Abstract
Purpose: To compare technique efficacy and safety of laser ablation (LA) and radiofrequency ablation (RFA) in treatment of benign thyroid nodules.
Materials and methods: Institutional review board approval was obtained, and patients’ consent was waived. 601 nodules were treated from May 2009 to December 2014 at eight centres, 449 (309 females, age 57 ± 14 years) with LA and 152 (107 females, age 57 ± 14 years) with RFA. A matched cohort composed of 138 patients from each group was selected after adjustment with propensity score matching. Factors influencing volume reduction at 6 and 12 months and complications were evaluated.
Results: No significant differences were observed in the baseline characteristics between groups after propensity score matching adjustment. Mean nodule reduction at 6 and 12 months was −67 ± 19% vs. −57 ± 21% (p < 0.001) − 70 ± 19% vs. −62 ± 22% (p = 0.001) in LA group and in RFA group, respectively. Nodules with volume >30 mL had significantly higher percentage volume reduction at 6 and 12 months (−69 ± 19 vs. −50 ± 21, p = 0.001) and (−73 ± 18 vs. −54 ± 23 8, p = 0.001) in the LA group than in the RFA group, respectively. In both groups, operator’s skills affected the results. Major complications occurred in 4 cases in each group (p = 0.116)
Conclusions: LA and RFA showed nearly similar outcome but LA was slightly more effective than RFA in large nodules. Operator’s skills could be crucial in determining the extent of nodule volume reduction regardless of the used technique.
Introduction
Over the last years, image-guided minimally-invasive techniques have been proposed to treat clinically relevant benign thyroid nodules (BTNs) [Citation1,Citation2]. Ultrasound-guided (US-g) thermal ablation with laser or radiofrequency energy is increasingly being used for non-surgical management of symptomatic non-functioning thyroid nodules that are benign at cytological assessment [Citation3]. Due to their debulking efficacy, substantial safety and low-cost as outpatient procedures, both laser ablation (LA) and radiofrequency ablation (RFA) are currently used in several thyroid referral centres. Microwave ablation (MWA) technique, on the other hand, represents an emerging but still not thoroughly assessed and well established ablative method for BNTs [Citation4–6].
Numerous uncontrolled and prospective randomised controlled trials with different treatment algorithms have confirmed the clinical effectiveness and safety of LA [Citation7–19] and of RFA in both solid thyroid nodules and in lesions with variable fluid component [Citation20–27]. However, the reported series of LA usually included patients with larger thyroid nodules and the series of RFA included patients with a higher cyst component [Citation2]. Thus, the results are difficult to be compared [Citation28]. To date, only one review including meta-analysis [Citation29] and a more recent paper on a small case series [Citation30] have reported direct comparisons between the two techniques with contradictory conclusions. While the first study concludes that RFA is superior to LA, the second highlights a substantial equivalence of the two techniques when performed by operators with the same expertise. Thus, pending prospective comparative studies, we retrospectively compared LA and RFA outcomes in the treatment of benign thyroid nodules in a large cohort of patients treated at different centres in Italy. In order to minimise the effect of potential confounders or selection bias, patients of each group were matched by applying the one-to-one propensity score matching [Citation31,Citation32]. The aim of our study was to compare in real clinical practice the efficacy and safety of LA and RFA in the treatment of benign thyroid nodules.
Methods
Patients
The institutional review board of each participating centre approved this retrospective study and waived the requirement for informed consent. All patients undergoing percutaneous thermal ablation for symptomatic BTNs from May 2009 to December 2014 at eight thyroid referral centres were retrospectively considered for the study. Four of these centres had specific expertise in RF technique while four of them used laser technology. Then, four centres have only used RFA technique and four laser technology. In four centres the procedures were performed by endocrinologists, in three by interventional radiologists, and in one by surgeons. The patients had refused surgery or had poor surgical indications because of age, cardiovascular risk, respiratory failure or because they were not eligible for general anaesthesia. No patient had undergone ethanol sclerotherapy or other percutaneous minimally invasive therapies. Inclusion criteria were as follows: solid nodule (uniformly compact or nearly completely solid, with a liquid component not exceeding 30%); evidence at ultrasound examination of a single or of a dominant nodule, clearly detectable in a multinodular goitre; serum TSH and thyroid hormones within normal range; benign cytological examination (class II of the Bethesda classification system) [Citation33] assessed by two separate FNAs; and presence of either local pressure symptoms or cosmetic concern. Patients were excluded from the treatment in case of suspicious or cytologically proven malignancy, altered serum TSH and thyroid hormones or coagulation disorders.
