Abstract
Purpose: To investigate the long-term outcome and prognostic factors of radiofrequency ablation (RFA) in recurrent hepatocellular carcinoma (HCC) after liver transplantation (LT).
Methods: From 2004 to 2014, 15 patients with 23 hepatic recurrent HCCs after LT underwent ultrasound-guided percutaneous RFA. There were 14 males and 1 female aged 54.3 ± 9.5 years old (37–78 years old). The average tumour size was 3.3 ± 1.2 cm (1.7–6.0 cm). Seven patients had a single HCC and eight had 2–4 HCCs. Regular follow-up after RFA was performed to assess local response rates and long-term survival rates. Survival results were generated using Kaplan–Meier estimates, and a multivariate analysis was performed using the Cox regression model.
Results: The technical success rate was 95.7% (22/23 tumours). The minor complication rate was 7.7% (2/26 sessions), and there were no major complications. The follow-up period was 27.4 ± 18.9 months (12–116 months). The local progression rate and intrahepatic new lesion rate were 13.0% (3/23 tumours) and 53.3% (8/15 patients), respectively. Extrahepatic metastasis was found in four patients (26.7%). The 1-, 3- and 5-year estimated overall survival rates were 71.8%, 35.9% and 26.9%, respectively. Additionally, the multivariate analysis revealed that serum α-fetoprotein (AFP) before RFA, tumour number and extrahepatic metastasis were significantly related to overall survival after RFA.
Conclusion: Ultrasound-guided percutaneous RFA of recurrent HCC after LT had a high technical success rate and local control. However, RFA cannot decrease the frequency of new tumours or extrahepatic metastasis. The AFP level and tumour number before RFA should be considered to predict the outcome.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignant tumours in the world, causing approximately 782 500 new cases and 745 500 deaths every year. The incidence of HCC has been increasing worldwide due to the spread of hepatitis B and C virus infections [Citation1,Citation2]. Currently, liver transplantation (LT) is regarded as the ideal treatment for the removal of existing tumours and replacement of chronically injured and multifocal precancerous livers [Citation3,Citation4]. However, there is a possibility of tumour recurrence after LT, and there has been an increasing trend in recurrence due to the expansion of LT criteria and immunosuppression intervention; the reported recurrence rates after LT are as high as 10–60% [Citation5–7]. Poor liver function reserve, widespread recurrent lesions and severe postoperative adhesions usually become obstacles for reoperation for most LT patients with recurrent tumours [Citation8,Citation9]. Thus, how to address recurrent HCC after LT has become the key question affecting long-term survival for patients [Citation10].
Previous studies have demonstrated that radiofrequency ablation (RFA) is a safe and effective local treatment method for patients with HCC and has some advantages such as minimal invasion, good curative effect, fewer complications and maximal preservation of normal liver parenchyma [Citation11–14]. Some centres have performed RFA as a first-line treatment, even for patients suitable for surgery [Citation15]. However, RFA therapy for hepatic recurrent HCC after LT is rarely reported [Citation16,Citation17]. The present study aimed to investigate the effectiveness, safety and clinical outcome of percutaneous RFA for recurrent HCC after LT and to analyse the prognostic factors.
Experimental procedures
This was a cohort study conducted as a retrospective analysis of a prospective database in a single centre. This clinical study was approved by the Institutional Review Board of our institution. Written informed consent was obtained from all patients before their RFA treatment.
Patients
From March 2004 to March 2014, there were 754 consecutive HCC patients who underwent ultrasound-guided percutaneous RFA in our centre. Of these patients, 15 with 23 recurrent HCCs after LT enrolled in this study. The inclusion criteria for RFA were as follows: (Citation1) a tumour size ≤6 cm and tumour number ≤4; (Citation2) an absence of significant direct tumour invasion of adjacent organs or tumour thrombi in the main or lobar portal system; (Citation3) a tumour not invading a main bile duct or being obviously exophytic; (Citation4) a tumour accessible via a percutaneous approach; (Citation5) an international standard ratio <1.6 and platelet count >50 000/μl; (Citation6) no extrahepatic metastasis or local extrahepatic metastasis with good control before RFA and (Citation7) a follow-up of at least one year after the first RFA treatment.
