Abstract
Background: In thyroid gland, radiofrequency ablation (RFA) has been applied to both recurrent cancers and benign nodules, although, according to the American Thyroid Association (ATA) and the Korean Society of Thyroid Radiology (KSThR) guidelines, surgery is the first-line treatment for follicular neoplasm. However, it has been argued that follicular neoplasm with lower risk of malignancy can be managed by close follow-up. In this study, we evaluated the effectiveness of RFA of small follicular neoplasms, examining reductions in volume and related clinical problems, and making observations over long-term follow-up.
Methods: We evaluated 10 follicular neoplasms in 10 patients who were treated with RF ablation between 2009 and 2011. A RF generator and an 18-gauge internally cooled electrode were used to perform complete ablation of the whole nodules. Changes in nodules or ablated zones on follow-up ultrasound, and complications during and after RF ablation were evaluated.
Results: The mean follow-up period was 66.4 ± 5.1 months (range: 60–76 months). In eight patients, single session of RF ablation was sufficient, while two patients required two sessions. There was a significant reduction in the mean volume (99.5 ± 1.0%) of lesions, with eight ablated lesions (8/10, 80%) disappearing completely on follow-up. No recurrences were found in any ablated zones at last follow-up. Transient mild neck pain (n = 6) occurred during the procedure without requiring any medication.
Conclusion: In addition to active surveillance, RF ablation may be an effective and safe alternative for the management of patients with small (<2 cm) follicular neoplasm suspected on thyroid biopsy and who strongly refuse surgery.
Introduction
The use of ultrasound has increased the number of incidentally detected thyroid nodules, with the prevalence of thyroid nodules reportedly ranging from 35% to 46%, with a 4.4% incidence of malignancy in non-palpable thyroid nodules [Citation1]. The incidence of thyroid follicular neoplasms is also increasing, with follicular adenoma (FA) being one of the most common thyroid nodule types, and follicular carcinoma (FC) accounting for 10–20% of all malignant thyroid nodules [Citation2]. Thyroid nodules showing follicular morphologic features include adenomatous nodules, FA, FC and the follicular variant of papillary thyroid carcinoma (FVPTC) [Citation3]. Differential diagnosis of carcinoma from adenoma is based on the presence of capsular, vascular or extrathyroidal tissue invasion, and nodal or distant metastases [Citation4,Citation5]. Because of the low sensitivity (25–42%) of fine-needle aspiration (FNA) [Citation6,Citation7], core needle biopsy (CNB) has been recommended as a better diagnostic tool for follicular neoplasm, as it has a lower false positive rate [Citation8,Citation9]. However, surgery, which has the ability to detect the presence of vascular and/or capsular invasion, still remains the definitive diagnosis and standard treatment for follicular neoplasm, despite the fact that up to 70% of follicular neoplasms are benign [Citation10].
Radiofrequency ablation (RFA) is a nonsurgical minimally invasive technique that has been widely used to treat hepatoma, as well as benign [Citation11] and malignant tumours [Citation12,Citation13]. In the thyroid gland, RFA has been applied to both recurrent cancers and benign nodules [Citation13]. Recently, ongoing studies have dealt with the RFA treatment of small papillary cancers in patients ineligible for surgery [Citation14,Citation15]. With increased life expectancy, conservative medical management and an increased incidence of follicular neoplasm, more patients are either reluctant to undergo surgery, or ineligible because of underlying medical conditions or old age; they may also refuse surgery even if it is possible. In an effort to expand on the indications for minimally invasive RFA in the conservative management of thyroid nodules and decrease the incidence of excessive surgery, the treatment of follicular neoplasms with RFA has been investigated [Citation16]. Dobrinja et al. [Citation16] insisted that follicular lesions/follicular neoplasm should not be recommended as it delays surgery in case of malignancy.
We report on our experience of 10 patients with follicular neoplasm and refusal for surgery. The aim of this study was to evaluate the effectiveness of RFA of follicular neoplasm performed with an internally cooled electrode, investigating both ablation efficacy, and the related clinical problems, over a follow-up period of 5 years.
Materials and methods
This retrospective study was approved by the institutional review board of Daerim St. Mary’s Hospital. A written informed consent document was obtained from all patients before the procedure.
