Abstract
Background: Vocal cord paresis (VCP) may occur following high intensity focused ultrasound (HIFU) of thyroid nodules. We hypothesised its occurrence relates to the distance of the focus point (FP) of the HIFU beams from the recurrent laryngeal nerve (RLN) and the thermal power that this point received. Their relationships were examined.
Methods: One hundred and three patients who underwent HIFU for symptomatic benign thyroid nodule from October 2015 to March 2017 were analysed. All treatment images were captured and were later watched by 2 reviewers to identify three FPs closest to the tracheoesophageal groove (TEG) on transverse sonographic view. TEG was taken as the RLN position. After identifying these FPs, their distance (mm) from the TEG, thermal power (W) used and depth from skin (mm) were recorded. These parameters were compared between those with and without VCP. VCP was defined as a cord with reduced or no movement.
Results: Four (3.9%) patients suffered from a unilateral VCP afterwards but they all recovered fully within 6 weeks. There were no significant differences in baseline characteristics and treatment efficacy between the two groups. The distance from TEG (OR = 1.706, 95%CI = 1.001 to 2.915, p = 0.050) was the only significant factor for VCP. None of the other variables including thermal power were significant.
Conclusions: The incidence of VCP was 3.9% (4/103) and they completely recovered within 6 weeks. The distance between the FP and the TEG was the only related factor for VCP. The safe distance between FP and TEG should be ≥1.1 cm.
Introduction
Thyroid nodules are common and although most are benign and remain relatively static, some do become large and cause local symptoms with time [Citation1–3]. In such scenario, thyroidectomy is usually indicated [Citation1,Citation2]. However, surgery is not only associated with complications but also with high cost and general anaesthesia. As a result, there has been a growing interest in exploring less invasive, non-surgical technique for benign thyroid nodules [Citation4–6]. For predominantly solid or solid nodules, thermal ablation techniques are highly effective [Citation4–6]. High intensity focused ultrasound (HIFU) is one of the ablation techniques which utilises focused ultrasound energy to induce thermal ablation. Studies have shown that it is effective in not only inducing significant nodule shrinkage but also in alleviating nodule-related symptoms [Citation7–9].
However, owing to the fact that the recurrent laryngeal nerve (RLN) that controls the movement of the vocal cord (VC) runs closely behind the thyroid lobe in the tracheoesophageal groove (TEG), heat-induced RLN injury leading to vocal cord paresis (VCP) is a known complication following various forms of thermal ablation of thyroid nodules [Citation10–13]. Indeed, VCP occurred not uncommonly following HIFU treatment of parathyroid diseases but fewer cases of VCP were reported in the treatment of benign thyroid nodules [Citation14–17] Furthermore, it remains unclear what factors might contribute to the occurrence of VCP during HIFU treatment.
Given that during treatment, HIFU beams are focused precisely on a small region to locally deposit high levels of energy and raise tissue temperature of up to 65–80 °C, we hypothesised that the risk of VCP may be associated with the distance between the focus point (FP) of the beams and the position of the RLN as well as the thermal power that the FP received. With a better understanding of their relationship, perhaps, VCP could be avoided during HIFU ablation. Therefore, the present study aimed to evaluate the incidence of VCP after HIFU ablation in benign thyroid nodules and to examine the association between the risk of VCP, distance between FP and RLN, and thermal power.
Methods
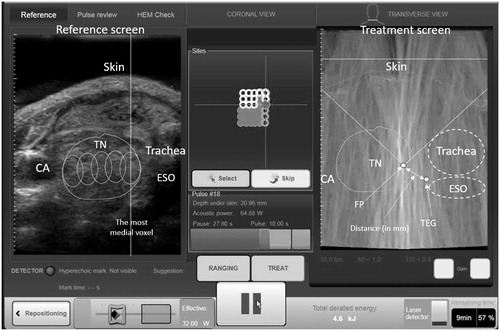
This retrospective analysis was approved by local institutional review board. All consecutive patients who underwent a single-session HIFU ablation for a symptomatic benign thyroid nodule from October 2015 to March 2017 were analysed. Over this period, HIFU ablation was only indicated for patients who had a cytologically confirmed benign thyroid nodule but did not wish to undergo surgery. Details on the eligibility of ablation had been previously described [Citation9,Citation17]. During the study period, all treatment images were automatically captured in real time from the very first HIFU pulse to the last HIFU pulse and stored in the ultrasound (USG)-guided HIFU device (EchoPulse; Theraclion, Paris, France). Since the RLN could not be visualised on USG, the ipsilateral TEG was taken as the landmark of the RLN (). By looking at the transverse sonographic view, it was possible to measure the actual distance between the FP and the position of the presumed RLN (). In addition, it was possible to record the actual amount of thermal power used and the perpendicular depth of the FP from the skin at each FP as the treatment head moved randomly until the entire nodule had been ablated (). For thermal power, two types were recorded. First was adapted power which was defined as the amount of power (measured in watts) delivered to the FP after taking into account of the perpendicular depth of the target from the skin and second was acoustic power which was defined as the amount of power (in watts) given from the treatment head to the FP without taking into account of the depth of the target.
