months compared with 5.0 months for patients with oligometastasis in other organs; the tumour size of the lung group (mean tumour size: 14.1 ± 8.8 mm) was smaller than the other organ group (mean tumour size: 23.1 ± 1.3 mm) (p < 0.001); and there was not significant difference of intrahepatic tumour status between them (p = 0.074). No significant difference was observed in the outcomes of patients in the oligometastatic group who had oligometastases in different extrahepatic organs.
Figure 2. Kaplan–Meier survival curves. (a) Overall survival (OS). (b) Disease-free survival (DFS). (c) Log-rank analysis of OS was stratified according to Child–Pugh score (HR =6.283, p < 0.001). (d) Log-rank analysis of OS was stratified according to the time to unoligometastatic progression (TTUP) (HR =0.136, p < 0.001).
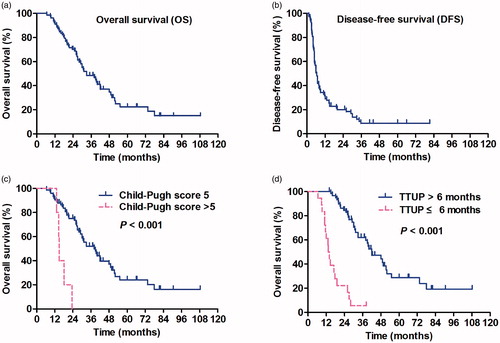
Figure 3. Kaplan–Meier survival curves. (a) Log-rank analysis of DFS was stratified according to the location of oligometastasis in the lung or elsewhere (HR =0.601, p = 0.035). (b) Log-rank analysis of OS was stratified according to the number of oligometastatic organs (HR =1.149, p = 0.734), (c) maximum diameter of oligometastases per patient (HR =1.199, p = 0.175) and (d) the number of oligometastases (HR =1.264, p = 0.388).
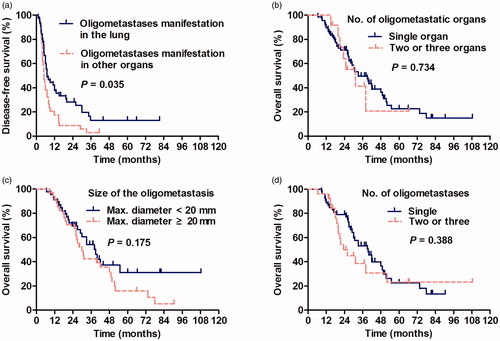
Table 3. Prognostic factors associated with overall and disease-free survival.
In the study cohort, 13 patients were treated with sorafenib. The median OS of those who were treated with sorafenib tended to be longer than that of patients who did not receive sorafenib (40.0 months vs. 31.2 months, respectively). However, this difference was not statistically significant (p = 0.521).
Complications
Although no procedural mortality occurred, major and minor complications occurred after 41 of the 95 RFA sessions (43.2%). Major complication was symptomatic pneumothorax, which required chest tube placement and which occurred in nine (9.5%, 9/95) RFA sessions in patients treated for lung metastases. Minor complications (33.7%, 32/95) included self-limited minor pneumothorax (n = 11), asymptomatic small haemothorax (n = 5), short-term abdominal pain (n = 4), hypertension (n = 3) and a low-grade fever (<38.5 °C) (n = 9). Abdominal pain developed in two patients from thermal damage of the adjacent peritoneum. No thermal injury of the gastrointestinal tract or bile duct was observed during RFA in any of the patients. Transient hypertension (SBP increase of >160 mm Hg) occurred during the RFA procedure in three patients, each of whom had adrenal gland metastasis. Their blood pressure returned to baseline levels after sublingual administration or injection of nitro glycerine.
Discussion
In solid tumours, metastasis is frequently classified as an advanced stage disease, and is accompanied with a hierarchy in terms of sites and numbers [Citation29]. In 1995, oligometastatic disease was first proposed by Hellman and Weichselbaum and was characterised by specific molecular cancer biology with a favourable prognosis [Citation11]. Those oligometastatic tumours early in the cascade of progression may have metastases that are limited in number and in types of organs affected; because the ability for metastatic growth has not been fully developed, and thus the site of such growth is restricted. Clearance of “early” metastasis, before the acquisition of widespread metastatic behaviour, might be curative and result in a “better-than-expected” prognosis [Citation10]. Our study represents the first large series that describes the clinical outcomes of oligometastatic HCC treated with RFA with a median OS of nearly 33.5 months. Notably, 13.9% (11/79) of patients survived without cancer recurrence, which indicates that RFA is a potentially curative treatment for patients with oligometastatic advanced HCC.