Patients
Data were retrospectively collected between January 2009 and December 2011. The inclusion criteria for RFA were as follows: diagnosed follicular neoplasm or suspicion of follicular neoplasm on CNB, reporting of any clinical symptom or cosmetic problem, thyroid nodule larger than 1 cm and smaller than 2 cm without suspicious malignant features such as spiculated/microlobulated margin, microcalcification, nonparallel shape or evidence of lymph node metastasis on ultrasonography (US), no clinical evidence of distant metastasis, refusal for surgery and a follow-up period of more than 5 years. Ultimately, 54 patients with follicular neoplasm (46 females, 8 males; mean age: 45.1 ± 10.5 years; range: 27–74) were consecutively enrolled (). Of the 54 patients, 30 declined any treatment, 11 had undergone surgery, three were lost during follow-up, and finally, 10 patients met the inclusion criteria. The baseline characteristics of 10 enrolled nodules from 10 patients are summarised in .
Table 1. Demographic characteristics of the enrolled patients.
Pre-ablation assessment
The US, CNB result and clinical concerns were evaluated for all patients. One radiologist (JYS) performed the US examination and CNB using a 10 MHz linear probe on a real-time US system (Aplio SSA-770A, Toshiba, Otawara-shi, Japan). Three orthogonal nodule dimensions (the largest dimension and two other perpendicular ones) were measured before initiating RFA. The volume of the nodule was calculated using the following equation: V = πabc/6, where V = volume, a = the largest dimension, and b and c = the other two perpendicular dimensions. The percentage volume reduction was calculated as ([initial volume − final volume] × 100)/initial volume. The composition of the nodules was subjectively assessed by the examiners, and classified as either solid (solid component >50%) or predominantly cystic (solid component between 10% and 50%). Tumour vascularity was classified with a four-point scale: 0 = no signal in the tumour, 1 = peripheral signal without any central vascular signal in the tumour, 2 = peripheral and central signals in <50% of the tumour and 3 = peripheral and central signals in >50% of the tumour.
At enrolment, patients were asked to rate pressure symptoms on a 10 cm visual analogue scale (0–10 cm), and the physician also recorded a cosmetic grade (1: no palpable mass; 2: invisible but palpable mass; 3: mass visible only to an experienced clinician and 4, easily visible mass).
RFA procedure
Patients were placed in the supine position with the neck extended, and two grounding pads were attached to both thighs. A RF generator (Cool-tip RF system, Radionics, Burlington, MA) and an 18-gauge 0.5 cm active-tip internally cooled electrode (Well-point RF; Taewoong Medical) were used. The patients were treated with 2% lidocaine (Huons, Hwasung, Korea) for local anaesthesia at the puncture site. To prevent serious haemorrhage, the vessels along the approach route were carefully evaluated. An electrode was inserted into the thyroid nodule under US guidance along the short axis of the nodule using the trans-isthmic approach method [Citation17]. The electrode tip was initially positioned in the deepest and most remote portion of the nodule, and, on the basis of previous experience, the nodules were treated with a “moving shot technique” beginning with 15 W of radiofrequency power. If a transient hyperechoic zone did not form at the electrode tip within 5–10 s, the power was increased in 5 W increments, reaching full capacity at 30 W. If the patient could not tolerate the pain during ablation, the power was reduced or turned off. During ablation, both thighs were checked frequently to prevent skin burn. Ablation was terminated when all imaginary units of the nodule had changed to transient hyperechoic zones [Citation17].
Post-treatment care
RFA was performed on an outpatient basis, and at the end of the procedure the patient was evaluated for complications and remained under observation for 1–2 h with compression of the neck lasting for 10–20 min.
Follow-up
Follow-up consisted of a US, and examination of clinical symptoms. A follow-up US examination was performed at 1 month, and then every year over the 5 year follow-up. On the US examination, changes in size, volume and vascularity were evaluated. Evaluation of symptoms and cosmetic grading were also performed. We defined “complete ablation” as complete treatment with no discernible lesion with same echogenicity as the index nodule, no vascularity on colour doppler US. Repeat RFA was performed according to the decisions of the clinician or patient, and a radiological finding of a viable nodule portion which did not fulfilled the definition of complete ablation. Complications were classified as major or minor according to classification of the Society of Interventional Radiology [Citation18].
