Abstract
Purpose: Initial studies of combinations of radioiodine therapy (RIT) and local ablative procedures for the treatment of thyroid nodules have shown promising results. The goal of this study was to evaluate the effectiveness of RIT combined with radiofrequency ablation (RFA) in patients with goitres and to determine which ablative procedure is the most suitable for a combined therapy.
Methods: Thirty patients with goitres were divided into two subgroups. A test group of 15 patients received combined therapy (RIT + RFA) and a control group of 15 patients received RIT mono therapy. All patients underwent assessments including ultrasound, laboratory evaluation (T3, T4, TSH, TG, TPOAb, TgAbTRAb) and scintigraphic imaging with Tc-99m-Pertechnetate. The 3-month volume reduction was used to evaluate therapy effectiveness.
Results: Combined therapy (subgroup 1) resulted in a significant (p < 0.05) thyroid volume reduction (22.3 ± 54 ml/32.2 ± 58.2%) with better performance (p > 0.05) than the control group (20.2 ± 32.2 ml/29.6 ± 42.1%). All patients became euthyroid after treatment. No major discomfort or complications occurred. A review of the literature investigating combinations of other local ablative procedures with RIT was performed to determine the most promising combination.
Conclusions: The present study confirms the positive experiences with the combined therapy of RIT and local ablative procedures shown in the current literature and approves this approach for the treatment of goitres with RFA + RIT. These findings, when confirmed by further studies, should expand the indication of combined therapy as a minimally invasive alternative to surgery.
Introduction
Goitres are a common problem of the thyroid [Citation1]. Many patients suffer from cosmetic issues, compression-based symptoms and local pain. The Medical standards for the treatment of benign thyroid disease are surgery or radioiodine therapy (RIT) [Citation2,Citation3]. Despite its proven effectiveness, RIT has strict limitations. Goitres of a larger volume might be critical in terms of radiation protection because higher doses of radioidine-131 could harm other organs and third parties [Citation4]. Therefore, the RIT of larger volume goitres has to be fractionated, and multiple attempts over a long period of time are necessary [Citation4]. RIT typically needs 3–12 months to reduce volume effectively [Citation5]. Many patients need a fast reacting therapy for quick relief of their compression-based symptoms or thyrotoxicosis. For those patients, surgery could help quickly improve quality of life [Citation6]. Nevertheless, surgery is associated with risks, especially for a growing number of older and multimorbid patients. Those risks lead to a higher demand for non- or minimally invasive treatments, which could result in fast symptom relief. There are several sonographically guided approaches that could possibly meet the requirements to replace surgery in carefully selected cases [Citation7,Citation8]. Early approaches, such as percutaneous alcohol injection therapy (PEIT), appear to have a limited indication for certain types of thyroid nodules. Laser ablative therapy (LAT) appears to be effective in other organs but is not supported by strong data concerning thyroidal use [Citation9–11]. Other thermoablative procedures, especially radiofrequency ablation (RFA) and microwave ablation (MWA), have great potential because of their broad indications, controllability and cost-effectiveness [Citation12–16]. Performed as mono therapy, those procedures are limited due to high treatment time in larger volume goitres. Despite their single-use limitations, thermoablative procedures have great potential when combined with RIT. The first studies of combined therapy show promising results [Citation9,Citation17]. The limitations of RIT contribute to the high number of surgical cases, especially in Germany [Citation18,Citation19], which could prove to be a major problem in the future. Demographic changes appear to strengthen the tendency to refuse surgery and the use of radiation [Citation20]. The perception of radiation-based therapy is mostly negative [Citation21], or at least critical [Citation22]. Therefore, several authors have demanded a better education for both patients and healthcare professionals [Citation23,Citation24], which emphasises the need for minimally invasive treatments that could reduce the number of surgical cases and required radiation doses. The present study evaluates the effectiveness of combined RFA-RIT treatment for volume reduction in goitres and provides a reference for other combined approaches described in the current literature. This study could help make RIT accessible to more patients, reduce the required dose of radioiodine-131 and, consequently, the number of surgeries in favour of minimally invasive treatments.
Methods
Patients
Thirty patients were divided into two subgroups. Subgroup 1 consisted of 15 patients (eight female mean age 51 years) who suffered from goitres and were treated with a combined therapy (RFA + RIT). The thyroid volume in this subgroup ranged from 12 to 170 ml, with a mean volume of 73 ml. A control group (subgroup 2) was established for retrospective comparison. This consisted of 15 patients (11 female mean age 66 years) who suffered from goitres and were treated with RIT alone. The mean thyroid volume in subgroup 2 ranged from 30 to 136 ml, with a mean volume of 72 ml. All patients refused surgery, fearing complications and suffering from compression-based symptoms such as swallowing problems and foreign body sensation. The inclusion criteria for this study were as follows: growing goitres, local compression symptoms, cosmetic concerns, high operative risks, previous thyroidectomy or refusal of surgery. The exclusion criteria were as follows: thyroids with retrosternal growth; histological evidence for follicular proliferation; malignancy; suspicious 99m-Methoxy-Isobutyl-Isonitril (MIBI) uptake; abnormal calcitonin measurements as evidence of medullary thyroid cancer or critical positions near vessels, nerves, oesophagus or trachea.