Figure 1. A treatment image captured before the ablation pulse. FP: focal point of the beams; TEG: tracheoesophageal groove; ESO: oesophagus; TN: thyroid nodule; CA: common carotid artery.
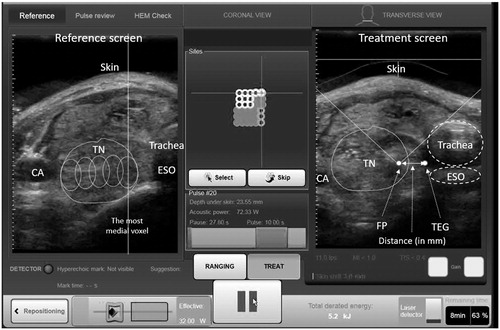
For the present study, two independent reviewers (H.L. and H.Y.) who were not directly involved with the study were instructed to examine all the stored real-time treatment images of eligible patients and to measure the distance of the three closest or nearest FPs from ipsilateral TEG. To reduce biases, both reviewers were unaware of the patient’s post-treatment VC status and were asked to watch the recorded treatment images independently. During the playback of the images, each reviewer had to identify the three specific FPs judged to be closest/nearest to the ipsilateral TEG. The rationale for using choosing three instead of just one FP was because it was anticipated that there might be differences in the choice of the three closest FPs between the two reviewers. In case of any discrepancies, they were resolved by consensus. Having chosen the three closest FPs, their respective distances from the TEG (in mm), adapted and acoustic powers (in watts) and also perpendicular depth from skin (in mm) were recorded. To look for association of VCP with any of these parameters, they were compared between those without VCP (group I) and those with VCP (group II).
Study eligibility
For the present study, the inclusions were (1) the patient had to have all his/her treatment images available for playback and review at the time of analysis; (2) during the playback and review, the relevant anatomical structures such as trachea and TEG had to be clearly seen such that a distance between the FP and the TEG could be measured. The exclusions were (1) any patients who underwent two or more treatments to one thyroid lobe within the same session; (2) any patients who did not undergo laryngoscopic assessment of the VCs before and/or after treatment; and (3) any patients with history of pre-existing VCP.
VC assessment
All patients underwent laryngscopic assessment of both VCs before and within the first week of treatment. VCP was defined as a VC which had reduced or no movement relative to the opposite side. For those with a VCP, they were reassessed every 6 weeks until the paralysed cord regained normal movement.
Treatment efficacy
Each nodule was graded by USG measurement at the day of treatment (baseline), 3rd month and 6th month. Nodule dimensions were measured using the LOGIQ e (GE Healthcare, Milwaukee, WI) scanner equipped with a 10–14 MHz linear matrix transducer. Three orthogonal diameters of the index nodule (its longest diameter and two other perpendicular diameters) were measured. In general, the longest diameter was the cranio-caudal dimension (length) of the nodule while the other two perpendicular diameters were the medio-lateral (width) and antero-posterior (depth) dimensions of the nodule. All measurements were made to the nearest 0.1 mm. To estimate nodule volume, we used the formula: volume (mL) = (width (in cm)×length (in cm) × depth (in cm)) × (π/6) where π was taken as 3.1416. The volume reduction ratio (VRR) was calculated based on the formula: [Baseline volume – volume at visit]/[Baseline volume] * 100. At 6th month, patients in the HIFU group had their nodule assessed clinically using the WHO grading system [Citation18].