Statistical analysis
Statistical analyses were performed using SPSS for Windows (version 23.0; SPSS, Inc., Chicago, IL). Variables at the time of each patient’s enrolment and at the last follow-up examination were compared using a Wilcoxon signed rank test. The level for statistical significance was defined as p < 0.05.
Results
Treatment characteristics
The treatment characteristics are summarised in . All patients were treated with a single session of RFA, except for two patients who were treated a second time, with one of these patients being retreated 2 months after the first session, and the other patient 9 months after the first session. The mean ablation time and power were 232.5 ± 123.5 s (110.0–420.0 s) and 18.5 ± 2.4 W (range, 15–20 W), respectively. For the second treatment sessions performed on two patients, the mean ablation time and power were 175.0 ± 35.4 s and 20.0 W, respectively. The mean total energy deposition was 4325.0 ± 2323.9 J (range: 1650.0–8400.0 J) for the primary ablations, and 3500.0 ± 707.1 J for the secondary ablations. The mean energy delivered per millilitre of pre-treatment nodule volume was 8397.5 ± 5203.8 J (range: 3975.8–19331.8 J) for the primary ablations, and 1348.2 ± 5922.9 J for the secondary ablations.
Table 2. Treatment characteristics of the 10 follicular neoplasms.
Ultrasonographic evaluation and clinical findings
The ultrasound features and clinical data at enrolment and follow-up evaluations are summarised in . The mean follow-up period in this study was 66.4 ± 5.1 months (range: 60–76 months). The mean volume reduction was 99.5% ± 1.0% (range: 97.4–100.0%) at the last follow-up (). Two patients who had undertreated lesions after 1st RFA session received additional RFA sessions at 2 and 9 months later. Two lesions were located adjacent to trachea and common carotid artery, respectively, could be safely and completely ablated with additional RFA sessions. Overall, eight lesions (8/10) were completely disappeared, while two lesions (2/10, 20%) showed 97.4% and 98.0% reduction, as minimal stable linear scar, on follow-up US. But, these two lesions had shown no discernible nodule, no vascularity and continuous volume decrease through the follow-up period, after one session of RFA. All of the nodules had a mainly solid composition (n = 10). During the RFA, the electrode was always clearly visible as a hyperechoic line on the US monitoring. After ablation, colour and power Doppler US showed significant reduction of the peripheral and/or intranodular vascular signals as a consequence of necrosis induced by RF (vascular scale: initial vs. 1 month vs. follow-up, 2.6 ± 0.7 vs. 0.6 ± 1.3, vs. 0, p = 0.0042). Four palpable lesions were more than 0.7 ml in volume and more anteriorly located than non-palpable nodules. The mean cosmetic grade improved from 1.4 ± 0.5 to 1.0 ± 0.0 (p = 0.0455). We had no lesion which caused symptoms, therefore symptomatic evaluation could not be evaluated due to relative small size <2 cm. The recurrence was not found in all ablated zones until last follow-up.
Figure 2. A 31-year-old woman with a right thyroid nodule proven to be a follicular neoplasm on core needle biopsy. (A) A well-defined hypoechoic solid thyroid nodule on the transverse US image. (B) An echogenic totally ablated thyroid nodule with a RF electrode (arrows) on the transverse US image. (C) One month after RFA, the nodule had decreased and there was no undertreated area. (D) Two years after RFA, the nodule had disappeared.
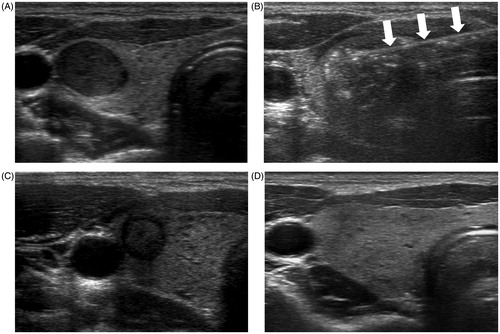
Table 3. Outcomes for the 10 follicular neoplasms after RF ablation.
Complications
Six patients reported mild neck pain without requiring any medication during the RFA procedure. The procedure was well tolerated by all patients and no patients required painkiller during or after RFA. No major complications requiring therapy, hospitalisation, adverse sequelae or death were encountered.