There were 14 men and 1 woman. Patients’ ages ranged from 37 to 78 years (mean, 54.3 ± 9.5 years). The tumour size was 3.3 ± 1.2 cm (1.7–6.0 cm). Seven patients had a single HCC and eight had 2–4 HCCs. Seven patients had intrahepatic recurrent HCC within one year, and the other eight patients had intrahepatic recurrent HCC beyond one year after LT. All these patients had previous liver cirrhosis, which was caused by hepatitis B in 12 patients, hepatitis C in 2, and alcohol abuse in 1. There were seven patients with an elevated AFP level before RFA treatment, and the highest value was 1000 ng/ml. The diagnosis of recurrent HCC was histologically proven at least in one lesion per case (). Before RFA, two patients suffered extrahepatic metastasis and had local control for the disease, including one patient with a surgical resection for right adrenal metastasis and one patient with radiotherapy for two lung metastatic lesions.
Table 1. Clinical profiles of 15 recurrent HCC patients after LT.
Preprocedural examination
All patients underwent a baseline evaluation, which included an enhanced CT or MRI scan of the abdomen and pelvis and electrocardiogram within one month before the treatment. Serum laboratory tests consisting of a complete blood count, coagulation profile, liver and kidney functional tests (such as alanine aminotransferase (ALT), aspartate aminotransferase (AST)) and serum tumour markers (such as α-fetoprotein (AFP)) were performed in the 2 weeks before the treatment. Contrast-enhanced ultrasound (CEUS) was regularly performed to confirm tumour coverage and tumour number prior to RFA. SonoVue (Bracco SpA, Italy) was used as the contrast agent in this study.
RFA method and technique
All RFA procedures were performed by two of four radiologists (YW, WW, CMH and YK), and all of them had more than 10 years of experience in ultrasound-guided interventional procedures. The tumour size, shape and border were obtained mainly using ultrasound scans, and enhanced CT/MRI was used as a reference. Currently, in clinical practice, most RFA devices can create an ablation sphere having a maximum diameter of up to 5 cm in the liver. When treating spherical tumours larger than 3 cm in diameter with 5 cm ablation spheres, we generally used multiple overlapping ablations based on a mathematical protocol [Citation11]. Ablative margins covered 0.5–1.0 cm beyond the original tumour with the exception of tumours. For these cases adjacent to major structures such as the diaphragm, gastrointestinal tract or gallbladder, the ablative margin was limited; thus, an individualised protocol was developed [Citation13]. Track ablation was performed when withdrawing the RFA electrode in all patients.
During RFA, intravenous moderate sedation provided by an anaesthesiologist was induced using 2.5–5.0 mg of midazolam (Roche; Basel, Switzerland) and 50–100 μg fentanyl (Fentaini; Renfu, Yichang, China). The patient was conscious when the RFA electrode was placed, and vital signs and oxygen saturation were continuously monitored during the procedure. After RFA, the patient was observed for one day at hospital and discharged the next day if no evidence of active bleeding was found.
Ultrasound-guided RFA instrument
In the present study, two types of RFA systems were used: multi-polar electrodes (Celon Lab Power, Germany) and multi-tined RITA electrodes (RITA Medical Systems, Martinez, CA). The RITA RFA system used in this study was a 460 kHz generator unit (model 1500, RITA Medical Systems), which can deliver a maximum power of 150 W through a 14-gauge electrode. The electrode contained nine hook-shaped prongs that could be deployed from the central needle cannula. Five of the nine prongs contained a thermocouple at the tip, and this array electrode (Starburst XL, RITA Medical Systems) enabled ablation of a 5.0 cm region. A sphere-like coagulation area 3–5 cm in diameter could be produced when the electrode was inserted into the tumour, and nine prongs were deployed from the cannula. The time to produce a 5 cm ablation sphere was approximately 20 min.
The Celon Lab Power system provides a maximum power output of 250 W (rated frequency, 470 ± 10 kHz) and can connect one to three 15 to 20 cm-long electrodes with an exposed tip of 3–4 cm. In bipolar and multipolar modes, the size and shape of the coagulation depend on the length, number, distance between, power and deploying time of the electrodes.