Assessments
All patients underwent assessments, including ultrasound, laboratory evaluation and scintigraphic imaging with either Tc-99m-Pertechnetate or Tc-99m-MIBI. The assessments were executed pre-therapy, post RFA and at the 3 month follow up (3MFU).
Ultrasound evaluation
B-mode ultrasound images conducted by the “Sonix Touch Ultrasound system®” (Ultrasonix Medical Corporation, Richmond, Canada) were used to evaluate the size, volume and composition of the target tissue.
Laboratory evaluation
The complete thyroid hormone status, including triiodothyronine (T3), thyroxine (T4), thyrotropin (TSH) and thyroglobulin (TG) was evaluated. This also included several antibody detection tests against thyroid peroxidase (TPOAb), thyroglobulin (TgAb) and thyrotropin receptor (TRAb). All tests were conducted with commercially available immunoradiometric assay (IRMA) or radioimmunoassay (RIA) kits and had the following reference ranges: T3: 1.0–3.3 nmol/l; T4: 55–170 nmol/l; TSH: 0.3–4.0 mE/l; Tg: 2–70 ng/ml; TPOAb: <50 IU/ml; TgAb: <50 IU/ml; TRAb: <1.5 IU/l.
Radioiodine therapy
For all patients who received combined therapy (RFA + RIT), individualised dosimetric calculations were performed according to actual consensus statements. A radioiodine uptake test (RIUT) was performed as described by Dietlein [Citation25]. A diagnostic radioiodine-131-dose of 2–4 MBq was administered to each patient one week prior to RIT. The biokinetic was followed 48 h and 96 h after administration with a calibrated gamma probe to evaluate individual effective half-life and extrapolated maximum accumulation (EMA). EMA is defined as extrapolation to t = 0 of the monoexponential iodine extraction curve of the thyroid. The individual dose of radioiodine-131 was then calculated according to the Marinelli equation [Citation26]. Thyroid medication and diet had to be carefully controlled because of their influences on the thyroidal radioiodine uptake.
Radiofrequency ablation
Equipment
RFA was performed with the CelonPower system® (Olympus Medical Systems Group) and cooled bipolar applicators. The system generates a variable output power from 1 to 250 W at a frequency of 470 kHz. Depending on the target area and the target volume, different probes could be used to achieve the target temperature of 60–110 °C. Due to the integrated “resistance controlled automatic power algorithm” (RCAP), a stable energy disposition and minimised procedure time is possible. The bipolar system is safe to use without neutral electrodes. It provides a current flow and, therefore, a steady energy disposition between the tips of the applicator probe.
Procedure
The positioning of the probe and the heat application were controlled via ultrasound. To minimise the perioperative risks, a trans-isthmic approach was chosen to position the probe. This procedure allows good visualisation of the probe and the protection of the critical structures in the “danger triangle” (triangular area around the recurrent laryngeal nerve, oesophagus and trachea). For good access to the thyroid, the patient had to be placed in a supine position with a hyperextended neck. After assuring antiseptic conditions, the thyroid was re-evaluated via B-mode ultrasound, and the puncture site was determined. Pain was controlled with local anaesthesia (1% Mepivacainhydrochloride (AstraZeneca, Wedel, Germany)) and analgesics (Novalgin® i.v.). A small scalpel incision (2–3 mm) enabled a smooth skin perforation. Permanent real-time ultrasound imaging was used to verify the correct positioning of the probe. Microbubbles and hyperechogenic areas visualised the heat transduced to the tissue. During the procedure, the integrity of the laryngeal nerve was controlled periodically by talking to the patient to detect phonation changes.
Combined therapy
US-guided RFA was performed as described above. Subsequently, RIT was initiated. To avoid over- or under-treatment all patients received the exact dose of radioiodine-131 calculated by individual dosimetry according to actual consensus statements. After three months tissue destruction was controlled with US.
Chronological overview
Enrolment assessments:
Anamnesis
Clinical examination
US evaluation
Laboratory evaluation
Scintigraphic imaging
Radioiodine uptake test one week before therapy:
(2–4 MBq) Biokinetic measurements after 48 h and 96 h
Calculation of EMA, effective half-life and resulting individual dose of radioiodine-131
Therapy:
Pre-RFA measurements: Identification of the target volume with US
RFA (CelonPower system® – 1–250 W at a frequency of 470 kHz)
Post-RFA measurements: Control of RFA effectiveness (microbubbles)
Administration of individually calculated dose of radioiodine-131
3 month follow up:
US examination of the tissue reduction
Laboratory evaluation
US was used to calculate the absolute volume reduction for each patient in ml. For a better comparability of both groups, the relative volume reduction was then calculated showing the volume reduction in % compared against the initial baseline-value (=100%).