Preoperative patient management
A general consensus among centres for clinical, ultrasound and laboratory assessment was based on the international guidelines criteria [Citation34,Citation35]. Thyroid sonographic evaluation was conducted in all centres by means of a commercially available US scanner, equipped with a 7.5–13.0 MHz linear transducer. The nodule volume was calculated with the ellipsoid formula (V = length × width × depth × 0.525). Serum TSH, FT4 and other laboratory controls were determined with commercially available immunoradiometric assay kits. Routine coagulation tests were performed before the procedure and included INR and platelet count. All centres operated according to the guidelines used for interventional procedures in other organs, such as liver, kidney or lung [Citation36,Citation37]. Only one session of treatment was scheduled for each nodule.
Technique
The treatment procedures followed those previously applied and published elsewhere [Citation7]. Briefly, in the case of laser technique, the number of 21 gauge (G) applicators (up to four) to be inserted is based on nodule size. Under US-g the introducer needles, and subsequently the optic fibres, are inserted into the target thyroid nodule along its longest axis. Each treatment is performed using diode lasers with a fixed-power protocol (3 W). Each illumination time ranges from a minimum of 400 s to a maximum of 600 s to achieve a total energy delivery between 1200 and 1800 Joules per fibre. Depending on the size of the nodule, one to three consecutive illuminations are performed with a pullback technique during the same treatment session.
For the radiofrequency technique, the procedure is based on the moving-shot technique as described elsewhere [Citation21]. Under US-g electrode-needles with a calibre ranging from 17 G to 19 G are inserted into the thyroid nodule along its short axis by using a trans-isthmic approach. Different zones of the lesion were ablated sequentially by moving the position of the electrode tip. The electrode was initially positioned in the deepest part of the nodule and was moved into the central and finally the superficial areas of the lesion. During the manoeuvre, the output power ranges from 40 to 80 W, and the exposure time was calculated mainly on the appearance of a transient hyperechoic area in each of the different zones undergoing ablation [Citation26,Citation27]. The number of insertions depends on the nodule size.
In different centres, the RFA procedures were performed by two endocrinologists with 4 and 6 years of experience, respectively, and by two interventional radiologists with 3 and 5 years of experience in the moving-shot technique, respectively. Laser procedures were performed by three endocrinologists with 1, 6 and 10 years of experience, respectively, and by a surgeon with 7 years of experience.
Data analysis
The nodule population was classified, in agreement with the available data in the literature [Citation11,Citation14,Citation27], into three groups according to the baseline volume. Nodules with initial volume ≤13 ml were defined as small, those between 13.1 to 30.0 ml as medium, and those >30.0 ml as large [Citation38].
Preoperative characteristics, age, sex, ablation time, energy deployed per nodule and results in terms of percentage volume reduction (PVR) at 6 and 12 months in RFA and LA groups were compared overall and according to the baseline nodule volume. In each centre, operators performing the ablation were involved in the assessment of the results and data collection. Previous clinical evidence consistently demonstrated that the major volume reduction of nodules occurs within the first six months after treatment (with significant improvement of local symptoms), followed by a minor reduction from the sixth to the twelfth month [Citation2,Citation39]. On the basis, technical success was defined as a ≥50% volume reduction at 6 months after a single treatment session. Three treatment variables were considered and correlated with a successful outcome: baseline nodule volume, total ablation time (from the initial targeting of applicator(s) into thyroid nodule to the final assessment after treatment) and total energy delivered per treatment session. Finally, we compared major and minor complications of the two treatment modalities, classifying them according to the time of occurrence as intraprocedural (during the thermal session), postprocedural (within 24 h), periprocedural (within 30 days) and late complications [Citation36,Citation37].