Real-time ultrasound systems, SSD-4000 (Aloka, Tokyo, Japan), Technos DU8 (Esaote, Milan, Italy) and LOGIC-E9 (GE Healthcare, Milwaukee, WI), were used for scanning with 3.5–5.0 MHz convex probes with needle-guide devices for ablation procedures.
Assessment of therapeutic efficacy and follow-up
To evaluate the technical success rate after RFA, we performed contrast-enhanced CT (CECT)/MRI one month after treatment. Subsequently, patients were followed with repeat ultrasound and CECT/MRI every 2–3 months during the first year and then a repeat ultrasound every 3 months and CT/MRI every 4–6 months were performed with lab tests. The period of follow-up ranged from 12 to 116 months (mean, 27.4 ± 18.9 months) after RFA. Residual tumour, local progression and new tumours were retreated if the patient was physically strong enough to tolerate another RFA session.
Definitions of terminology
Definitions are based on the standardisation by the International Working Group on Image-Guided Tumor Ablation [Citation18]. Technical success was defined when the tumour was treated according to the protocol and was completely replaced by RFA zones at the immediate follow-up. Local tumour progression was diagnosed when a follow-up exam demonstrated findings of interval development/growth of the tumour along the margin of the ablation zone where the RFA had been technically effective. New tumour development was defined by a lesion with similar characteristics but not contacting the original ablation zone in the liver. Cancer seeding was regarded as extrahepatic recurrence.
A major complication was defined as an event that leads to substantial morbidity and disability, thereby increasing the level of care, or results in hospital admission or substantially lengthens the hospital stay. All other complications were regarded as minor.
Statistical analysis
SPSS 21.0 software (SPSS, Chicago, IL) was used for statistical analysis in our study. p < 0.05 was considered to indicate a significant difference. All continuous data are provided as the means ± standard deviations. Overall survival duration was counted in months from the date of initial RFA to death. Survival curves were evaluated using the Kaplan–Meier model and compared using the log-rank test. 15 potential prognostic factors were considered in this study, and their classification scores are listed in . A univariate analysis was performed for each variable. All significant variables were subjected to multivariate analysis to assess their value as independent prognostic factors with Cox proportional hazards models (conditional LR).
Table 2. Potential prognostic variables and their class scores in RFA of recurrent HCC patients after LT.
Results
Early response
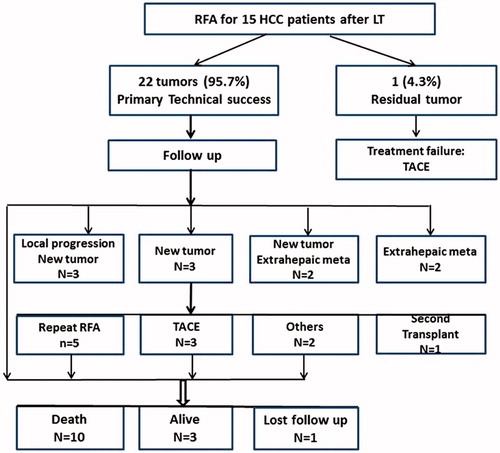
Evaluated by CECT/MRI one month after the initial procedure, primary technical success was achieved in 22 of 23 tumours (95.7%). Residual tumour was observed in one nodule in one patient due to being close to the large portal vein (). This patient with residual tumour declined an additional RFA session because lung metastasis occurred and then received transcatheter arterial chemoembolization (TACE). This patient has survived for 10 months after RFA.
Local tumour progression and new tumour development
During the follow-up period, local progression developed in three tumours at 3–8 months after RFA. Among these three tumours, one tumour was larger than 3 cm, one tumour had multiple foci before initial RFA and the third tumour had both multiple foci and elevated AFP level before RFA. Two tumours were retreated by RFA and the other one combing with intrahepatic new tumours were received TACE. The local progression rate and intrahepatic new tumour rate were 13.0% (3/23 tumours) and 53.3% (8/15 patients), respectively. Extrahepatic metastasis was found in four patients (26.7%) and included bone metastasis (n = 2), lung metastasis (n = 1) and peritoneum metastasis (n = 1). Five patients underwent repeated RFA sessions (2–6 sessions) due to local progression or new tumours ( and ). A total of eight intrahepatic new tumours were ablated. Of the seven patients with an elevated AFP level before RFA, six (85.7%) showed a decrease in AFP level 1–2 months after RFA treatment.