Pain score
Pain during the procedure was measured on a 10-point scale ranging from no pain = 0 to the most imaginable pain = 10.
Statistical analysis
All data were recorded with Microsoft Excel. Non-parametric tests (Wilcoxon–Mann–Whitney-U and Wilcoxon-matched pairs test) were performed to analyse the data. The BiAS® programme version 11.02 (1989–2016) for Windows was used.
Literature research
A systematic literature search in various databases was supplemented using Endnote X5® (Thomson Reuters, New York, NY) and Google scholar® (Google Inc., Mountain View, CA). Three review authors independently screened and selected the literature and included eligible studies. The literature search considered studies of combined therapies including RIT and a local ultrasound-guided minimal invasive ablative procedure. The different procedures are described in the following section.
Ablative procedures
RFA
RFA uses heat generated from high alternating electric current oscillating between 200 and 1200 kHz. The RF waves passing through the electrode agitate tissue ions around the electrode. The moving ions create frictional heat. In addition to the frictional heat, conduction heat from the ablated area could cause slower damage to the tissue in places far from the electrode tip. This process of thermal injury secondary to friction and conduction heat is the basic mechanism of RFA [Citation27].
Microwave ablation
Microwave radiation refers to the region of the electromagnetic spectrum with frequencies from 900 to 2450 MHz. As water molecules are not symmetrically charged (oxygen is negatively charged, and hydrogen is positively charged), they can be flipped due to the charge of the electromagnetic wave. This flipping results in movement of the water molecules (up to 2–5 billion times a second) and the creation of frictional heat, inducing cellular death via coagulation necrosis similar to RFA in the histopathological examination [Citation28].
Percutaneous ethanol injection therapy
Alcohol diffusion into cells causes immediate dehydration of cytoplasmic proteins and, consequentially, coagulation necrosis followed by fibrosis. When entering smaller blood vessels, alcohol diffusion induces necrosis of endothelial cells and platelet aggregation, which leads to thrombosis and ischaemia of the ablated tissue (Tumor Ablation Principles and practice/Springer 2010).
Laser ablative therapy
The principle of LAT or more specifically laser induced thermo therapy (LITT) is the generation of heat through the absorption of photon energy in the target tissue. The energy could either be produced by an Nd:YAG-Laser (Neodymium–Yttrium–Aluminium–Granat), emitting photons with a wavelength of 1064 nm, or by a diode-laser with a wavelength of 800–940 nm. The ablation area can be controlled by modulation of the intensity and the time of the treatment [Citation11].
Results
Comparability of test and control groups
To guarantee comparability, both groups were tested using a Wilcoxon–Mann–Whitney test for significant differences in terms of age, gender and thyroid volume. p values were designated p < 0.05. The groups did not show statistically significant differences in thyroid volume, age or gender (p < 0.05). Comparability was assured.
Thyroid volume
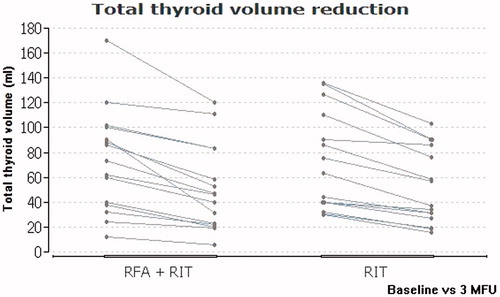
After three months, the combined therapy (subgroup 1) resulted in a mean absolute thyroid volume reduction of 22.3 ± 54 ml and a mean relative reduction of 32.2 ± 58.2%. The RIT mono therapy (subgroup 2) led to a mean absolute thyroid volume reduction of 20.2 ± 32.2 ml. This finding entails a mean relative thyroid volume reduction of 29.6 ± 42.1%. The volume reduction after three months was significant in both cases (p < 0.05) and did not differ significantly between the two groups (p > 0.05).
provides an overview of the data evaluated in this study. shows a comparison of the thyroid volume reduction in both subgroups. The differences in thyroid volume changes between the two subgroups concerning absolute and relative values are shown in and , respectively.
Figure 2. A comparison of the relative thyroid volume reduction due to combined therapy (RFA + RIT) in both subgroups.
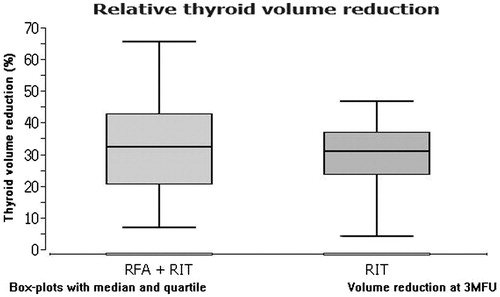
Table 1. An overview of the data evaluated in this study.
Complications and pain score
No major complications occurred. Pain during the procedure was measured on a 10-point scale ranging from no pain =0 to the most imaginable pain =10. No patient suffered major pain during the RFA procedure. Two patients described mild pain with a score of three. All other patients described minor discomfort represented by a score of one or two.