To minimise the effect of potential confounders on selection bias, propensity scores were generated by using binary logistic regression to estimate the probability that a patient would undergo RFA instead of LA. Independent variables that entered into the propensity model included sex, age and baseline nodule volume.
Statistical analysis
Statistical analyses were performed using statistical software (SPSS, version 22.0 for Windows; SPSS, Chicago, Ill). All data were first analysed for normality of distribution using the Kolmogorov–Smirnov test of normality. One-to-one matching between the treatment groups was accomplished by using the nearest-neighbour matching method [Citation31,Citation32,Citation40]. Briefly, the distribution of propensity scores was evaluated by treatment group to examine for sufficient overlap among the groups to ensure comparability. Continuous variables were expressed as mean ± SD, categorical variables displayed as frequencies and the appropriate parametric (student t-test) or non-parametric test (Mann–Whitney U-test or χ2 test) was used to assess significance of the differences between subgroups. A p value of less than 0.05 was considered statistically significant.
Results
Baseline characteristics of LA and RFA groups
Six hundred and one nodules in 601 euthyroid patients were ablated percutaneously through US-g thermal therapy. Four hundred and forty-nine (mean age 57 ± 14) underwent LA while 152 (mean age, 57 ± 14) underwent RFA. The baseline characteristics of all patients are shown in . A number of cases treated in each centre are reported in . The mean total baseline volume of the nodules treated with LA did not show statistically significant differences (21.5 ± 16.5 vs. 24.6 ± 17.9 ml, respectively; p = 0.065) compared with nodules treated with RFA. Patients with baseline nodule volume >30 ml who underwent RFA were significantly older (65 ± 14 vs. 58 ± 15 years, respectively; p = 0.028) than those who underwent LA. The mean baseline nodule volume in group of patients with nodules ≤13 ml was significantly greater in patients who underwent RFA than in patients who underwent LA (9.9 ± 2.9 vs. 7.4 ± 3.1 ml, respectively; p< 0.001) ().
Figure 1. The figure shows the operator’s role in determining the extent of nodule volume reduction using laser light (A) or radiofrequency energy (B).
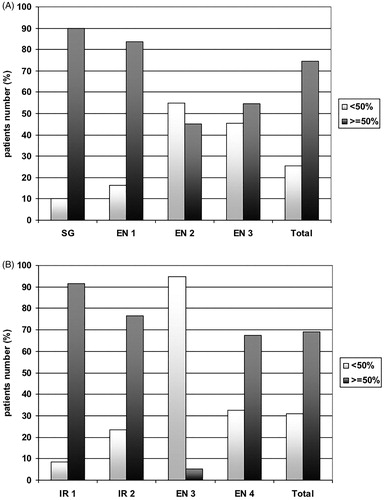
Table 1. Baseline characteristics and results in all study patients according to baseline nodule volume group before propensity score matching.
Volume reduction according to baseline nodule volume
Mean nodule volume decreased from 21.5 ± 16.5 at baseline to 8.7 ± 7.7 (p < 0.001) and to 8.0 ± 7.2 ml (p < 0.001) at 6 and 12 months, respectively, after treatment in patients who underwent LA. Mean nodule volume decreased from 24.5 ± 17.9 ml at baseline to 11.3 ± 10.7 (p < 0.001) and to 9.9 ± 9.5 ml (p < 0.001) at 6 and 12 months, respectively, after treatment (p < 0.001) in patients who underwent RFA. There were no statistically significant differences between the two treatment groups.
Volume reduction in nodules >30 ml at both 6 and 12 months was significantly higher (p = 0.003 and p = 0.033, respectively) in LA group (from mean 45.1 ± 16.6 ml baseline volume to 17.6 ± 9.8 ml and to 16.0 ± 9.3 ml, respectively) than in RFA group (from mean 49.0 ± 20.3 ml baseline volume to 24.6 ± 13.3 ml and to 21.5 ± 11.5 ml, respectively). The total energy delivered per treatment/session was significantly higher in nodules treated with RFA than in nodules treated with LA (64.6 ± 58 vs. 5.8 ± 2.7 kJ, p = 0.001). There was no statistically significant difference in ablation time between the two techniques. All results are summarised in .