Discussion
All 10 patients found RFA of a thyroid nodule to be tolerable. At the 5-year follow-up, eight follicular neoplasms had completely disappeared, while the other two were considerably decreased in volume (more than 97% volume reduction), and remained as scar-like lesions. There were no recurrences or distant metastases during the follow-up period. Our results suggest that RFA is an effective and safe procedure for patients with a small (<2 cm) follicular neoplasm, and who are at high surgical risk or refuse to undergo surgery.
According to the current guidelines of the American Thyroid Association (ATA) [Citation19] and the Korean Society of Thyroid Radiology (KSThR), surgery is a standard treatment for follicular neoplasm [Citation20]. The advantages of surgical therapy are immediate resolution of the symptoms, removal of the nodule, treatment of rare cases of carcinoma [Citation21] and a decrease in patient anxiety due to the possibility of malignancy. However, surgery inevitably has some complications, which include permanent hypothyroidism, permanent hypoparathyroidism, thyroid hormone dependence, permanent recurrent laryngeal nerve paralysis and the risk of an unsightly scar [Citation22,Citation23], which can substantially affect a patient’s quality of life. Moreover, although the ultimate purpose of surgery for follicular neoplasm is to distinguish carcinomas from adenomas [Citation24], patients may possibly undergo unnecessary surgery because of high false positive rate of FNA. When used for diagnosis of follicular neoplasm, FNA has a relatively high false positive rate (22.2–35%), which includes thyroiditis or nodular goitre following surgery [Citation25,Citation26]. With regard to this false positive rate, CNB seems to be superior to FNA, with a lower false positive rate of 0–18.2% [Citation27,Citation28] and 4.2% [Citation9] as nodular hyperplasia after surgery. Up to 80% of thyroid nodules diagnosed as a follicular neoplasm turn out to be benign upon histological examination, with about 20% being revealed as malignant [Citation29]. In the present study, all the nodules in the patients who underwent surgery were confirmed as being benign (10 as FA and one as nodular hyperplasia). Many investigators have attempted to determine the highest risk of harbouring invasive carcinoma according to preoperative patient and tumour characteristics [Citation30,Citation31]. Male gender, older age (>50 years) and a nodule size greater than 2 cm are used as clinical variables; however, there are still limitations when these are used for identifying malignancy [Citation32]. Additionally, distant metastases occur in less than 10% of patients with differentiated FC [Citation33], and minimally invasive FC has an excellent prognosis, as distant metastases are rare [Citation34]. With consideration of the relatively high false positive results of FNA/CNB, the inaccurate preoperative predictors of invasiveness, the low rates of malignancy and metastasis and the possible surgical complications, the management of follicular neoplasms remains controversial. The ATA guideline [Citation19] also states that diagnostic surgical excision is the long-established standard of care for the management of FN/SFN cytology nodules. However, it also suggests that, after consideration of clinical and sonographic features, molecular testing may be used to supplement malignancy risk assessment data, in lieu of proceeding directly with surgery. Informed patient preference and feasibility should be considered in clinical decision-making; however, in patients with surgical ineligibility, there is a lack of guidance for the management plan, apart from observation, which can increase patient anxiety and may not be accepted by many patients.
Minimally invasive RFA is not only an effective treatment, but also a reasonably safe option for recurrent [Citation35–38] and primary thyroid cancers [Citation14,Citation15], especially in patients ineligible for surgery and requiring conservative management. Thus, RFA could be an alternative to surgery; however, current RFA guidelines from Korea [Citation20] and Italy [Citation39] do not recommend RFA for treating follicular neoplasm, as there is no evidence of any treatment benefit from RFA. Recently, Dobrinja et al. [Citation16] investigated RFA in six patients with Thy3 (follicular lesions/follicular neoplasm) nodules, and found recurrence in two patients with nodules with a volume >20 ml. After surgery, these nodules were diagnosed as a minimally invasive FC and a follicular neoplasm of indeterminate malignant behaviour. Although current guidelines do not recommended RFA, it is an excellent method for control of local tumours. Furthermore, RFA does not require general anaesthesia and does not induce scarring, and there are no reports of procedure-related deaths [Citation17,Citation40,Citation41]. The present study suggests that RFA could be safely and effectively applied to small (<2 cm) follicular neoplasms in patients ineligible for surgery. We treated follicular neoplasms smaller than 2 cm that were confirmed by CNB, and were without evidence of suspicious US features [Citation42], lymph nodes or distant metastases. With lower false positive rates [Citation9] and the possibility of gaining immunocytochemistry and genetic information for the diagnosis of follicular neoplasm [Citation43,Citation44], CNB and RFA may play complementary roles in reducing the need for unnecessary diagnostic surgery. RFA with the “moving shot” technique also allows the complete ablation of the lesion, including the safety margin, which can result in an absence of local tumour recurrence, as presented in a previous study [Citation14]. Indeed, surgical resection with a tumour-free margin is the mainstay of therapy for local recurrence [Citation21]. Accordingly, RFA may supplement the current choices that consist of either surgery or observation.