Hormone level change
All patients became euthyroid after treatment. The mean T3 values decreased significantly from a mean of 2.6–1.6 nmol/l (p < 0.05). T4 showed a slight increase after thermoablation but showed decreased values after 3 months. The changes were not significant (p > 0.05). The TSH values increased significantly (p < 0.05) from a mean of 0.7–2.6 mIU/l.
Literature review
A comparison of the different combined therapy approaches in the current literature is shown in .
Table 2. A complete overview of the combined therapy approaches in the current literature. It evaluates the essential parameters in order to make the different studies comparable.
Discussion
RFA has proven to be a safe and controllable minimally invasive treatment that could be used effectively to reduce thyroid volume [Citation29–31]. However, RIT is still the standard treatment for this indication [Citation18]. The limitations of RIT are caused by the slow reaction time of the tissue and radiation protection [Citation4]. Multiple attempts or higher doses of radioiodine-131 could be a strain for the patient and the patient’s surroundings [Citation4]. Expensive precautionary measures are necessary to obey German radiation protection laws [Citation4,Citation25]. In cases of thyrotoxicosis or acute symptoms, a fast responding therapy is needed to restore quality of life [Citation6,Citation32]. In those cases, surgery is the standard treatment [Citation2]. As a result, the surgical case numbers are relatively high, especially in Germany [Citation18,Citation19]. The complications related to this fact are already visible today and will increase in the future as the numbers of multi morbid patients and refusals of surgeries rise [Citation20]. Thermo ablation cannot remove thyroid tissue immediately but induces necrosis and involution of the thyroid tissue, which results in a fast volume reduction and improvements in clinical symptoms, with a similar effectiveness to that of thyroid surgery [Citation33]. RFA as a minimally invasive treatment could be performed predictably on patients who refuse surgery and on high-risk patients for whom surgery would be too dangerous to perform [Citation12,Citation29,Citation30,Citation32,Citation34,Citation35]. The combination of a local ablative procedure with RIT could help make this treatment safer and available to a higher number of patients. The first approaches of combined therapy showed promising results [Citation9,Citation10,Citation17,Citation36].
An early prospective randomised study by Zingrillo et al. from 2003 [Citation10] described the combined therapy of PEIT and RIT in comparison to RIT single therapy in large (>4 cm) toxic thyroid nodules (TTNs). Twenty-two patients with large TTNs were divided into two subgroups. Subgroup A (11 patients) received RIT alone, whereas subgroup B (11 patients) was treated with PEIT (2 months pre-RIT). The injection of 95% sterile ethanol in weekly intervals in doses of 4–8 ml led to a significantly higher nodule volume reduction than the volume reduction of the RIT alone group without decreasing the radioiodine uptake. After 12 months, the patients in both groups were clinically euthyroid, but patients treated with the combined therapy showed significantly (p < 0.01) better local symptom improvement than patients treated with RIT alone. The authors declared a decrease in the administered dose of radioiodine because of significant shrinkage of the nodule due to pre-RIT therapy; they considered the combined therapy useful "when marked shrinkage of the nodule is required to relieve local symptoms or when the reduction of the radioiodine dose can prevent hospitalisation". Despite the promising results, PEIT is limited to primary cystic nodules [Citation37–39] and can be associated with severe side effects, such as ethyl toxic necrosis of the larynx [Citation40]. This side-effect is unacceptable when seeking a therapy for high-risk patients and for people in fear of operative risks.
A pilot study by Chianelli et al. [Citation9] from 2014 combined (LAT) with (RIT) to assess the efficacy with respect to the rapidity of control of local symptoms, hyperthyroidism and administered dose of radioiodine-131 in patients who refused or had contraindications to surgery. Fifteen patients with large autonomous nodules (mean 27.7 ml) treated with LAT followed by RIT were compared to a group of 17 patients with large autonomous nodules (mean 29.4 ml) treated only with RIT (target dose 200 Gy). Thyroid scans with Tc-99m-pertechnetate were performed before, 1 month and 1 year after therapy. Ultrasound assessment was performed before and 1 month and 1 and 2 years after treatment. A symptom score (SYS) was used to evaluate the comfort of the treatment. No differences were observed in baseline nodule volume, but volume- and symptom reduction occurred faster and at a greater scale with combined therapy at each time point, leading to an overall nodule volume reduction of 71.3% (LAT + RIT) vs. 47.4% (RIT alone) after 24 months. In three cases, no RIT was needed after LAT. The authors declared this combined therapy to be a suitable method to reduce the radioiodine-131 dose and to achieve a quicker improvement of local symptoms and of biochemical hyperthyroidism [Citation9]. LAT has proven its effectiveness in various indications, such as liver [Citation41], lung [Citation42] and brain tumours [Citation43] as well as for thyroidal treatments [Citation9]. Despite its effectiveness and low risk [Citation41,Citation44], very few centres are using this technique because of its high cost [Citation11].