Technical results
Although not statistically significant, the rate of technical success was higher in LA- than in RFA-treated patients (75% vs. 69%; p = 0.197). The baseline volume was higher in PVR ≥50% group than in PVR <50% group (p = 0.006) in the LA-treated while this finding was not observed in the RFA-treated patients. Total energy delivered was higher in PVR ≥50% group than in PVR <50% group, both in LA-treated patients (p = 0.033) and in RFA-treated patients (p = 0.010). Finally, ablation time was longer in the PVR ≥50% than in PVR <50% group, both in all study patients (p = 0.001) and in only RFA treated-patients (p = 0.002). summarises all these data. shows the distribution of the number of patients with a nodule volume reduction higher or lower than 50% at 6 months in the LA (panel A) and in the RFA (panel B) groups according to the different centres/operators. In both groups, operators with interventional training (surgeons or interventional radiologists) had the highest rate of >50% volume reduction.
Table 2. Predictive factors for percentage volume reduction (PVR) at 6th month in all study patients and according to LA or RFA treatment before propensity score matching.
Comparison of volume reduction rates between LA group and RFA group after one-to-one propensity score matching
We trimmed the sample by removing 325 patients (RFA, n = 14; LA, n = 311) with non-overlapping propensity score distribution. Therefore, adjusted comparisons by propensity scores were based on data from 138 patients for each treatment arm. Confounding factors were well matched between the LA and RFA groups: mean age 57 ± 14 vs. 57 ± 13 (p = 0.932), baseline nodule volume 21.9 ± 13.3 vs. 21.5 ± 11.5 (p = 0.760), and female subjects 69% vs. 72% (p = 0.572), respectively. Therefore, after this matching the resulting two patient groups had similar baseline characteristics. After this adjustment, mean nodule reduction at 6 and 12 months was −67 ± 19% (mean volume 7.5 ± 6.6 ml) vs. −57 ± 21% (9.6 ± 7.5 ml) (p < 0.001) and −70 ± 19% (6.6 ± 6.2 ml) vs. −62 ± 22% (8.6 ± 7.8 ml) (p = 0.001) in LA vs. RFA group. Nodules with volume >30 ml had significantly higher percentage volume reduction at 6 and 12 months in the LA group than in the RFA group (−70 ± 19% vs. 62 ± 22, p = 0.001). No difference in total ablation time was detected (17.7 ± 6.3 vs. 18.7 ± 13.6 min (p = 0.436), while a lower release of energy in the LA group compared to the RFA group was confirmed (6.1 ± 2.7 vs. 61.6 ± 51.4kJ, respectively; p = 0.001) (). According to the PVR ≥50% response, the rate of volume reduction was greater in LA than in RFA group (86% vs. 70% and 92% vs. 78%) at 6 and 12 months, respectively. In , we report the mean nodule reduction at 6 and 12 months according to baseline nodule volume group in all patients before (A) and after (B) propensity score adjustment. Again, in both groups the propensity score-matching analysis confirmed the operators’ role in determining a volumetric reduction greater than 50% at six months.
Figure 2. The figure shows the mean nodule reduction at 6 months according to baseline nodule volume group in all patients before (A) and after (B) propensity score adjustment. After propensity score-matching analysis, the higher rate of PVR in LA group in comparison with RFA group, appears confirmed in large nodules (>30 ml) thyroid nodules, appears clearly confirmed.
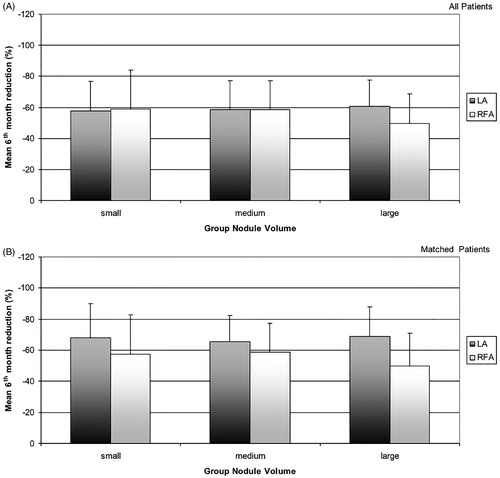
Table 3. Results in all study patients according to baseline nodule volume group after propensity score matching.