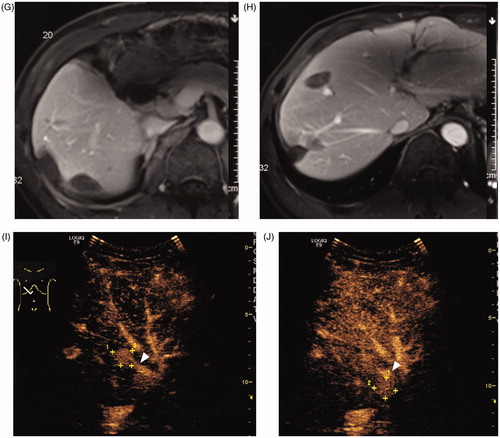
Figure 2. A 56-year-old man had intrahepatic recurrence after LT of hepatocellular carcinoma. (A) Contrast-enhanced CT showed a 2.2 cm tumour (arrow) in segment V of liver. (B) Intercostal ultrasound showed that two bipolar RFA electrodes were placed parallel to the edge of tumour. (C) Contrast-enhanced CT in 4 months after RFA showed ablation area had no viability (arrow). (D) Intercostal ultrasound in 8 months after initial RFA showed the second RFA was performed at the new tumour in Segment V. (E) Intercostal ultrasound in 15 months after initial RFA showed the third RFA was performed at the new tumour in Segment VIII. (F) Subcostal ultrasound in 31 months after initial RFA showed the fourth RFA was performed at the new tumour in Segment IV. (G–H) Contrast-enhanced MRI in 36 months after initial RFA showed there were multiple ablation areas in the liver and had no viability. (I–J) Contrast-enhanced ultrasound in 58 months after initial RFA showed there were two new tumours (+) in the liver and had close relationship to main branch of portal vein (▴). This case was considered not suitable for RFA and recommended to TACE treatment. So far, this patient has received six session of RFA and has survived more than 5 years.
Survival outcome and prognosis analysis
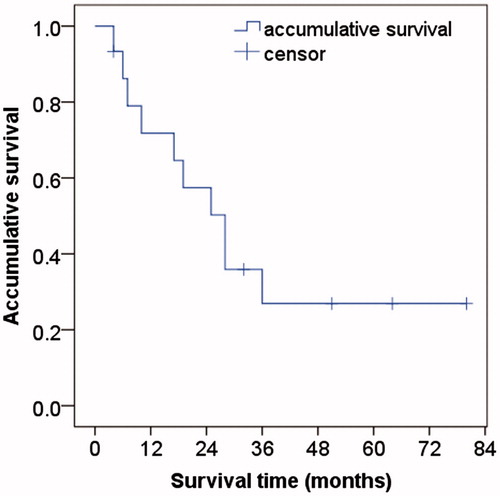
Follow-up ranged from 12 to 116 months with an average of 27.4 ± 18.9 months. In all, 10 patients died, one received second transplants, and three were alive without transplants. The remaining one patient (6.7%) was lost to follow-up (64 months before lost) due to immigration to another country. Overall, eight deaths were related to HCC progression and two were unrelated to liver disease. The overall estimated 1-, 3- and 5-year survival rates were 71.8%, 35.9% and 26.9%, respectively. The median overall survival period was 28 months (). Based on tumour size, subgroup analysis identified higher overall survival rates in patient with ≤3.0 cm tumours (mean: 46.7 ± 11.2 months) than patients with 3.1–6.0 cm tumours (22.4 ± 7.4 months), but the difference was not statically significant (p = 0.168, log-rank test). The results also showed similar overall survival in patient with tumour >5 mm from major vascular structures (mean: 32.6 ± 7.9 months) than patients with tumour close to major vascular structures (35.5 ± 14.3 months) (p = 0.702).
