Abstract
Objective: The cerebellum is extremely sensitive to heat-induced injury because of the abundance of Purkinje cells. This study explored the influence of temperature on cerebellar metabolite levels by magnetic resonance spectroscopy (MRS).
Materials and Methods: Ten healthy volunteers were recruited to undergo a MRS examination in the summer and winter. Twenty-four ordinary patients with fever underwent the same examination during fever and after recovery. All MR spectras were recorded from the cerebellum. Metabolites ratios including N-acetyl aspartate/creatine (NAA/Cr), choline/creatine (Cho/Cr), and N-acetyl aspartate/choline (NAA/Cho) were calculated.
Results: There are no significant differences in cerebellar metabolite levels in the 10 healthy volunteers during summer and winter (p > .05). For the 24 patients with fever, the cerebellar Cho/Cr ratios during fever were increased compared with those after recovery (p = .043).
Conclusions: In summary, MRS plays a role in the evaluation of the influence of temperature on cerebellar metabolite levels. It was observed that cerebellar metabolite levels are not influenced by seasonal temperature but are influenced by body temperature.
Introduction
The cerebellum contains an abundance of Purkinje cells. Varying degrees of heat-induced cerebellar injury have been reported [Citation1–5]. A previous study has confirmed the selective vulnerability of Purkinje cells to heat-induced injury and revealed the neuropathology of heat stroke due to a severe loss of Purkinje cells associated with the expression of heat-shock protein 70 by Bergmann glia [Citation6]. Proton magnetic resonance spectroscopy (MRS) is a non-invasive technique that has been increasingly used in brain research. MRS gives information on the chemical/metabolic composition of the brain [Citation7]. As described in detail previously [Citation8], heat stroke with a body temperature of over 40 °C can lead to a decrease in cerebellar N-acetyl aspartate/creatine (NAA/Cr) ratios. This study used MRS to investigate whether cerebellar metabolite levels are influenced by seasonal temperature or by body temperature (<40 °C).
Materials and methods
This study was approved by the ethics committee of Binzhou Medical University. All participants provided their written informed consent to participate in this study. This consent procedure was also approved by the ethics committee of Binzhou Medical University. Ten healthy volunteers (five male and five female) were recruited to undergo a MRS examination during summer and winter. They were chosen from a healthy population with no fever. Their average age was 30 (range 26–35) years. Twenty-four ordinary patients (9 male and 15 female) with fever were selected to undergo the same examination during fever and after recovery. They were chosen from patients suffering from an upper respiratory tract infection or pneumonia. The exclusion criterion was encephalitis. Their body temperature ranged from 38 to 40 °C during fever. Their average age was 36 (range 27–45) years.
MRS examinations were performed using a SIEMENS Avanto 1.5-T magnetic resonance scanner (Siemens Healthcare, Berlin, Germany) with a standard quadrature head coil. A standard 2D chemical-shift imaging point-resolved spectroscopy (CSI-PRESS) (Siemens Healthcare, Berlin, Germany) was used with the following parameters: repetition time (TR) = 1500 ms, echo time (TE) = 135 ms, Fov 160 × 160 mm2, thickness = 15 mm, average = 4. All MR spectras were recorded from the cerebellum between the fourth ventricle and the skull. A rectangular volume of interest (A ≫ P 30 mm, R ≫ L 70 mm, and F ≫ H 15 mm) was placed to cover the cerebellum between the fourth ventricle and the skull [Citation8]. The spectras were analysed using a manufacturer-supplied software package. The processing of spectras including filtering, base line correction, and curve fitting were automatically accomplished. All data processing was done with the same parameters and automated. Metabolites ratios including NAA/Cr, choline/creatine (Cho/Cr), and N-acetyl aspartate/choline (NAA/Cho) were calculated and averaged for each participant.
Statistical analysis was performed using the Statistical Package for the Social Sciences (SPSS, version 17) (SPSS Inc., Chicago, IL). Paired samples t-test was performed to evaluate differences between the metabolites ratios of the 10 healthy volunteers during summer and winter. Similar analyses were performed to evaluate the differences between the metabolites ratios of 24 ordinary patients during fever and after recovery. The differences were considered statistically significant at p < .05.
Results
There are no significant differences in cerebellar metabolite levels of the 10 healthy volunteers during summer and winter (p > .05) (). For the 24 ordinary patients with fever, the cerebellar Cho/Cr ratios were increased during fever compared with those after recovery (t = 2.138; p = .043) ().
Table 1. Cerebellar metabolite ratios (mean ± standard deviation) of 10 healthy volunteers during summer and winter.
Table 2. Cerebellar metabolite ratios (mean ± standard deviation) of 24 ordinary patients during fever and after recovery.
Cerebellar metabolite levels were not influenced by seasonal temperature (), but can be influenced by body temperature. Elevated body temperature increased cerebellar Cho/Cr ratios ().
Figure 1. Healthy volunteer during summer and winter. (a) Proton MR spectra image of the cerebellum obtained in a 28-year-old healthy man during summer. (b) Proton MR spectra image of the cerebellum obtained in the same man during winter. The image shows no difference with the previous image.