Complications
No immediate or late changes in thyroid function were observed. Thyroid-stimulating hormone, (TSH) free triiodothyronine (FT3) and free thyroxine (FT4) serum levels remained stable. No significant changes were observed in anti-thyroglobulin (TgAb) and anti-thyroperoxidase (TPOAb) antibodies titres during follow up, except for a single patient with a large nodule who developed autoimmune hyperthyroidism six months after RF ablation. reports the incidence of major and minor complications and side effects in both groups.
Table 4. Major, minor complications and side effects in each group of 138 patients.
Discussion
Several case reports and prospective randomised trials have established the clinical effectiveness of US-g thermal ablation procedures for the management of symptomatic BTNs [Citation39]. Presently, a still unresolved issue concerns the comparison of the two mainly used techniques – laser and RF – in terms of technique efficacy and safety in real clinical practice. One recent systematic review tried to compare the results of RFA and LA from the data of the published literature, and concluded that RFA appears to be superior to LA in reducing benign solid thyroid nodule volume [Citation29]. However, in this paper, the considered studies had very small number of patients (range 10–21), papers regarding LA were all significantly older than paper regarding RFA, and 6 out of 8 considered papers on LA were performed by the same author. A more recent paper compared results of LA and RFA performed by the same equipe of operators, and found no significant differences among the two techniques, suggesting that the two techniques might be similarly effective when performed by operators with the same expertise. However, this study was based on a limited experience of a single equipe of operators [Citation30]. For this reason, our comparative study was performed on a consecutive population of patients treated in thyroid referral centres and its outcomes reflect the clinical findings of the everyday activities of well-trained operators working in this field. To our knowledge, this is the first large retrospective cohort study to date which compares safety and technique efficacy of RFA and LA in the percutaneous treatment of benign solid thyroid nodules by using propensity score matching with power analysis and statistically simulating randomised controlled trials [Citation31].
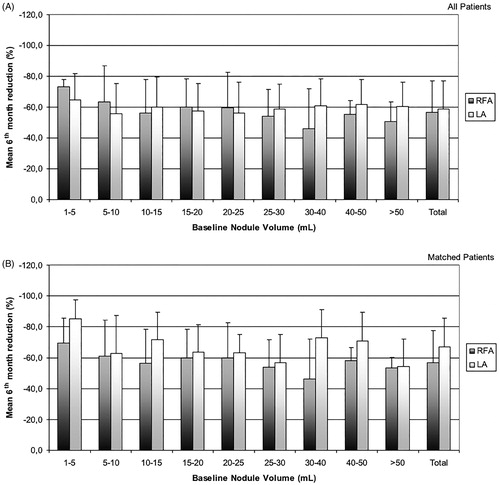
This study leads to some interesting considerations. The first is that both percutaneous thermal ablation procedures were highly effective in inducing a significant decrease in thyroid nodule volume. Both techniques were equally effective in small and medium size nodules, while the laser technique showed a slightly greater efficacy than RFA in nodules larger than 30 ml ( and ). Based on previous studies on thyroid tissue and in other organs [Citation13,Citation41–43], this different outcome could be due to the simultaneous use of multiple (up to four) laser sources in large nodules, with a more homogeneous distribution of heat energy in the target area and thus with greater treatment efficacy. Moreover, with the trans-isthmic approach it might be more difficult to treat the deeper part of large nodules, which can be conversely easily reached by a direct puncture of the nodule on its long axis. After adjustment by the propensity score matching, the higher rate of PVR in the LA group for large thyroid nodules was confirmed (as represented in and in ). Notably, both techniques produced a rather wide variability in nodule volume reduction (). This results in a relevant overlap between the two procedures. Although the statistical analysis shows a significant difference between LA and RFA, in clinical practice the different efficacy of the two techniques seems to be of minor importance. A randomised controlled trial (RCT) could confirm this specific finding. To this end, we are currently conducting an ongoing Phase III trial and we are recruiting patients to prospectively assess the effectiveness and safety of LA compared to RFA (ClinicalTrials.gov identifier: NCT02714946) in order to avoid the potentially confounding bias present in this retrospective study.