A recent study by Korkusuz et al. from 2016 evaluated the effectiveness of combined MWA + RIT in patients with benign thyroid nodules in a 3MFU [Citation36]. Fifteen patients with nodules and either goitre, Grave’s disease or autonomous tissue with no previous surgeries or RIT were included. All patients underwent pre-therapy assessments, post-MWA assessments and assessments at 3MFU, including US (B-mode ultrasound), laboratory tests (T3, T4, TSH, TG, TPOAb, TgAb, TRAb) and functional imaging with cm-pertechnetate (in some cases Tc-99m-MIBI). US-guided MWA was performed under local anaesthesia (1% Scandicain®) and sterile conditions using 14–16 gauge probes. RIT was executed with I-131 aiming at target activities of 150 Gy (goitre) or 250 (Grave’s disease). Combined therapy led to a mean volume reduction of 26.4 ml ±7.9 ml, reflecting a mean relative reduction of 30.5%±4.6% (p < 0.05). The mean absolute reduction of the radioiodine-131 dose was 563 ± 102 MBq, reflecting a relative reduction of 26.6 ± 4.8%. This led to a mean reduction of hospitalisation time reduction of 2 ± 0.7 days and a relative reduction of 30.9 ± 19.9%. TSH levels remained within the normal range in 13 out of 15 patients. Two patients showed TSH levels below the normal range, but all patients were clinically euthyroid. No serious complications occurred, and the authors concluded the combination of MWA and RIT was “an effective approach for the treatment of large benign nodular goitres” [Citation36].
The current study evaluated the effectiveness of combined therapy (RFA + RIT) in patients with goitres who refused or had contraindications to surgery. The thyroid volume reduction was used to compare therapy effectiveness. All patients suffered from goitres with inhomogeneous uptake in thyroid scintigraphy. The goitres contained partially hyper and hypofunctional areas but did not have any clearly circumscribed hot or cold nodules. Fifteen patients were included in the test group receiving RFA + RIT. A control group of 15 patients with the same diagnosis as the test group was established. The groups did not show statistically significant differences in thyroid volume, age or gender (p > 0.05). Comparability was assured. A complete thyroid hormone status, including triiodothyronine (T3), thyroxine (T4), thyrotropin (TSH), thyroglobulin (TG) thyroid, and antibody detection tests against peroxidase (TPOAb), thyroglobulin (TgAb) and thyrotropin receptor (TRAb) were performed at enrolment, post RFA and at the 3MFU. Sonographic and functional imaging with Tc-99m-pertechnetate were implemented. The tissue destruction of thermoablation was controlled sonographically. The combined therapy led to a significant thyroid volume reduction of 22.3 ± 54 ml (32.2 ± 58.2%) (p < 0.05) and achieved slightly better but not statistically different results (p > 0.05) than the RIT mono therapy, which led to a significant thyroid volume reduction of 20.2 ± 32.2 ml (29.6 ± 42.1%, p < 0.05). This result clearly shows the effectiveness of the combined therapy, even in goitres with hyper and hypofunctional tissue. The inhomogeneous tissue treated in this study did not decrease therapy effectiveness and proves the combination of RFA + RIT has broad applications. Combined therapy could be a benefit to RIT in general when used to reduce the RIT target volume. This could reduce the required dose of radioiodine-131, making RIT accessible for a larger number of patients. However, the largest potential of a combined approach would be in large goitres with cold nodules.
Cold nodules could not be treated effectively with RIT because of the low radioiodine uptake. Exceptionally high doses of radioiodine-131 would be needed to reach the required target dose in the nodule. Instead of choosing surgery, as an alternative treatment to RIT, a combination therapy approach with ultrasound-guided ablative therapy could help these patients in a minimally invasive way. An initial case study has already approved this approach [Citation17]. Happel et al. [Citation17] described the use of a combined therapy in this indication due to refusal of surgery. The patient suffered from a 2.6 ml hyperfunctional thyroid nodule in the caudal right lobe, which could be treated with RIT. Tc-99m-pertechnetate imaging showed a 3.6 ml nodule in the central left lobe of the thyroid to be hypofunctional. Due to the low radioiodine uptake of hypofunctional nodules, surgery was planned. The patient refused surgical intervention and MWA was used to ablate the hypofunctional nodule. Both nodules were identified as indifferent. An absolute volume reduction due to RIT of 1.5 ml (down from 2.6 ml) and to 1.5 ml (down from 3.6 ml) due to MWA made it possible to classify this approach as “a suitable, safe and minimally invasive alternative to surgical intervention”.
The current study could approve the efficiency of combined therapy on a larger scale. All patients could be treated effectively without major complications or negative effects. RFA in combination with RIT in goitres led to significant thyroid volume reduction (p < 0.05), with results that are comparable to other combined approaches ().