Figure 3. Kaplan–Meier curve showing an overall 5-year survival after radiofrequency ablation in recurrent hepatocellular carcinoma after liver transplantation. The overall estimated 1, 3, 5 year survival rates were 71.8%, 35.9% and 26.9%, respectively.
The factors associated with overall survival are reported in . Univariate analysis identified four factors that were related to the post-RFA overall survival rate: serum AFP before RFA, number of tumours, number of RFA sessions and extrahepatic metastasis. In the multivariate analysis, AFP before RFA (OR = 9.718, p = 0.028), number of tumours (OR = 7.626, p = 0.024) and extrahepatic metastasis (OR = 55.232, p = 0.017) were independent prognostic factors for overall survival after RFA ().
Table 3. Multivariate analysis of prognostic factors with cox proportional hazards in RFA of 15 HCC patients after LT.
Complications
No procedure-related deaths or major complications occurred in this group. The minor complication rate was 7.6% (2/26 sessions); complications included moderate pleural effusion (n = 1) and mild dilation of the biliary duct (n = 1).
Discussion
Currently, the management of recurrent HCC after LT seems to be a losing battle with the increase of LT. Surgical resection can be considered the most promising and potentially curative treatment (Citation6). Unfortunately, poor functional liver reserve, widespread recurrent lesions, or severe postoperative adhesions seriously affected the operation [Citation8]. In LT recipients, the use of immunosuppressant may hinder wound healing and thus result in a higher probability of infective complications. In addition to surgical resection, various treatments are also in use, including TACE and re-transplantation. Re-transplantation would be curative therapy for failure of first transplantation. But, the body damage and cost would be a burden for patients and it was not guaranteed to avoid the new recurrence. However, variable vascular anatomy in a graft liver or severe adhesion increases the difficulty of catheter insertion when the catheter is negotiated through the arterial anastomosis, and incomplete tumour necrosis still is an important tissue for TACE efficacy. Sung et al. [Citation19] reported that intrahepatic recurrence or extrahepatic metastasis occurred in 21 of 28 patients (75.0%) during a 3-month follow-up period and in 26 of 28 patients (92.9%) during a 6-month period following TACE. Extrahepatic metastasis was noted in 18 of 28 patients (64.3%). The 1-, 3- and 5-year survival rates following TACE were 47.9, 6.0 and 0%, respectively. Re-transplantation is limited by graft scarcity, and the long-term survival benefit of HCC patients remains controversial due to immunosuppression intervention. Andorno et al. [Citation20] reported that the difference in overall patient survival for patients who received re-transplantation or not was not significant. Considering the complications, re-transplantation may cause hepatic artery thrombosis and primary function failure, thus leading to irreversible hepatic graft failure [Citation21]. Furthermore, how to ensure the life quality of patients and delay the growth rate of tumour after LT is of great significance.
It is generally confirmed that RFA, as a minimally invasive treatment, is a safe and effective therapy for primary HCC in our institution and other centres. Moreover, percutaneous thermal ablation can avoid reoperation, protect the residual liver function and achieve a comparable efficacy as surgery in HCC patients [Citation11–14,Citation22–24]. Several studies have been published on RFA in recurrent HCC after hepatectomy. Choi et al. reported overall 1-, 2- and 3-year survival rates of 82%, 72% and 54%, respectively. They considered RFA for recurrent HCC after surgery to be beneficial [Citation25]. Our previous study showed overall 1-, 2- and 3-year survival rates were 88.9%, 81.5% and 72.4%, respectively, in the late recurrence group after hepatectomy and suggested aggressive therapy, such as RFA, might lengthen survival in recurrent-HCC patients after hepatectomy, especially in patients with late recurrence [Citation26]. However, RFA therapy for recurrent HCC after LT is rarely reported. At present, only one study (11 patients) and one case report have been published showing that RFA therapy is an alternative if surgical resection is contraindicated or technically infeasible. Additionally, another study on microwave ablation therapy was conducted to analyse the short-term outcome for 11 patients with hepatic recurrent HCC after LT, in which the 2-year overall survival rate was only 15.3% [Citation16,Citation17,Citation27]. Besides, a case-control study between RFA and TACE reported the 4-year survival rate, showing 33% and 25% in the RFA group (n = 6) and TACE group (n = 21), respectively [Citation28]. To our knowledge, there was no research to report the long-term (5 years) outcome of percutaneous RFA in recurrent HCC after LT.