Figure 3. The figure shows the wide variability in volume reduction with both the techniques according to baseline nodule volume, both before (A) and after (B) propensity score adjustment.
A second and the most relevant finding, as shown in , is the role of the operator in determining the efficacy and the extent of nodule volume reduction. The influence of the operator experience on the treatment outcome was confirmed, regardless of whether laser light or radiofrequency energy was used. The proper targeting of nodules in close proximity to vital structures such as neck vessels, trachea and laryngeal nerve requires a good manual skill in placing the devices correctly in an anatomical region as small as the neck. This problem underscores the importance of appropriate training and learning curve, which should be longer for operators who are not confident with minimally invasive procedures. This issue should be better clarified by a study addressed to the learning curve of individual operators for thyroid minimally invasive techniques.
Thirdly, we observed that to achieve a comparable volume reduction in small and medium size nodules, the operators used more energy (about ten times more) with the radiofrequency technique than with laser technology ( and . This finding could be explained by the different modalities of production and distribution of thermal energy within the tissue to be ablated by means of the two techniques. The last data, however, do not seem to be relevant in clinical practice because they are inherent to the technique used but do not cause the undesired effects on thyroid tissue that are described in hepatic thermal ablation [Citation44–46].
The two procedures appeared both safe and well tolerated, with a similar complications rate (). The easiness of use of techniques seems to be subjective and in most cases is dependent on the clinical circumstances and on the devices available in the institution where the operators work.
Finally, since costs are becoming more and more important in decision making, may be useful to consider the costs of the two techniques. Taking into account the rental of generators in both techniques, the cost of a single laser applicator is about U.S.$250 per session (in most cases two applicators are needed) while the cost of an RFA electrode is about U.S.$800 per session. Both treatments may be performed on outpatients by an operator and a nurse. In both procedures, the cost of dressings, including local anaesthetics and pain-killers, is quite low.
Our study has some limitations. First, we used a retrospective approach; therefore, inherent selection bias was unavoidable, even with the propensity score analysis. Second, this was a multicentre study with only eight participating centres, even if each of them had a large volume of percutaneous RFA or LA ablation. Moreover, operators were involved in the results assessment and data collection, and some possible bias should be taken into account. Therefore, we believe that careful consideration is needed before generalising our results to other settings.
In summary, this study confirms the efficacy and safety in different clinical settings of US-g thermal ablation procedures consistently with previous reports. RFA and LA techniques seem to provide similar results, with a high success rate and low risk of major complications. The only difference was observed in the efficacy of LA in comparison with RFA in treatment of large size nodules. This finding seems to be, at least in part, due to the variable skill and experience of the operators. So the operator’s specific manual ability in ablative techniques appears crucial, regardless of the chosen technique.
Declaration of interest
No potential conflict of interest was reported by the authors.
References
- Gharib H, Hegedus L, Pacella CM, et al. (2013). Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 98:3949–57.
- Papini E, Pacella CM, Hegedus L. (2014). Thyroid ultasound (US) and US-assisted procedures: from the shadows into an array of applications. Eje 170:R1–R15.
- Gharib H, Papini E, Garber JR, et al. (2016). American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical guidelines for clinical practice for the diagnosis and management of thyroid nodules–2016 update. Endocr Pract 22:622–39.
- Feng B, Liang P, Cheng Z, et al. (2012). Ultrasound-guided percutaneous microwave ablation of benign thyroid nodules: experimental and clinical studies. Eur J Endocrinol 166:1031–7.
- Yue W, Wang S, Wang B, et al. (2013). Ultrasound guided percutaneous microwave ablation of benign thyroid nodules: safety and imaging follow-up in 222 patients. Eur J Radiol 82:e11–16.