Lam CM et al retrospectively analysed nine HCC patients with a solitary pulmonary metastasis suitable for curative pulmonary resection, and none of these patients had concomitant intrahepatic recurrence. The median OS was 42 months after resection of the solitary pulmonary metastasis [Citation16]. In this study, the median OS in HCC patients with EHM treated by RFA was 33.5 months, which is close to that in those treated by curative resection. The fact that the patients of Lam's study were healthier at baseline, with only a solitary site of EHM, whereas 26 of 79 of our patients had multiple sites of EHM. In another study by Hiraki T [Citation30], RFA was performed in 21 HCC patients with one or two pulmonary metastases, which did not result in viable intrahepatic recurrence; the OS rates were 100% at 1 year and 84% at 3 years. In our study, for each of the oligometastatic organs including the lungs, lymph nodes, bones, adrenal glands and pleura and peritoneum, the OS rates were 91% at 1 year and 48% at 3 years. The lower 3 years OS in our study may be related to the differences in tumour burden or to an inherent difference between the characteristics of metastatic disease to the lung, as evaluated in the Hiraki T's study, to other sites, as evaluated in our study.
In the multivariate analysis, a better Child–Pugh classification and a long-term oligometastatic state were found to be positive prognostic indicators of OS. Child–Pugh classification, as an indicator of baseline liver function, is an independent prognostic factor of OS in patients with HCC, which was also found to be related to improved OS in previous reports of HCC patients with lymph node or lung metastases [Citation2,Citation7,Citation30–32]. Moreover, with respect to the hypothesis that the intrinsic characteristics of the tumour precisely affect the prognosis of oligometastatic HCC, long-term TTUP may represent the dormancy or indolent characteristics of the tumour, and a longer survival time might be achieved compared with those with rapid progression [Citation10]. In our study, 86.1% (68/79) patients experienced disease recurrence after curative RFA of oligometastases. The progression status of most patients (77.9%) have still satisfied the enrolment criteria, which means that all those recurrent sites could be treated repeatedly with curative RFA, but the rest of subset rapidly progresses and spreads as polymetastases. We believe that those patients did not have true oligometastases due to micrometastases or aggressive features, and, therefore, might not being benefited from RFA or other local regional treatment, systemic therapy are needed.
The subgroup analysis of patients with oligometastases in the lung revealed that those patients had a longer median DFS compared with those with oligometastases in other organs; the difference of intrahepatic tumour status between the two groups did not reach statistical significance. A possible reason for this might be that the mean size of the lung metastases of patients enrolled in our study was smaller than those of the other organs, which resulted in a greater likelihood of complete ablation [Citation33].
Among the fatal cases in this study, 70.6% patients died of hepatic functional deterioration caused by progressive intrahepatic HCC, which accounted for the majority of deaths. This finding demonstrates the importance of the control of intrahepatic tumours and the maintenance of the liver function. In previous studies, the use of RFA for lung metastasis and lymph node metastasis resulted in a median OS of 21 and 13 months, respectively [Citation18,Citation19]. The difference in these survival times between them may be mainly due to varying status of viable intrahepatic lesions of enrolled patients. Therefore, effectively control of intrahepatic lesions is crucial prior to RFA for oligometastases.
As a minimally invasive procedure, percutaneous CT-guided RFA can be safely and repeatedly used in cases of oligoprogression or oligorecurrence of HCC [Citation34]. No death was related to the RFA procedure itself. The major complication observed was symptomatic pneumothorax, which required chest tube placement; this complication occurred in RFA sessions for lung metastases, which was otherwise well tolerated and easily controlled. Percutaneous CT-guided RFA offers several advantages over local radiotherapy and surgical resection. Most notably, it is minimally invasive, able to be repeated and is associated with increased preservation of surrounding tissues, especially in oligoprogression or oligorecurrence. Local radiotherapy and surgical resection, have been reported to cause major complications and are associated with high mortality and fatal gastrointestinal bleeding rates (3.8 and 40.0% of patients, respectively) [Citation32,Citation35]. Comparing with radiotherapy and surgical resection, RFA, as minimally invasive method, may acquire a relatively less disadvantage in subset of rapid progression-oligometastasis state patients.