In this study, we investigated the outcome of RFA and analysed prognostic factors in 15 patients with hepatic recurrent HCC after LT. The 1-, 3- and 5-year overall survival rates in this group were 71.8%, 35.9% and 26.9%, respectively. The technical success rate in this group was 95.7%. The minor complication rate was 7.6%, and there were no major complications. Our study showed that RFA therapy is a favourable method for hepatic recurrent HCC after LT with a high technical success rate and low complication rate. Additionally, it is worth mentioning that RFA can be repeated many times easily and safely to treat residual tumours or new intrahepatic lesions. In our study, five patients underwent two to six sessions of RFA with no major complications, which is almost impossible to achieve for surgery. The patients who underwent multiple sessions of RFA had better overall survival, which meant these patients tended to have close follow-up and retreatment times. Currently, some of these patients have achieved a long survival period over 116 months and are still alive, which has not been reported previously. However, in patients with a highly carcinogenic liver in which new nodules frequently appear, second LT may be another choice.
Evaluation of the prognostic factors in such cases is also of great importance for selecting RFA patients, planning RFA protocols and improving prognosis. Poor overall survival was associated with serum AFP level before RFA, tumour number and extrahepatic metastasis.
As previously reported in primary HCC, we observed that the high serum AFP level before RFA was an independent prognostic factor of HCC recurrence. The normal AFP level is less than 20 ng/mL in the blood of a healthy adult. Serum levels of AFP can be regarded not only as a tumour marker of the presence of HCC but also as a predictor of hypercarcinogenic potential, especially for patients with chronic hepatitis. Thus, even if there is no obvious cancer, elevation of serum AFP levels indicates that hepatocytes have become dedifferentiated and begun to develop towards a carcinogenic state [Citation29–32]. Equally, in the present cohort, the influence of AFP levels on the prognosis after RFA therapy confirmed that LT patients with high serum AFP levels had a significantly poorer overall survival.
Our data also confirmed that the number of recurrent tumours after LT was significantly correlated with survival rates after RFA. According to previous studies, the number of tumours is related to the degree of microvascular invasion and reflects the invasive biological behaviour of these tumours. When more than three nodules were noticed in the liver, intrahepatic metastasis or multifocal HCC may have appeared. Moreover, the meta-analysis containing 468 patients showed that the hazard ratio for overall survival was reduced by 1.23 (95%CI = 1–1.53) in patients with multiple nodules vs. patients with a single nodule [Citation33]. Therefore, the number of recurrent tumours after LT should be considered in predicting the outcome after RFA treatment. Our subgroup analysis identified higher overall survival rates in patient with ≤3.0 cm tumours than patients with larger tumours. The difference was not statically significant due to limited samples. We considered the tumours < 3 cm in size would be ideal candidates for RFA of recurrent HCC after LT.
Not surprisingly, extrahepatic metastasis was also an important influential factor in patient prognosis. In the present study, extrahepatic metastasis after RFA had a negative effect on overall survival based on both univariate and multivariate analyses. Our results with regard to extrahepatic metastasis were compatible with the results from the study by Ng et al. [Citation34], in which patients with extrahepatic metastasis had a worse overall survival with a 1-year survival rate of 18.3%, and all patients died within 14 months after RFA. Thus, the occurrence of extrahepatic metastasis during the follow-up period indicated that the patient’s prognosis became worse and local or systematic therapy should be considered.
There were several limitations to our study. First, we could not completely exclude the influence of selection bias in the study because the data were obtained from a single centre. As a specialised unit, more patients with unfavourable tumour conditions for RFA may have been referred to us than to other centres. Second, our sample size was small because of limited LT patients. Liver donor sources are still rare, which is an obstacle in the further development of LT. Additionally, antiviral therapy has been a concern for longer survival time in recent years and should be further analysed in the next study [Citation35,Citation36].