HIFU treatment
All treatments were performed by one person (B.H.L.) with >2 years of experience using the same USG-guided HIFU device. This device comprised an energy generator, a treatment head, a skin cooling device and a touchscreen interface for planning. The treatment head incorporated an image transducer (7.5 MHz, 128 elements, linear array) and HIFU transducer (3 MHz, single element, 60 mm in diameter). After positioning, patients were sedated with diazepam (10–15 mg) and pethidine (50–100 mg). Under USG guidance, the treatment head was positioned until the entire index nodule was within the treatable depth. The device computer (Beamotion version no. TUS 3.2.2, Theraclion, Paris, France) automatically divided the nodule into multiple ablation subunits (voxels). Each voxel measured approximately 7.3 mm in thickness and 5 mm in width and received a continuous 8-s pulses of HIFU energy followed by 30–40 s of cooling time before the beam was moved to the adjacent voxel. This cycle continued until all planned voxels were ablated. To ensure safety, nearby structures like the carotid artery, trachea and skin were marked out on the treatment screen before the start of treatment by the operator. To avoid inadvertent heat injury to important surrounding structures, the device automatically selected the following safety margins: (a) 0.5 cm from the skin, (b) at least 0.5 cm from the trachea and RLN and (c) 0.2 cm from the ipsilateral carotid artery and cancelled any voxels which were within these distances. A laser-based movement detector enabled immediate power interruption when the patient moved or swallowed during ablation. To avoid skin burn, the skin was cooled by a balloon (filled with 10 °C liquids) at the tip of the treatment head. All ablations started at 204 J/pulse and increased up to 280 J/pulse until hyperechoic marks appeared at the focal point (). Oral diet was resumed immediately afterwards and patients were allowed to go home two hours after treatment.
Statistical analysis
Continuous variables were expressed as mean ± SD and groups were compared using the Mann–Whitney U test. Chi-square tests were used to compare categorical variables. Binary logistic regression model was performed to estimate the effect of treatment parameters on risk of VCP after HIFU ablation. All statistical analyses were performed using STATA Version 13.0 (StataCorp LP, College Station, TX). All significance tests were two-tailed and those with a p values <0.05 were considered statistically significant.
Results
Altogether 109 patients completed single-session HIFU ablation of a symptomatic benign thyroid nodule. Of these, 3 patients (2.8%) received two sequential HIFU treatments on the same side as they had two large nodules within the same thyroid lobe. There were no patients who required bilateral treatment in the same session. Three (2.8%) patients were not able to be analysed because their stored treatment images were found to be missing or incomplete at the time of analysis. After excluding these patients, 103 (94.5%) patients were eligible for analysis.
All 103 patients had normal bilateral VCs before treatment (at baseline) but in the first week after treatment, 4 (3.9%) were found to have a unilateral VCP (group II). All 4 patients complained of immediate voice change after treatment (i.e. on day 0 following treatment). On laryngoscopic examination, all 4 (100.0%) patients had a complete paralysed VC on the same side as their treatment. However, by the time of the first laryngoscopic reassessment (i.e. 6 week), they all regained full movement. By 6th week, both VCs were able to adduct to the midline and there was no perceptive deficit in any of the patients’ voice. There were no other treatment-related complications such as local infection, skin burn or thyroid dysfunctions afterwards. Redness and swelling on the treatment side were noted in 38 (36.9%) patients but they all resolved in the first week.
shows a comparison of the baseline characteristics between the group I and II. Age at treatment, sex ratio, body mass index, WHO nodule grade, location and side of nodule, nodule dimensions and volume as well as serum TSH and FT4 were not significantly different between the two groups (p > 0.05).
Table 1. A Comparison of baseline characteristics between patients who did not suffer from vocal cord paresis (group I) and those who did (group II) following single-session high intensity focused ultrasound (HIFU) treatment.
Treatment efficacy
At 3rd month, the overall mean VRR after single-session HIFU was 51.14 ± 18.63% (Median = 51.42%; Range = 17.09–90.33%) while at 6th month, the overall mean VRR was 72.43 ± 15.99% (Median = 74.71%; Range = 31.45–98.00%). The proportion of patients with ≥50% VRR at 3rd and 6th months were 58.3% and 92.2%, respectively. compares the treatment efficacy between group I and II. Both groups had similar VRR at 3rd and 6th month following HIFU. The proportion of patients with ≥50% VRR did not differ significantly in the two groups at 3rd and 6th month (p = 0.638 and p = 1.000, respectively). In terms of WHO nodule grades, at 6th month, most patients (56.3%) had WHO grade 1 b while at baseline, most patients (61.2%) had WHO grade 2.