- Yang YL, Chen CZ, Zhang XH. (2014). Microwave ablation of benign thyroid nodules. Future Oncol 10:1007–14.
- Pacella CM, Bizzarri G, Spiezia S, et al. (2004). Thyroid tissue: US-guided percutaneous laser thermal ablation. Radiology 232:272–80.
- Papini E, Guglielmi R, Bizzarri G, Pacella CM. (2004). Ultrasound-guided laser thermal ablation for treatment of benign thyroid nodules. Endocr Pract 10:276–83.
- Dossing H, Bennedbaek FN, Hegedus L. (2006). Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules: one versus three treatments. Thyroid 16:763–8.
- Cakir B, Topaloglu O, Gul K, et al. (2006). Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Investig 29:876–84.
- Valcavi R, Riganti F, Bertani A, et al. (2010). Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 20:1253–61.
- Gambelunghe G, Fede R, Bini V, et al. (2013). Ultrasound-guided interstitial laser ablation for thyroid nodules is effective only at high total amounts of energy: results from a three-year pilot study. Surg Innov 20:345–50.
- Pacella CM, Mauri G, Achille G, et al. (2015). Outcomes and risk factors for complications of laser ablation for thyroid nodules: a multicenter study on 1531 patients. J Clin Endocrinol Metab 100:3903–10.
- Achille G, Zizzi S, Di Stasio E, et al. (2016). Ultrasound-guided percutaneous laser ablation in treating symptomatic solid benign thyroid nodules: our experience in 45 patients. Head Neck 38:677–82.
- Dossing H, Bennedbaek FN, Hegedus L. (2005). Effect of ultrasound-guided interstitial laser photocoagulation on benign solitary solid cold thyroid nodules – a randomised study. Eur J Endocrinol 152:341–5.
- Gambelunghe G, Fatone C, Ranchelli A, et al. (2006). A randomized controlled trial to evaluate the efficacy of ultrasound-guided laser photocoagulation for treatment of benign thyroid nodules. J Endocrinol Investig 29:RC23–6.
- Papini E, Guglielmi R, Bizzarri G, et al. (2007). Treatment of benign cold thyroid nodules: a randomized clinical trial of percutaneous laser ablation versus levothyroxine therapy or follow-up. Thyroid 17:229–35.
- Dossing H, Bennedbaek FN, Hegedus L. (2011). Long-term outcome following interstitial laser photocoagulation of benign cold thyroid nodules. Eur J Endocrinol 165:123–8.
- Negro R, Salem TM, Greco G. (2016). Laser ablation is more effective for spongiform than solid thyroid nodules. A 4-year retrospective follow-up study. Int J Hyperthermia 32:822–8.
- Kim YS, Rhim H, Tae K, et al. (2006). Radiofrequency ablation of benign cold thyroid nodules: initial clinical experience. Thyroid 16:361–7.
- Jeong WK, Baek JH, Rhim H, et al. (2008). Radiofrequency ablation of benign thyroid nodules: safety and imaging follow-up in 236 patients. Eur Radiol 18:1244–50.
- Sung JY, Kim YS, Choi H, et al. (2011). Optimum first-line treatment technique for benign cystic thyroid nodules: ethanol ablation or radiofrequency ablation? AJR Am J Roentgenol 196:W210–14.
- Lim HK, Lee JH, Ha EJ, et al. (2013). Radiofrequency ablation of benign non-functioning thyroid nodules: 4-year follow-up results for 111 patients. Eur Radiol 23:1044–9.
- Baek JH, Kim YS, Lee D, et al. (2010). Benign predominantly solid thyroid nodules: prospective study of efficacy of sonographically guided radiofrequency ablation versus control condition. AJR Am J Roentgenol 194:1137–42.
- Huh JY, Baek JH, Choi H, et al. (2012). Symptomatic benign thyroid nodules: efficacy of additional radiofrequency ablation treatment session-prospective randomized study. Radiology 263:909–16.
- Deandrea M, Sung JY, Limone P, et al. (2015). Efficacy and safety of radiofrequency ablation versus observation for nonfunctioning benign thyroid nodules: a randomized controlled international collaborative trial. Thyroid 25:890–6.