Overall, our study had some limitations. The first and greatest limitation was its retrospective nature. Second, we recognised that not all the extrahepatic lesions were subjected to pathologic examination. Third, laboratory and molecular determinants were limited in the identification of the oligometastatic state at the beginning; otherwise, it would have been beneficial to divide the distant metastasistic tumours into the oligometastasis and rapid progression-oligometastasis groups [Citation36,Citation37]. Finally, the non-comparative nature of this study limits its ability to demonstrate a survival benefit of RFA.
In conclusion, our results support the existence of oligometastases in HCC patients. RFA can also be safely and repeatedly used in cases of oligoprogression or oligorecurrence with favourable outcomes. Patients who experience the oligometastatic state for a longer period and those with a better Child–Pugh stage had a more favourable prognosis. The oligometastatic state of HCC patients with EHM might help clinicians stratify appropriate candidates in M staging (using the TNM system) or C staging (using the BCLC system) according to a distinct clinical prognosis, which was recently confirmed in metastatic nasopharyngeal carcinoma and lung cancer [Citation38,Citation39]. Ideally, prospective randomised clinical trials should evaluate the role of RFA in oligometastatic HCC.
Supplemental File
Download MS Power Point (2 MB)Acknowledgements
We thank Zhen-Feng Zhang, MD, PhD and Peng-Hui Zhou, MD, PhD, for providing writing assistance of the manuscript.
Disclosure statement
No potential conflicts of interest were disclosed.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65:87–108.
- Bruix J, Reig M, Sherman M. (2016). Evidence-based diagnosis, staging, and treatment of patients with hepatocellular carcinoma. Gastroenterology 150:835–53.
- Li Z, Zhang K, Lin SM, et al. (2016). Radiofrequency ablation combined with percutaneous ethanol injection for hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 33:237–46.
- Katyal S, Oliver JH III, Peterson MS, et al. (2000). Extrahepatic metastases of hepatocellular carcinoma. Radiology 216:698–703.
- Senthilnathan S, Memon K, Lewandowski RJ, et al. (2012). Extrahepatic metastases occur in a minority of hepatocellular carcinoma patients treated with locoregional therapies: analyzing patterns of progression in 285 patients. Hepatology 55:1432–42.
- Uchino K, Tateishi R, Shiina S, et al. (2011). Hepatocellular carcinoma with extrahepatic metastasis: clinical features and prognostic factors. Cancer 117:4475–83.
- Bruix J, Sherman M. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Nakano M, Tanaka M, Kuromatsu R, et al. (2015). Sorafenib for the treatment of advanced hepatocellular carcinoma with extrahepatic metastasis: a prospective multicenter cohort study. Cancer Med 4:1836–43.
- Sohn W, Paik YH, Cho JY, et al. (2015). Sorafenib therapy for hepatocellular carcinoma with extrahepatic spread: treatment outcome and prognostic factors. J Hepatol 62:1112–21.
- Weichselbaum RR, Hellman S. (2011). Oligometastases revisited. Nat Rev Clin Oncol 8:378–82.
- Hellman S, Weichselbaum RR. (1995). Oligometastases. J Clin Oncol 13:8–10.
- White ML, Atwell TD, Kurup AN, et al. (2016). Recurrence and survival outcomes after percutaneous thermal ablation of oligometastatic melanoma. Mayo Clin Proc 91:288–96.
- Tomlinson JS, Jarnagin WR, DeMatteo RP, et al. (2007). Actual 10-year survival after resection of colorectal liver metastases defines cure. J Clin Oncol 25:4575–80.
- Collen C, Christian N, Schallier D, et al. (2014). Phase II study of stereotactic body radiotherapy to primary tumor and metastatic locations in oligometastatic nonsmall-cell lung cancer patients. Ann Oncol 25:1954–9.
- Liu M, Huang GL, Xu M, et al. (2017). Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia 1–11. [Epub ahead of print]. doi: 10.1080/02656736.2017.1278622.
- Lam CM, Lo CM, Yuen WK, et al. (1998). Prolonged survival in selected patients following surgical resection for pulmonary metastasis from hepatocellular carcinoma. Br J Surg 85:1198–200.