However, it is also important not to make a hasty inference concerning RFA as an alternative management method. Huh et al. stated that, because of incomplete treatment of nodule margins and unresolved clinical problems, thyroid nodules >20 ml require more than one session of RFA [Citation45]. In a study by Dorinja et al. [Citation16], two patients had a recurrence in nodules with a volume >20 ml. According to a study by Paramo et al. [Citation46], the incidence of malignancy in follicular neoplasm is higher if the nodule size is larger than 4 cm, which suggests that larger nodules have a higher probability of being malignant. In the present study, there were no recurrences during the 5-year follow-up, which may be because the volumes of the follicular neoplasms (ranging from 0.2 to 1.6 ml) were smaller than in the previous study [Citation16]. Additionally, FC metastasises to distant organs such as lung and bone, rather than lymph nodes, and its prognosis is worse than that of papillary carcinoma [Citation34]. The presence of metastasis is an independent factor for poor survival [Citation47], and careful evaluation of potential metastases should precede treatment. Therefore, the possibility of recurrence or the requirement for additional RFA sessions must be kept in mind with nodules of a large volume, and careful pre-procedural evaluation and long-term follow-up are required to eliminate the possibility of metastasis. Current studies to determine the appropriate indication between observation and interventional management approaches such as RFA are insufficient. However, RFA may still be suggested as a limited treatment in the management of small follicular neoplasms in patients ineligible for surgery.
Active surveillance of small papillary carcinoma has been in the spotlight, with a Japanese observational study [Citation48] and the ATA guideline describing “low risk” as an absence of clinically evident metastases, local invasion and aggressive cytological evidence [Citation19]. Some studies suggest that follicular neoplasms with a lower risk of malignancy can be managed by close follow-up and repeated FNA, provided that patients are willing to accept a small risk of cancer [Citation10]. However, in patients who are reluctant to undergo surgery or active surveillance with regular follow-up, RFA could be safely and effectively applied.
This study has several limitations. Firstly, its retrospective design may have caused a selection bias. Secondly, this study involved a small number of cases and a follow-up period of no longer than 5 years.
To our knowledge, this study is the first evaluation of RFA of follicular neoplasms with a 5-year follow-up period without recurrence. Further studies are needed to clarify whether RFA can be recommended as a treatment for patients with follicular neoplasm. Nevertheless, the results suggest that, in addition to surgery and active surveillance, RFA represents an effective local tumour control method for small (<2 cm) follicular neoplasms.
Declaration of interest
The authors have no conflicts of interest to declare.
References
- Leenhardt L, Hejblum G, Franc B, et al. (1999). Indications and limits of ultrasound-guided cytology in the management of nonpalpable thyroid nodules. J Clin Endocrinol Metab 84:24–8.
- Gilliland FD, Hunt WC, Morris DM, Key CR. (1997). Prognostic factors for thyroid carcinoma. A population-based study of 15,698 cases from the surveillance, epidemiology and end results (seer) program 1973–1991. Cancer 79:564–73.
- Duggal R, Rajwanshi A, Gupta N, Vasishta RK. (2011). Interobserver variability amongst cytopathologists and histopathologists in the diagnosis of neoplastic follicular patterned lesions of thyroid. Diagn Cytopathol 39:235–41.
- Goldstein RE, Netterville JL, Burkey B, Johnson JE. (2002). Implications of follicular neoplasms, atypia, and lesions suspicious for malignancy diagnosed by fine-needle aspiration of thyroid nodules. Ann Surg 235:656–62. discussion 62-4.
- Dosen D, Turic M, Smalcelj J, et al. (2003). The value of frozen section in intraoperative surgical management of thyroid follicular carcinoma. Head Neck 25:521–8.