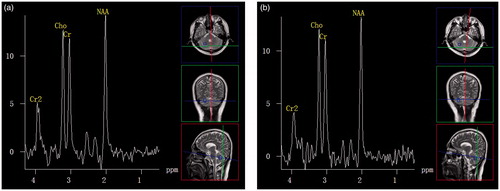
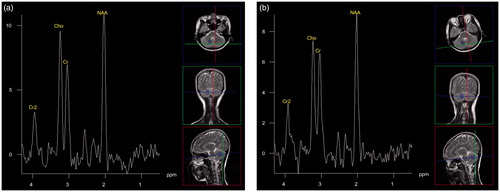
Figure 2. Ordinary patient during fever and after recovery. (a) Proton MR spectra image of the cerebellum obtained in a 26-year-old woman with a cold during fever. The image shows a high Cho peak. (b) Proton MR spectra image of the cerebellum obtained in the same woman after recovery. The image shows a normal Cho peak.
Discussion
Although the cerebellum is extremely sensitive to heat-induced changes [Citation6], this study showed that seasonal temperature does not influence cerebellar metabolite levels. Seasonal temperature changes may not affect the brain temperature. Body temperature regulation is a fundamental homeostatic function that involves numerous involuntary effector responses and is governed by the preoptic anterior hypothalamus [Citation9,Citation10]. An increased warm signal input may activate heat loss effectors, while an increased cold signal input may increase heat production [Citation11].
NAA peaks are considered as neuronal activity markers. Hyperthermia (over 40 °C) can lead to decreased cerebellar NAA peaks [Citation8] because of a series of pathophysiological changes including severe diffuse loss of Purkinje cells, osmotic demyelination, and degeneration of Purkinje cells axons [Citation6,Citation12,Citation13]. This study showed that NAA peaks were not affected within the limits of elevated temperature.
This study also found that elevated body temperature led to increased cerebellar Cho/Cr ratios, which are considered as transport-function markers of the cell membrane. Increased cerebellar Cho/Cr ratios with elevated body temperature may be explained by temperature effects on cell metabolism [Citation14]. It may be more conducive to the exchange of substances between the inner and the outer of cell membrane with increased of the body temperature. However, this is not consistent with a previous study [Citation8]. The inconsistency in the Cho/Cr ratio is due to different comparison methods. The previous study compared heat stroke patients and normal controls. This study compared the same person at different timeframes. Metabolites ratios can also be influenced by age.
In summary, MRS plays a role in the evaluation of cerebellar metabolite levels varying with temperature.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Lee JS, Choi JC, Kang SY, et al. (2009). Heat stroke: increased signal intensity in the bilateral cerebellar dentate nuclei and splenium on diffusion-weighted MR imaging. AJNR Am J Neuroradiol 30:E58.
- Albukrek D, Bakon M, Moran DS, et al. (1997). Heat-stroke-induced cerebellar atrophy: clinical course, CT and MRI findings. Neuroradiology 39:195–7.
- Murcia-Gubianas C, Valls-Masot L, Rognoni-Amrein G. (2012). Brain magnetic resonance imaging in heat stroke. Med Intensiva 36:526.
- Mahajan S, Schucany WG. (2008). Symmetric bilateral caudate, hippocampal, cerebellar, and subcortical white matter MRI abnormalities in an adult patient with heat stroke. Proc (Bayl Univ Med Cent) 21:433–6.
- Ookura R, Shiro Y, Takai T, et al. (2009). Diffusion-weighted magnetic resonance imaging of a severe heat stroke patient complicated with severe cerebellar ataxia. Intern Med 48:1105–8.
- Bazille C, Megarbane B, Bensimhon D, et al. (2005). Brain damage after heat stroke. J Neuropathol Exp Neurol 64:970–5.
- Manganas LN, Zhang X, Li Y, et al. (2007). Magnetic resonance spectroscopy identifies neural progenitor cells in the live human brain. Science 318:980–5.
- Li J, Zhang XY, Wang B, et al. (2015). Multivoxel proton magnetic resonance spectroscopy in heat stroke. Clin Radiol 70:37–41.
- Hanegan JL, Williams BA. (1973). Brain calcium: role in temperature regulation. Science 181:663–4.
- Nakamura K. (2011). Central circuitries for body temperature regulation and fever. Am J Physiol Regul Integr Comp Physiol 301:R1207–28.
- Nomoto S, Shibata M, Iriki M, Riedel W. (2004). Role of afferent pathways of heat and cold in body temperature regulation. Int J Biometeorol 49:67–85.
- McNamee T, Forsythe S, Wollmann R, Ndukwu IM. (1997). Central pontine myelinolysis in a patient with classic heat stroke. Arch Neurol 54:935–6.
- Liu K, Li B, Qian S, et al. (2015). Altered interhemispheric resting state functional connectivity during passive hyperthermia. Int J Hyperthermia 8:840–9.
- Ducommun P, Rueux P-A, Kadouri A, et al. (2002). Monitoring of temperature effects on animal cell metabolism in a packed bed process. Biotechnol Bioeng 77:838–42.