The ideal ablative procedure for a combined therapy should be safe, effective and available. Due to the small number and different designs of the studies, it is difficult to identify the ideal constellation. However, PIET could not be recommended because of its limitation to mainly cystic nodules [Citation37,Citation38] and its potentially severe side effects [Citation40]. LIT is safe and effective [Citation45,Citation46] but has limited availability due to high costs [Citation11]. Thermoablative approaches, such as RFA and MWA are cost effective and work predictably in every kind of nodule [Citation47–50]. RFA has been well researched over a long period of time. This technique was first used in liver surgery for the ablation of hepatocellular carcinomas [Citation11,Citation51]. Even tumours of the suprarenal gland could be safely ablated in this way [Citation49]. The effectiveness for thyroidal use has been proven in numerous studies [Citation12,Citation52–56]. The efficiency of RFA appears to be slightly superior to that of LAT [Citation46,Citation31,Citation57] and comparable to MWA. Overall, the side effects are mild, controllable and reversible [Citation56]. No life-threatening complications have yet been reported in thyroidal use of RFA [Citation56]. Some authors consider RFA to be as effective as surgery [Citation12,Citation29] or even demand RFA to replace surgery as the first-line treatment for goitres because it preserves thyroid function, has fewer complications and leads to reduced hospitalisation time [Citation30]. Therefore, RFA appears to be a good choice for combined therapy with RIT. Still, RFA has some disadvantages. It creates only heat in a small area around the tip of the electrode, and this requires higher temperatures or special electrodes (LeVeen® electrode) to reach bigger ablation fields. At temperatures between 60 and 100 °C, nearly immediate tissue coagulation is induced with irreversible damage caused to the tissue, but temperatures greater than 100–110 °C result in tissue vaporisation and carbonisation [Citation58]. Carbonised tissue serves as an isolator, which prevents heat spread and reduces RFA effectiveness [Citation59]. In terms of heat penetration in the tissue, MWA has theoretical advantages to RFA [Citation28] and performs well in comparison [Citation60,Citation61], but the data must be confirmed [Citation60].
Conclusions
The present study evaluated the effectiveness of combined RFA and RIT treatment in goitres and compared the 3MFU data to a single RIT control group with the same diagnosis (goitre). The combined therapy led to a significant reduction in thyroid volume (p < 0.05), showing better effectiveness than the control group (p > 0.05). All patients were clinically euthyroid after treatment, and no major complications or discomfort were recorded. A review of the current literature concerning local ultrasound-guided ablative approaches in combination with RIT showed good results overall with percutaneous ethanol injection therapy, laser ablation therapy, MWA and radiofrequency ablation. Major complications or side effects did not occur in any of the studies. The ablative treatments led to a quicker volume reduction, with effective relief in compression-based symptoms. Local ablative treatments appear to be an appropriate addition to RIT, but the ideal ablative procedure has yet to be chosen. LIT works effectively but is too costly and inferior to RFA. PEIT is limited in terms of the indication and has potentially dangerous side effects. RFA is proven, cheap and effective but appears to be inferior to MWA in terms of the size of the ablation field and treatment time.
The present study supports the positive experiences with combined approaches of RIT with minimally invasive ultrasound-guided ablative treatments in goitres and proves the effectiveness of combined therapy (RFA + RIT) for goitres with inhomogeneous uptake in thyroid scintigraphy. The increased effectiveness due to combined therapy could make RIT safer by reducing the required dose of radioiodine-131. These results, when supported by stronger data, should encourage physicians to lower the number of surgeries by choosing non-invasive alternatives for carefully selected patients.
| Abbreviations | ||
| RFA | = | radiofrequency ablation |
| RIT | = | radioiodine-131 therapy |
| US | = | ultrasound |
| MWA | = | microwave ablation |
| PEIT | = | percutaneous ethanol injection therapy |
| LITT | = | laser-induced thermo therapy |
| LAT | = | laser ablative therapy |
| RIUT | = | radioiodine uptake test |
| EMA | = | extrapolated maximum accumulation |
| RCAP | = | resistance controlled automatic power algorithm |
Disclosure statement
The authors declare that there is no conflict of interest.
References
- Reiners C, Wegscheider K, Schicha H, et al. (2004). Prevalence of thyroid disorders in the working population of Germany: ultrasonography screening in 96,278 unselected employees. Thyroid 14:926–32.
- Papi G, Corsello SM, Pontecorvi A. (2014). Clinical concepts on thyroid emergencies. Front Endocrinol 5:102.
- Yano Y, Sugino K, Akaishi J, et al. (2011). Treatment of autonomously functioning thyroid nodules at a single institution: radioiodine therapy, surgery, and ethanol injection therapy. Ann Nucl Med 25:749–54.
- Meier DABD, Becker DV, Clarke SEM, et al. (2016). Procedure guideline for therapy of thyroid disease with 131Iodine. J Nucl Med 43:856–61.
- Bachmann J, Kobe C, Bor S, et al. (2009). Radioiodine therapy for thyroid volume reduction of large goitres. Nucl Med Commun 30:466–71.