- Yoon JH, Kim EK, Hong SW, et al. (2008). Sonographic features of the follicular variant of papillary thyroid carcinoma. J Ultrasound Med 27:1431–7.
- Shih SR, Shun CT, Su DH, et al. (2005). Follicular variant of papillary thyroid carcinoma: Diagnostic limitations of fine needle aspiration cytology. Acta Cytol 49:383–6.
- Na DG, Baek JH, Jung SL, et al. (2017). Core needle biopsy of the thyroid: 2016 consensus statement and recommendations from Korean Society of Thyroid Radiology. Korean J Radiol 18:217–37.
- Yoon RG, Baek JH, Lee JH, et al. (2014). Diagnosis of thyroid follicular neoplasm: Fine-needle aspiration versus core-needle biopsy. Thyroid 24:1612–7.
- Gulcelik NE, Gulcelik MA, Kuru B. (2008). Risk of malignancy in patients with follicular neoplasm: Predictive value of clinical and ultrasonographic features. Arch Otolaryngol Head Neck Surg 134:1312–5.
- Mauri G, Cova L, Monaco CG, et al. (2017). Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia 33:295–9.
- Gazelle GS, Goldberg SN, Solbiati L, Livraghi T. (2000). Tumor ablation with radio-frequency energy. Radiology 217:633–46.
- Dupuy DE, Monchik JM, Decrea C, Pisharodi L. (2001). Radiofrequency ablation of regional recurrence from well-differentiated thyroid malignancy. Surgery 130:971–7.
- Kim JH, Baek JH, Sung JY, et al. (2017). Radiofrequency ablation of low-risk small papillary thyroidcarcinoma: preliminary results for patients ineligible for surgery. Int J Hyperthermia 33:212–9.
- Sun J, Liu X, Zhang Q, et al. (2016). Papillary thyroid carcinoma treated with radiofrequency ablation in a patient with hypertrophic cardiomyopathy: a case report. Korean J Radiol 17:558–61.
- Dobrinja C, Bernardi S, Fabris B, et al. (2015). Surgical and pathological changes after radiofrequency ablation of thyroid nodules. Int J Endocrinol 2015:576576.
- Jeong WK, Baek JH, Rhim H, et al. (2008). Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–50.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Haugen BR, Alexander EK, Bible KC, et al. (2016). 2015 American Thyroid Association Management Guidelines for adult patients with thyroid nodules and differentiated thyroid cancer: the American Thyroid Association Guidelines task force on thyroid nodules and differentiated thyroid cancer. Thyroid 26:1–133.
- Na DG, Lee JH, Jung SL, et al. (2012). Radiofrequency ablation of benign thyroid nodules and recurrent thyroid cancers: consensus statement and recommendations. Korean J Radiol 13:117–25.
- McHenry CR, Phitayakorn R. (2011). Follicular adenoma and carcinoma of the thyroid gland. Oncologist 16:585–93.
- Gharib H. (2004). Changing trends in thyroid practice: understanding nodular thyroid disease. Endocr Pract 10:31–9.
- Linos D, Economopoulos KP, Kiriakopoulos A, et al. (2013). Scar perceptions after thyroid and parathyroid surgery: comparison of minimal and conventional approaches. Surgery 153:400–7.
- Yoo C, Choi HJ, Im S, et al. (2013). Fine needle aspiration cytology of thyroid follicular neoplasm: cytohistologic correlation and accuracy. Korean J Pathol 47:61–6.
- Dabelic N, Matesa N, Matesa-Anic D, Kusic Z. (2010). Malignancy risk assessment in adenomatoid nodules and suspicious follicular lesions of the thyroid obtained by fine needle aspiration cytology. Coll Antropol 34:349–54.
- Deveci MS, Deveci G, LiVolsi VA, Baloch ZW. (2006). Fine-needle aspiration of follicular lesions of the thyroid. Diagnosis and follow-up. CytoJournal 3:9.
- Nasrollah N, Trimboli P, Guidobaldi L, et al. (2013). Thin core biopsy should help to discriminate thyroid nodules cytologically classified as indeterminate. A new sampling technique. Endocrine 43:659–65.