Table 2. A Comparison of 3- and 6-month treatment efficacy between patients who did not suffer from vocal cord paresis (group I) and those who did (group II) following single-session high intensity focused ultrasound (HIFU) treatment.
compares the overall treatment parameters and treatment details of the three nearest/closest voxels from TEG between groups I and II. In terms of overall treatment, the number of voxels treated per nodule was similar between the two groups (58.3 vs. 55.7, p = 0.306). However, the mean adapted energy per voxel within each nodule was significantly higher in group II than group I (320.7 J vs. 284.2 J, p = 0.045). As a result, despite the fewer number of voxels treated, the total energy delivered per nodule tended to be higher (17.9 KJ vs. 16.1 KJ, p = 0.295) and the treatment time tended to be longer in group II than group I (70.7 min vs. 65.2 min, p = 0.379).
Table 3. A Comparison of treatment parameters between patients who did not suffer from vocal cord paresis (group I) and those who did (group II) following single-session high intensity focused ultrasound (HIFU) treatment.
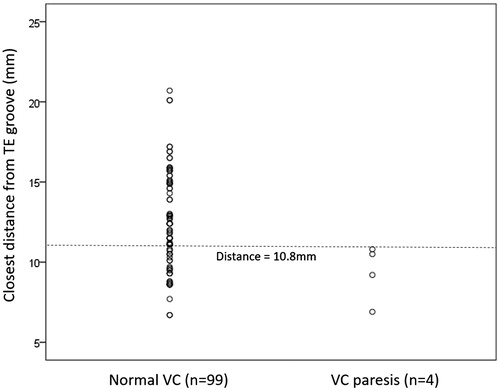
In the first closest voxel, the mean distance from FP to TEG was found to be significantly shorter in group II than I (9.4 mm vs. 12.5 mm, p = 0.025). Among the 4 patients in group II, their distances to the TEG were 6.9 mm, 9.2 mm, 10.5 mm and 10.8 mm, respectively, while in group I, the distance to the TEG ranged between 6.7–20.7 mm. There were 29 (29.3%) patients in group I with distances ≤10.8 mm from TEG. shows the distribution of closest distances between the two groups. In terms of depth from skin, adapted and acoustic powers, they were similar between the two groups. Similar findings were observed in the second closest voxel from TEG. In the third closest voxel from TEG, none of the parameters reached statistical significance between the two groups (p = 0.087).
Figure 3. A scatterplot comparing distances from tracheoesophageal groove between those without and with vocal cord paresis.
By logistic regression, only the distance of closest FP from TEG (OR = 1.706, 95%CI = 1.001 to 2.915, p = 0.050) was a significant factor for VCP (see ). None of the other parameters reached statistical significance.
Table 4. A univariate analysis of factors leading to vocal cord palsy after single-session high intensity focused ultrasound (HIFU).
Discussion
In agreement with the initial hypothesis, our data confirmed that the distance from the closest FP to the ipsilateral TEG was significantly associated with subsequent risk of VCP (OR = 1.706 (95%CI = 1.001–2.915), p = 0.05). In the univariate comparison, the mean distance of the closest FP was significantly shorter for those with VCP than those without VCP (9.4 mm vs. 12.5 mm, p = 0.025). This finding is consistent with the general belief that VCP is caused by heat spreading from the hottest point (i.e. the FP of the beams during treatment) to the RLN and therefore, by positioning the FP of the beams too close to the TEG (i.e. the presumed position of the RLN) during treatment, the risk of VCP would increase. This is also consistent with the experience in other forms of image-guided thermal ablation of thyroid nodules (such as radiofrequency ablation). In a large ablation series, it was noted that patients with nodules either abutting or located close to the course of the RLN were at greater risk of VCP [Citation19]. Similarly, of those with VCP, they all recovered within 3 months (i.e. temporary only) [Citation19].