In conclusion, this study demonstrated that RFA, as a safe and effective treatment option, can achieve favourable outcomes and few complications for patients with intrahepatic tumour recurrence after LT. However, other therapy options, such as TACE, might be more appropriate for patients with multiple lesions, high AFP levels and extrahepatic metastasis before treatment. Further investigations, such as multicentre clinical studies, are necessary for a reliable evaluation of prognostic factors in patients with intrahepatic tumour recurrence after LT.
| Abbreviations | ||
| HCC | = | hepatocellular carcinoma |
| LT | = | liver transplantation |
| RFA | = | radiofrequency ablation |
| AFP | = | α-fetoprotein |
| MRI | = | magnetic resonance imaging |
| CT | = | computed tomography |
| CEUS | = | contrast-enhanced ultrasound |
| CECT | = | contrast-enhanced computed tomography |
| ALT | = | alanine aminotransferase |
| AST | = | aspartate aminotransferase |
| TACE | = | trans-catheter arterial chemoembolization |
| OR | = | odds ratio |
| MC | = | Milan criteria |
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Venook AP, Papandreou C, Furuse J, de Guevara LL. (2010). The incidence and epidemiology of hepatocellular carcinoma: a global and regional perspective. Oncologist 15:5–13.
- Torre LA, Bray F, Siegel RL, et al. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65:87–108.
- Xu DW, Wan P, Xia Q. (2016). Liver transplantation for hepatocellular carcinoma beyond the Milan criteria: a review. World J Gastroenterol 22:3325–34.
- Morise Z, Kawabe N, Tomishige H, et al. (2014). Recent advances in the surgical treatment of hepatocellular carcinoma. World J Gastroenterol 20:14381–92.
- Rubin J, Ayoub N, Kaldas F, Saab S. (2012). Management of recurrent hepatocellular carcinoma in liver transplant recipients: a systematic review. Exp Clin Transplant 10:531–43.
- Chok K. (2015). Management of recurrent hepatocellular carcinoma after liver transplant. World J Hepatol 7:1142–8.
- Chok KS, Chan SC, Cheung TT, et al. (2011). Late recurrence of hepatocellular carcinoma after liver transplantation. World J Surg 35:2058–62.
- Minagawa M, Makuuchi M, Takayama T, Kokudo N. (2003). Selection criteria for repeat hepatectomy in patients with recurrent hepatocellular carcinoma. Ann Surg 238:703–10.
- Zhou B, Shan H, Zhu KS, et al. (2010). Chemoembolization with lobaplatin mixed with iodized oil for unresectable recurrent hepatocellular carcinoma after orthotopic liver transplantation. J Vasc Interv Radiol 21:333–8.
- Perkins JD. (2007). Recurrent hepatocellular carcinoma is a problem we need to tackle. Liver Transpl 13:1057–61.
- Chen MH, Yang W, Yan K, et al. (2004). Large liver tumors: protocol for radiofrequency ablation and its clinical application in 110 patients-mathematic model, overlapping mode, and electrode placement process. Radiology 232:260–71.
- Yan K, Chen MH, Yang W, et al. (2008). Radiofrequency ablation of hepatocellular carcinoma: long-term outcome and prognostic factors. Eur J Radiol 67:336–47.
- Chen MH, Wei Y, Yan K, et al. (2006). Treatment strategy to optimize radiofrequency ablation for liver malignancies. J Vasc Interv Radiol 17:671–83.
- Yang W, Yan K, Wu GX, et al. (2015). Radiofrequency ablation of hepatocellular carcinoma in difficult locations: Strategies and long-term outcomes. World J Gastroenterol 21:1554–66.
- Kim YS, Lim HK, Rhim H, et al. (2013). Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 58:89–97.
- Huang J, Yan L, Wu H, et al. (2016). Is radiofrequency ablation applicable for recurrent hepatocellular carcinoma after liver transplantation?. J Surg Res 200:122–30.
- Ho CK, Chapman WC, Brown DB. (2007). Radiofrequency ablation of recurrent hepatocellular carcinoma in a patient after liver transplantation: two-year follow-up. J Vasc Interv Radiol 18:1451–3.