- Mishra A, Sabaretnam M, Chand G, et al. (2013). Quality of life (QoL) in patients with benign thyroid goiters (pre- and post-thyroidectomy): a prospective study. World J Surg 37:2322–9.
- Hegedüs L. (2009). A new nonsurgical therapy option for benign thyroid nodules? Nat Rev/Endocrinol 5:476–78.
- Mauri G, Sconfienza LM. (2017). Percutaneous ablation holds the potential to substitute for surgery as first choice treatment for symptomatic benign thyroid nodules. Int J Hypertherm 33:301–2.
- Chianelli M, Bizzarri G, Todino V, et al. (2014). Laser ablation and 131-iodine: a 24-month pilot study of combined treatment for large toxic nodular goiter. J Clin Endocrinol Metabol 99:E1283–6.
- Zingrillo M, Modoni S, Conte M, et al. (2003). Percutaneous ethanol injection plus radioiodine versus radioiodine alone in the treatment of large toxic thyroid nodules. J Nucl Med 44:207–10.
- Vogl TJ, Mack M, Eichler K, et al. (2011). Interventionelle Thermoablation von malignen Lebertumoren und Lebermetastasen: Vergleich von Radiofrequenzablation (RFA), laserinduzierter Thermotherapie (LITT) und Mikrowellenablation (MWA). Hessisches Ärzteblatt 10:606–15.
- De Bernardi IC, Floridi C, Muollo A, et al. (2014). Vascular and interventional radiology radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: literature review. La Radiol Med 119:512–20.
- Heck K, Happel C, Grunwald F, Korkusuz H. (2015). Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hypertherm 31:560–7.
- Kohlhase KD, Korkusuz Y, Groner D, et al. (2016). Bipolar radiofrequency ablation of benign thyroid nodules using a multiple overlapping shot technique in a 3-month follow-up. Int J Hypertherm 32:511–16.
- Mader OM, Tanha NF, Mader A, et al. (2017). Comparative study evaluating the efficiency of cooled and uncooled single-treatment MWA in thyroid nodules after a 3-month follow up. Eur J Radiol Open 4:4–8.
- Korkusuz Y, Mader OM, Kromen W, et al. (2017). Cooled microwave ablation of thyroid nodules: initial experience. Eur J Radiol 85:2127–32.
- Happel C, Korkusuz H, Kranert WT, Grünwald F. (2014). Combination of ultrasound guided percutaneous microwave ablation and radioiodine therapy for treatment of hyper- and hypofunctioning thyroid nodules. Nuklearmed Nucl Med 53:N48–9.
- Reiners C, Dietlein M, Luster M. (2015). Stationäre nuklearmedizinische Therapie 2010 bis 2012 in Deutschland. Nuklearmed Nucl Med 54:61–8.
- Verburg FA. (2015). Is thyroid surgery performed too often in Germany? Nuklearmed Nucl Med 54:101–5.
- Aizer AA, Chen MH, Parekh A, et al. (2014). Refusal of curative radiation therapy and surgery among patients with cancer. Int J Radiat Oncol Biol Phys 89:756–64.
- Freudenberg LS, Beyer T, Muller SP, et al. (2006). Evil radioactivity. Subjective perception of radioactivity in patients with thyroid disease prior to treatment with radioiodine. Nuklearmed Nucl Med 45:229–34.
- von Muller F, Happel C, Reinhardt J, et al. (2014). Evaluation of fear of radiation and isolation before and after radioiodine therapy. Thyroid 24:1151–5.
- Freudenberg L, Muller S, Beyer T, Bockisch A. (2009). Subjective perception of radioactivity – no change post successful treatment with radioiodine. Nuklearmed Nucl Med 48:84–8.
- Freudenberg LS, Beyer T. (2011). Subjective perception of radiation risk. J Nucl Med 52 Suppl 2:29S–35S.
- Dietlein M. (2007). Procedure guideline for radioiodine test (Version 3). Nuklearmed Nucl Med 5:198–202.
- Marinelli LD. (1949). Dosage determination in the use of radioactive isotopes. J Clin Investig 28:1271–80.
- Goldberg SN, Gazelle GS. (2001). Radiofrequency tissue ablation: physical principles and techniques for increasing coagulation necrosis. Hepato-gastroenterology 48:359–67.
- Simon CJ, Dupuy DE, Mayo-Smith WW. (2005). Microwave ablation: principles and applications. Radiographics 25 Suppl 1:S69–S83.
- Bernardi S, Dobrinja C, Fabris B, et al. (2014). Radiofrequency ablation compared to surgery for the treatment of benign thyroid nodules. Int J Endocrinol 2014:934595.
- Che Y, Jin S, Shi C, et al. (2015). Treatment of benign thyroid nodules: comparison of surgery with radiofrequency ablation. AJNR Am J Neuroradiol 36:1321–5.
- Dong Gyu Na JHL. (2012). Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 13:117–25.
- Huh JY, Baek JH, Choi H, et al. (2012). Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session – prospective randomized study. Radiology 263:909–16.