- Nakagawa T, Kamiyama T, Nakanishi K, et al. (2006). Pulmonary resection for metastases from hepatocellular carcinoma: factors influencing prognosis. J Thorac Cardiovasc Surg 131:1248–54.
- Li X, Wang J, Li W, et al. (2012). Percutaneous CT-guided radiofrequency ablation for unresectable hepatocellular carcinoma pulmonary metastases. Int J Hyperthermia 28:721–8.
- Pan T, Xie QK, Lv N, et al. (2017). Percutaneous CT-guided radiofrequency ablation for lymph node oligometastases from hepatocellular carcinoma: a propensity score-matching analysis. Radiology 282:259–70.
- Jung SM, Jang JW, You CR, et al. (2012). Role of intrahepatic tumor control in the prognosis of patients with hepatocellular carcinoma and extrahepatic metastases. J Gastroenterol Hepatol 27:684–9.
- Simon R, Norton L. (2006). The norton-Simon hypothesis: designing more effective and less toxic chemotherapeutic regimens. Nat Clin Pract Oncol 3:406–7.
- Sasaki K, Shindoh J, Margonis GA, et al. (2017). Effect of background liver cirrhosis on outcomes of hepatectomy for hepatocellular carcinoma. JAMA Surg 152:e165059.
- Gao F, Gu Y, Huang J, et al. (2012). Radiofrequency ablation of retroperitoneal metastatic lymph nodes from hepatocellular carcinoma. Acad Radiol 19:1035–40.
- Hiraki T, Gobara H, Takemoto M, et al. (2006). Percutaneous radiofrequency ablation combined with previous bronchial arterial chemoembolization and followed by radiation therapy for pulmonary metastasis from hepatocellular carcinoma. J Vasc Interv Radiol 17:1189–93.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology 273:241–60.
- Li X, Fan W, Zhang L, et al. (2011). CT-guided percutaneous microwave ablation of adrenal malignant carcinoma: preliminary results. Cancer 117:5182–8.
- Yamamoto A, Nakamura K, Matsuoka T, et al. (2005). Radiofrequency ablation in a porcine lung model: correlation between CT and histopathologic findings. AJR Am J Roentgenol 185:1299–306.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Gupta GP, Massague J. (2006). Cancer metastasis: building a framework. Cell 127:679–95.
- Hiraki T, Yamakado K, Ikeda O, et al. (2011). Percutaneous radiofrequency ablation for pulmonary metastases from hepatocellular carcinoma: results of a multicenter study in Japan. J Vasc Interv Radiol 22:741–8.
- Lee DY, Park JW, Kim TH, et al. (2015). Prognostic indicators for radiotherapy of abdominal lymph node metastases from hepatocellular carcinoma. Strahlenther Onkol 191:835–44.
- Zeng ZC, Tang ZY, Fan J, et al. (2005). Consideration of role of radiotherapy for lymph node metastases in patients with HCC: retrospective analysis for prognostic factors from 125 patients. Int J Radiat Oncol Biol Phys 63:1067–76.
- Tiong L, Maddern GJ. (2011). Systematic review and meta-analysis of survival and disease recurrence after radiofrequency ablation for hepatocellular carcinoma. Br J Surg 98:1210–24.
- Timmerman RD, Bizekis CS, Pass HI, et al. (2009). Local surgical, ablative, and radiation treatment of metastases. CA Cancer J Clin 59:145–70.
- Chen SW, Wang S, Wang B, et al. (2011). Metachronous pulmonary and adrenal metastases after liver transplantation for hepatocarcinoma. World J Surg Oncol 9:156.
- Uppal A, Ferguson MK, Posner MC, et al. (2014). Towards a molecular basis of oligometastatic disease: potential role of micro-RNAs. Clin Exp Metastasis 31:735–48.
- Uppal A, Wightman SC, Mallon S, et al. (2015). 14q32-encoded microRNAs mediate an oligometastatic phenotype. Oncotarget 6:3540–52.
- Shen L, Li W, Wang S, et al. (2016). Image-based multilevel subdivision of M1 category in TNM staging system for metastatic nasopharyngeal carcinoma. Radiology 280:805–14.
- Eberhardt WE, Mitchell A, Crowley J, et al. (2015). The IASLC lung cancer staging project: proposals for the revision of the M descriptors in the forthcoming eighth edition of the TNM classification of lung cancer. J Thorac Oncol 10:1515–22.