- Goldberg SN, Grassi CJ, Cardella JF, et al. (2005). Image-guided tumor ablation: standardization of terminology and reporting criteria. Radiology 235:728–39.
- Ko HK, Ko GY, Yoon HK, Sung KB. (2007). Tumor response to transcatheter arterial chemoembolization in recurrent hepatocellular carcinoma after living donor liver transplantation. Korean J Radiol 8:320–7.
- Immordino G, Bottino G, De Negri A, et al. (2014). Predictability and survival in liver replantransplantation: monocentric experience. Transplant Proc 46:2290–2.
- Masior L, Grat M, Krasnodebski M, et al. (2016). Prognostic factors and outcomes of patients after liver retransplantation. Transplant Proc 48:1717–20.
- Cho YK, Kim JK, Kim WT, Chung JW. (2010). Hepatic resection versus radiofrequency ablation for very early stage hepatocellular carcinoma: a Markov model analysis. Hepatology 51:1284–90.
- Bruix J, Sherman M. American Association for the Study of Liver Diseases (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Wang JH, Wang CC, Hung CH, et al. (2012). Survival comparison between surgical resection and radiofrequency ablation for patients in BCLC very early/early stage hepatocellular carcinoma. J Hepatol 56:412–18.
- Choi D, Lim HK, Kim MJ, et al. (2004). Recurrent hepatocellular carcinoma: percutaneous radiofrequency ablation after hepatectomy 1. Radiology 230:135–41.
- Yang W, Chen MH, Yin SS, et al. (2006). Radiofrequency ablation of recurrent hepatocellular carcinoma after hepatectomy: therapeutic efficacy on early-and late-phase recurrence. AJR Am J Roentgenol 186:S275–S83.
- Zhai H, Liang P, Yu XL, et al. (2015). Microwave ablation in treating intrahepatic recurrence of hepatocellular carcinoma after liver transplantation: an analysis of 11 cases. Int J Hyperthermia 31:863–8.
- Kim SS, Kang TW, Song KD, et al. (2017). Radiofrequency ablation and transarterial chemoembolisation as first-line treatment for recurrent hepatocellular carcinoma or isolated intrahepatic recurrent hepatocellular carcinoma in transplanted livers. Clin Radiol 72:141–9.
- Toro A, Ardiri A, Mannino M, et al. (2014). Effect of pre- and post-treatment α-fetoprotein levels and tumor size on survival of patients with hepatocellular carcinoma treated by resection, transarterial chemoembolization or radiofrequency ablation: a retrospective study. BMC Surg 14:40.
- Arrieta O, Cacho B, Morales-Espinosa D, et al. (2007). The progressive elevation of alpha fetoprotein for the diagnosis of hepatocellular carcinoma in patients with liver cirrhosis. BMC Cancer 7:28.
- Dohi C, Nouso K, Miyahara K, et al. (2016). Potential of alpha-fetoprotein as a prognostic marker after curative radiofrequency ablation of hepatocellular carcinoma. Hepatol Res 46:916–23.
- Yamamoto K, Imamura H, Matsuyama Y, et al. (2009). Significance of alpha-fetoprotein and des-γ-carboxy prothrombin in patients with hepatocellular carcinoma undergoing hepatectomy. Ann Surg Oncol 16:2795–804.
- Germani G, Gurusamy K, Garcovich M, et al. (2011). Which matters most: number of tumors, size of the largest tumor, or total tumor volume?. Liver Transpl 17:S58–S66.
- Ng KK, Poon RT, Lo CM, et al. (2008). Analysis of recurrence pattern and its influence on survival outcome after radiofrequency ablation of hepatocellular carcinoma. J Gastrointest Surg 12:183–91.
- Zheng S, Chen Y, Liang T, et al. (2006). Prevention of hepatitis B recurrence after liver transplantation using lamivudine or lamivudine combined with hepatitis B Immunoglobulin prophylaxis. Liver Transpl 12:253–8.
- Wu CY, Chen YJ, Ho HJ, et al. (2012). Association between nucleoside analogues and risk of hepatitis B virus–related hepatocellular carcinoma recurrence following liver resection. JAMA 308:1906–13.