However, in contrast to the initial hypothesis, we did not find the level of adapted or acoustic power to be related to the subsequent risk of VCP. There were no significant differences in adapted or acoustic power at the three closest sites from TEG between the two groups (p > 0.05). The clinical implication of these findings is that in HIFU ablation, keeping a safe distance from important landmarks like the TEG might be more important in avoiding inadvertent thermal-induced neural injury than simply lowering the ablation power.
In terms of what constitutes a safe distance from TEG, our data found that there were no patients with VCP when the distance was ≥1.1 cm while all 4 patients who suffered VCP had distances <1.1 cm. Therefore, it would appear that a safe distance of ≥1.1 cm would ensure a complete safety of the RLN (see ). However, there are several caveats with this recommendation. First, despite having no patients with VCP when distances had reached ≥1.1 cm, there was still a large overlap in distances between those with and without VCP (). In fact, following this recommendation, there were only 4/33 (or 12.1%) patients who would suffer a VCP when the distances were <1.1 cm and so, implementing this in actual practice might be difficult. Furthermore, by implementing such a margin of 1.1 cm, it is inevitable that some proportions of the nodule would be left untreated or under-treated and this may lead to nodule regrowth in the future [Citation20].
Although it was previously advocated that the FP of the beams should be at least 0.3 cm from the trachea and 0.2 cm from the ipsilateral carotid artery, our data suggested that a larger safe distance might be necessary to minimise risk of VCP [Citation7]. Perhaps, one other point to note is the fact that apart from direct injury from heat, swelling of the nodule by heat may also lead to compression of the RLN resulting in VCP [Citation11]. This may have explained for the transient nature of this type of injury.
Another finding worth highlighting was the fact that our data did not find a significant association between VRR and risk of VCP. Despite the significantly lower mean adapted power in group I (284.2 J/voxel vs. 320.7 j/voxel, p = 0.045), the VRR at 3rd and 6th month did not differ significantly from group II. In fact, to the contrary, the VRR appeared slightly greater in group I. This finding is consistent to our previous observation that after attaining to a certain threshold of total energy, no additional nodule shrinkage was observed with higher ablation power [Citation21]. This may suggest that perhaps, less power or energy could be given to some parts of the nodule where they are deemed too close to TEG without compromising efficacy.
Although other complications such as nodule rupture, abscess formation, hypothyroidism, Horner’s syndrome, brachial plexus injury, haematoma and skin burn were previously reported from other forms of thermal ablation [Citation10–13,Citation19], they were not observed in the present cohort. However, in our earlier experience, one patient did suffer subclinical hypothyroidism 3 months following HIFU ablation and was not included in the present study [Citation22].
Despite these findings, we would like to acknowledge several shortcomings. First, our study was a relatively small study and so, our results were prone to type II errors (i.e. some of the non-significant findings might have been due to inadequate power of the study). Second, we could not rule out the possibility that injury to the RLN might have been a result of accumulative insults from several FPs close to the RLN instead of a single insult from the closest FP alone. Nevertheless, the distances in the two closest FPs from TEG were shorter in group II than group I. Third, the study did not consider the location variability of the RLN as up to 30% of RLNs may lie outside the TEG [Citation23].
Conclusion
VCP occurred uncommonly (3.9%) following single-session HIFU ablation of symptomatic benign thyroid nodule with all of the injuries being temporary (≈6 weeks) in nature. The actual distance from the FP of the HIFU beams to the ipsilateral TEG appeared to be the most significant risk factor for subsequent VCP. This finding is likely to contribute to the safety framework for therapeutic HIFU ablation.
Acknowledgements
We would like to thank Ms. Heather Lam and Mr. Hill Yu for helping with reviewing the treatment images and making the relevant measurements from the images. Also we would like to thank Mr. Hill Yu for his help with reminding patients for follow-up visits and clinical data collection.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Gharib H, Papini E, Garber JR, AACE/ACE/AME Task Force on Thyroid Nodules, et al. (2016). American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: 2016 update. Endocr Pract 22:622–39.
- Haugen BR, Alexander EK, Bible KC, et al. (2016). 2015 American Thyroid Association management guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133.
- Durante C, Costante G, Lucisano G, et al. (2015). The natural history of benign thyroid nodules. JAMA 313:926–35.
- Gharib H, Hegedüs L, Pacella CM, et al. (2013). Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 98:3949–57.