- Jeong WK, Baek JH, Rhim H, et al. (2008). Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–50.
- Ha JH, Ba JH, Lee JH. (2011). The efficacy and complications of radiofrequency ablation of thyroid nodules. Curr Opin Endocrinol Diabetes Obes 18:310–14.
- Park KW, Shin JH, Han BK, et al. (2011). Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol 18:2564–8.
- Korkusuz H, Happel C, Koch DA, Gruenwald F. (2016). Combination of ultrasound-guided percutaneous microwave ablation and radioiodine therapy in benign thyroid disease: a 3-month follow-up study. RoFo: Fortschritte Auf Dem Gebiete Der Rontgenstrahlen 188:60–8.
- Ferreira MC, Piaia C, Cadore AC. (2016). Percutaneous ethanol injection versus conservative treatment for benign cystic and mixed thyroid nodules. Arch Endocrinol Metabol 60:211–16.
- Reverter JL, Alonso N, Avila M, et al. (2015). Evaluation of efficacy, safety, pain perception and health-related quality of life of percutaneous ethanol injection as first-line treatment in symptomatic thyroid cysts. BMC Endocr Disord 15:73.
- Sung JY, Baek JH, Kim KS, et al. (2013). Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 269:293–300.
- Mauz PS, Stiegler M, Holderried M, Brosch S. (2005). Complications of ultrasound guided percutaneous ethanol injection therapy of the thyroid and parathyroid glands. Ultraschall Med 26:142–5.
- Mack MG, Lehnert T, Eichler K, Vogl TJ. (2005). MR-guided laser ablation. Magn Reson Imaging Clin N Am 13:583–94.
- Vogl TJ, Naguib NN, Lehnert T, Nour-Eldin NE. (2011). Radiofrequency, microwave and laser ablation of pulmonary neoplasms: clinical studies and technical considerations–review article. Eur J Radiol 77:346–57.
- Atsumi H, Matsumae M. (2005). Laser interstitial thermo therapy (LITT) for brain tumors. Nihon Rinsho Japan J Clin Med 63Suppl 9:495–8.
- Pacella CM, Mauri G, Achille G, et al. (2015). Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metabol 100:3903–10.
- Negro R, Salem TM, Greco G. (2016). Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int J Hypertherm 32:822–8.
- Mauri G, Cova L, Monaco CG, et al. (2017). Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hypertherm 33:295–9.
- Korkusuz H, Happel C, Heck K, et al. (2014). Percutaneous thermal microwave ablation of thyroid nodules. Preparation, feasibility, efficiency. Nuklearmed Nucl Med 53:123–30.
- Korkusuz H, Nimsdorf F, Happel C, et al. (2015). Percutaneous microwave ablation of benign thyroid nodules. Functional imaging in comparison to nodular volume reduction at a 3-month follow-up. Nuklearmed Nucl Med 54:13–19.
- Warmuth M. (2012). Radiofrequenzablation bei benignen und malignen Veraenderungen endokriner Organe (Schilddruese und Nebenniere). Ludwig Boltzmann Institut Fuer Health Technology Assessment (LBI-HTA) 56:1–48.
- Li XL, Xu HX, Lu F, et al. (2016). Treatment efficacy and safety of ultrasound-guided percutaneous bipolar radiofrequency ablation for benign thyroid nodules. Br J Radiol 89:20150858.
- Goldberg SN, Gazelle GS, Compton CC, et al. (2000). Treatment of intrahepatic malignancy with radiofrequency ablation: radiologic-pathologic correlation. Cancer 88:2452–63.
- Fuller CW, Nguyen SA, Lohia S, Gillespie MB. (2014). Radiofrequency ablation for treatment of benign thyroid nodules: systematic review. Laryngoscope 124:346–53.
- Baek JH, Kim YS, Lee D, et al. (2010). Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 194:1137–42.
- Young-Sun Kim HREA. (2006). Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 16:361–7.
- Baek JH, Jeong HJ, Kim YS, et al. (2008). Radiofrequency ablation for an autonomously functioning thyroid nodule. Thyroid 18:675–6.
- Spiezia S, Garberoglio R, Milone F, et al. (2009). Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 19:219–25.
- Ha EJ, Baek JH, Kim KW, et al. (2015). Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and Bayesian network meta-analysis. J Clin Endocrinol Metabol 100:1903–11.
- Goldberg SN. (2001). Radiofrequency tumor ablation: principles and techniques. Eur J Ultrasound 13:129–47.
- Shin JH, Beak JH, Ha EJ, Lee JH. (2012). Radiofrequency ablation of thyroid nodules: basic principles and clinical application. Int J Endocrinol 2012:7 pages.
- Yu J, Liang P, Yu X, et al. (2011). A comparison of microwave ablation and bipolar radiofrequency ablation both with an internally cooled probe: results in ex vivo and in vivo porcine livers. Eur J Radiol 79:124–30.
- Wright AS, Sampson LA, Warner TF, et al. (2005). Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 236:132–9.