- Cesareo R, Pasqualini V, Simeoni C, et al. (2015). Prospective study of effectiveness of ultrasound-guided radiofrequency ablation versus control group in patients affected by benign thyroid nodules. J Clin Endocrinol Metab 100:460–6.
- Wong KP, Lang BH. (2013). Use of radiofrequency ablation in benign thyroid nodules: a literature review and updates. Int J Endocrinol 2013:428363.
- Ha EJ, Baek JH, Kim KW, et al. (2015). Comparative efficacy of radiofrequency and laser ablation for the treatment of benign thyroid nodules: systematic review including traditional pooling and bayesian network meta-analysis. J Clin Endocrinol Metab 100:1903–11.
- Mauri G, Cova L, Monaco CG, et al. (2017). Benign thyroid nodules treatment using percutaneous laser ablation (PLA) and radiofrequency ablation (RFA). Int J Hyperthermia 33:295–9.
- McDonald RJ, McDonald JS, Kallmes DF, Carter RE. (2013). Behind the numbers: propensity score analysis-a primer for the diagnostic radiologist. Radiology 269:640–5.
- Haukoos JS, Lewis RJ. (2015). The Propensity Score. Jama 314:1637–8.
- Bongiovanni M, Spitale A, Faquin WC, et al. (2012). The Bethesda system for reporting thyroid cytopathology: a meta-analysis. Acta Cytol 56:333–9.
- Cooper DS, Doherty GM, Haugen BR, et al. (2009). Revised American Thyroid Association management guidelines for patients with thyroid nodules and differentiated thyroid cancer. Thyroid 19:1167–214.
- Gharib H, Papini E, Paschke R, et al. (2010). American Association of Clinical Endocrinologists, Associazione Medici Endocrinologi, and European Thyroid Association medical guidelines for clinical practice for the diagnosis and management of thyroid nodules: executive summary of recommendations. J Endocrinol Investig 33:51–6.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of Interventional Radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria–a 10-year update. J Vasc Interv Radiol 25:1691–705 e1694.
- Livraghi T, Goldberg SN, Lazzaroni S, et al. (2000). Hepatocellular carcinoma: radio-frequency ablation of medium and large lesions. Radiology 214:761–8.
- Papini E, Pacella CM, Misischi I, et al. (2014). The advent of ultrasound-guided ablation techniques in nodular thyroid disease: towards a patient-tailored approach. Best Pract Res Clin Endocrinol Metab 28:601–18.
- Takuma Y, Takabatake H, Morimoto Y, et al. (2013). Comparison of combined transcatheter arterial chemoembolization and radiofrequency ablation with surgical resection by using propensity score matching in patients with hepatocellular carcinoma within Milan criteria. Radiology 269:927–37.
- Pacella CM, Stasi R, Bizzarri G, et al. (2008). Percutaneous laser ablation of unresectable primary and metastatic adrenocortical carcinoma. Eur J Radiol 66:88–94.
- Pacella CM, Francica G, Di Lascio FM, et al. (2009). Long-term outcome of cirrhotic patients with early hepatocellular carcinoma treated with ultrasound-guided percutaneous laser ablation: a retrospective analysis. J Clin Oncol 27:2615–21.
- Mauri G, Cova L, Tondolo T, et al. (2013). Percutaneous laser ablation of metastatic lymph nodes in the neck from papillary thyroid carcinoma: preliminary results. J Clin Endocrinol Metab 98:E1203–7.
- Rozenblum N, Zeira E, Bulvik B, et al. (2015). Radiofrequency ablation: inflammatory changes in the periablative zone can induce global organ effects, including liver regeneration. Radiology 276:416–25.
- Rozenblum N, Zeira E, Scaiewicz V, et al. (2015). Oncogenesis: an “off-target” effect of radiofrequency ablation. Radiology 276:426–32.
- Velez E, Goldberg SN, Kumar G, et al. (2016). Hepatic thermal ablation: effect of device and heating parameters on local tissue reactions and distant tumor growth. Radiology 281:782–92.