- Sung JY, Baek JH, Kim KS, et al. (2013). Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 269:293–300.
- Wong KP, Lang BH. (2013). Use of radiofrequency ablation in benign thyroid nodules: a literature review and updates. Int J Endocrinol 2013:428363.
- Korkusuz H, Sennert M, Fehre N, et al. (2014). Local thyroid tissue ablation by high-intensity focused ultrasound: effects on thyroid function and first human feasibility study with hot and cold thyroid nodules. Int J Hyperthermia 30:480–5.
- Kovatcheva RD, Vlahov JD, Stoinov JI, Zaletel K. (2015). Benign solid thyroid nodules: us-guided high-intensity focused ultrasound ablation-initial clinical outcomes. Radiology 276:597–605.
- Lang BH, Woo YC, Wong CK. High intensity focused ultrasound (HIFU) treatment for symptomatic benign thyroid nodules: a prospective study. Radiology 2017;161640. doi: 10.1148/radiol.2017161640.
- Valcavi R, Riganti F, Bertani A, et al. (2010). Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 20:1253–61.
- Papini E, Rago T, Gambelunghe G, et al. (2014). Long-term efficacy of ultrasound-guided laser ablation for benign solid thyroid nodules. Results of a three-year multicenter prospective randomized trial. J Clin Endocrinol Metab 99:3653–9.
- Yue W, Wang S, Yu S, Wang B. (2014). Ultrasound-guided percutaneous microwave ablation of solitary T1N0M0 papillary thyroid microcarcinoma: initial experience. Int J Hyperthermia 30:150–7.
- Wang B, Han ZY, Yu J, et al. (2017). Factors related to recurrence of the benign non-functioning thyroid nodules after percutaneous microwave ablation. Int J Hyperthermia 33:459–64.
- Kovatcheva RD, Vlahov JD, Stoinov JI, et al. (2012). High-intensity focused ultrasound (HIFU) treatment in uraemic secondary hyperparathyroidism. Nephrol Dial Transplant 27:76–80.
- Kovatcheva R, Vlahov J, Stoinov J, et al. (2014). US-guided high-intensity focused ultrasound as a promising non-invasive method for treatment of primary hyperparathyroidism. Eur Radiol 24:2052–8.
- Leenhardt L, Rouxel A, Menegaux F, Esnault O. (2013). An open-label, randomized, controlled study of the effectiveness and safety of a high intensity focused ultrasound device compared with observation in patients with non-malignant cold thyroid nodules. Endocrine Abstracts 32:P1013.
- Lang BH, Wong CKH, Ma EPM. Single-session high intensity focused ablation (HIFU) versus open cervical hemithyroidectomy for benign thyroid nodule: analysis on early efficacy, safety and voice quality. Int J Hyperthermia 2017. [Epub ahead of print]. doi: 10.1080/02656736.2017.1305127.
- Zimmermann M, Saad A, Hess S, et al. (2000). Thyroid ultrasound compared with World Health Organization 1960 and 1994 palpation criteria for determination of goiter prevalence in regions of mild and severe iodine deficiency. Eur J Endocrinol 143:727–31.
- Baek JH, Lee JH, Sung JY, et al. (2012). Complications encountered in the treatment of benign thyroid nodules with us-guided radiofrequency ablation: a multicenter study. Radiology 262:335–42.
- Sim JS, Baek JH, Lee JY, Cho WJ, Jung SI. Radiofrequency ablation of benign thyroid nodules: depicting early sign of regrowth by calculating vital volume. Int J Hyperthermia. 2017. [Epub ahead of print]. doi: 10.1080/02656736.2017.1309083.
- Lang BHH, Woo YC, Chiu KW. Single-session high-intensity focused ultrasound treatment in large-sized benign thyroid nodules. Thyroid 2017;27:714–721.
- Lang BHH, Woo YC, Chiu KW. High-intensity focused ablation (HIFU) of single benign thyroid nodule rarely alters underlying thyroid function. Int J Hyperthermia 2017. [Epub ahead of print]. doi: 10.1080/02656736.2017.1318456.
- Henry BM, Sanna B, Graves MJ, et al. (2017). The reliability of the tracheoesophageal groove and the ligament of berry as landmarks for identifying the recurrent laryngeal nerve: a cadaveric study and meta-analysis. Biomed Res Int 2017:4357591.