- Sung JY, Na DG, Kim KS, et al. (2012). Diagnostic accuracy of fine-needle aspiration versus core-needle biopsy for the diagnosis of thyroid malignancy in a clinical cohort. Eur Radiol 22:1564–72.
- Baloch ZW, Fleisher S, LiVolsi VA, Gupta PK. (2002). Diagnosis of “follicular neoplasm”: a gray zone in thyroid fine-needle aspiration cytology. Diagn Cytopathol 26:41–4.
- Sclabas GM, Staerkel GA, Shapiro SE, et al. (2003). Fine-needle aspiration of the thyroid and correlation with histopathology in a contemporary series of 240 patients. Am J Surg 186:702–9; discussion 9-10.
- Giorgadze T, Rossi ED, Fadda G, et al. (2004). Does the fine-needle aspiration diagnosis of "Hurthle-cell neoplasm/follicular neoplasm with oncocytic features" denote increased risk of malignancy? Diagn Cytopathol 31:307–12.
- Choi YJ, Yun JS, Kim DH. (2009). Clinical and ultrasound features of cytology diagnosed follicular neoplasm. Endocr J 56:383–9.
- Schlumberger M, Leboulleux S. (2015). Treatment of distant metastases from follicular cell-derived thyroid cancer. F1000Prime Rep 7:22.
- Ito Y, Hirokawa M, Higashiyama T, et al. (2007). Prognosis and prognostic factors of follicular carcinoma in Japan: importance of postoperative pathological examination. World J Surg 31:1417–24.
- Baek JH, Kim YS, Sung JY, et al. (2011). Locoregional control of metastatic well-differentiated thyroid cancer by ultrasound-guided radiofrequency ablation. Am J Roentgenol 197:W331–6.
- Monchik JM, Donatini G, Iannuccilli J, Dupuy DE. (2006). Radiofrequency ablation and percutaneous ethanol injection treatment for recurrent local and distant well-differentiated thyroid carcinoma. Ann Surg 244:296–304.
- Park KW, Shin JH, Han BK, et al. (2011). Inoperable symptomatic recurrent thyroid cancers: preliminary result of radiofrequency ablation. Ann Surg Oncol 18:2564–8.
- Kim JH, Yoo WS, Park YJ, et al. (2015). Efficacy and safety of radiofrequency ablation for treatment of locally recurrent thyroid cancers smaller than 2 cm. Radiology 276:909–18.
- Garberoglio R, Aliberti C, Appetecchia M, et al. (2015). Radiofrequency ablation for thyroid nodules: which indications? The first Italian opinion statement. J Ultrasound 18:423–30.
- Deandrea M, Limone P, Basso E, et al. (2008). US-guided percutaneous radiofrequency thermal ablation for the treatment of solid benign hyperfunctioning or compressive thyroid nodules. Ultrasound Med Biol 34:784–91.
- Spiezia S, Garberoglio R, Milone F, et al. (2009). Thyroid nodules and related symptoms are stably controlled two years after radiofrequency thermal ablation. Thyroid 19:219–25.
- Shin JH, Baek JH, Chung J, et al. (2016). Ultrasonography diagnosis and imaging-based management of thyroid nodules: revised Korean Society of Thyroid Radiology consensus statement and recommendations. Korean J Radiol 17:370–95.
- Moses W, Weng J, Sansano I, et al. (2010). Molecular testing for somatic mutations improves the accuracy of thyroid fine-needle aspiration biopsy. World J Surg 34:2589–94.
- Nikiforov YE, Steward DL, Robinson-Smith TM, et al. (2009). Molecular testing for mutations in improving the fine-needle aspiration diagnosis of thyroid nodules. J Clin Endocrinol Metab 94:2092–8.
- Huh JY, Baek JH, Choi H, et al. (2012). Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session – prospective randomized study. Radiology 263:909–16.
- Paramo JC, Mesko T. (2008). Age, tumor size, and in-office ultrasonography are predictive parameters of malignancy in follicular neoplasms of the thyroid. Endocr Pract 14:447–51.
- Lo CY, Chan WF, Lam KY, Wan KY. (2005). Follicular thyroid carcinoma: the role of histology and staging systems in predicting survival. Ann Surg 242:708–15.
- Ito Y, Oda H, Miyauchi A. (2016). Insights and clinical questions about the active surveillance of low-risk papillary thyroid microcarcinomas. Endocr J 63:323–8.