Abstract
Background: Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) is an emerging surgical procedure for peritoneal carcinomatosis (PC). CRS/HIPEC is a complicated treatment that requires multi-disciplinary teamwork (MDT), which may be lacking when establishing a CRS/HIPEC programme. Herein, we report our preliminary treatment outcomes with the early implementation of an MDT model for CRS/HIPEC.
Methods: From April 2015 to December 2016, 45 patients with a diagnosis of PC who received CRS/HIPEC were reviewed retrospectively in a single institution in Taiwan.
Results: Among the 45 patients, CRS was mainly performed by laparotomy (n = 42), and only three patients with limited PC underwent laparoscopic CRS. The first 13 patients received treatment before the MDT had been established (group 1), and the other 32 patients were treated after the MDT had been established (group 2). The highest peri-HIPEC body temperature in group 2 was significantly lower than that in group 1 (36.8 °C vs. 37.5 °C, p < 0.001). Overall, eight patients experienced major complications. The trend of a lower major complication rate was observed after the MDT model had been implemented (30.7% in group 1 vs. 12.4% in group 2, p = 0.202). Pre-CRS/HIPEC abdominal pain significantly increased the risk of post-operative major complications (p = 0.017).
Conclusions: Our experience suggests that the early implementation of an MDT model when establishing a CRS/HIPEC programme at a single institution may result in a higher complete cytoreduction rate and lower major complication rate, and also shorten the learning curve of this complicated procedure.
Introduction
Peritoneal carcinomatosis (PC) is a serious oncological condition in patients with metastatic malignancy, for which cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) has become the treatment of choice. Spratt and colleagues reported the first case of CRS/HIPEC in a patient with pseudomyxoma peritonei (PMP) in 1980 [Citation1]. Since then, CRS/HIPEC has consistently shown dramatic improvements in outcomes in selected patients with PC, PMP, and peritoneal mesothelioma [Citation2]. In highly selected patients with colorectal cancer, CRS/HIPEC has shown benefits in disease-free and overall survival compared to systemic chemotherapy alone, with a 5-year survival rate of up to 45% for patients with no residual tumour after completing CRS [Citation3,Citation4]. Furthermore, in patients with PMP receiving CRS/HIPEC, the survival rate beyond 10 years has been reported to reach 63% [Citation5].
Despite the survival benefits from CRS/HIPEC, complications have been reported with major morbidity and 30-day mortality rates of 20–50% and 1–10%, respectively [Citation4–10]. In addition, the procedure has a steep learning curve, and it has been reported that at least 130 procedures are necessary to gain sufficient experience to overcome incomplete CRS, major morbidities, and procedure-related mortality [Citation7,Citation8]. Surgical training and technical guidance at experienced centres may help staff to achieve a better performance when establishing new programmes [Citation10,Citation11]; however, most experienced centres are located in Europe and North America with only a few institutions in Japan and one in South East Asia [Citation9].
In Taiwan, CRS/HIPEC has been performed in several pioneer hospitals since 1999 [Citation12,Citation13]. However, only individual surgeons or small surgical teams practice CRS/HIPEC in these hospitals, and it is still an unfamiliar surgical procedure in Taiwan. Multi-disciplinary teamwork (MDT) for CRS/HIPEC has been suggested to improve complication and survival rates [Citation14]; however an established MDT model in a new CRS/HIPEC programme is uncommon.
We performed the first CRS/HIPEC procedure at our hospital in April 2015; however, we encountered difficulty in managing procedure-related major complications in the subsequent cases. After a thorough review and consensus, we established an MDT programme in April 2016. In this study, we report our preliminary results of CRS/HIPEC and explore the role of the early implementation of an MDT model in improving treatment outcomes.
Materials and methods
Study overview
This is a retrospective analysis of our CRS/HIPEC database of patients who visited Chang-Gung Memorial Hospital at Chiayi. We reviewed 45 patients with PC (primary or metastatic) who received CRS/HIPEC from April 2015 to December 2016. This study was approved by the Institutional Review Board of Chang Gung Memorial Hospital (201600885B0C601).
Process of setting up a CRS/HIPEC programme with an MDT model
CRS/HIPEC was performed by different surgeons in the first 13 patients with PC from April 2015 to January 2016. After a thorough review and consensus, two lead members were selected to participate in the CRS/HIPEC educational advanced course held by the European Society of Surgical Oncology in March 2016 in Hamburg, and our CRS/HIPEC MDT programme was then established in April 2016.
The MDT consisted of three proctologists, one medical oncologist, one gynaeco-oncologist, one general surgeon, two radiologists, one thoracic surgeon, one pulmonologist, two anaesthesiologists and one case manager. A biweekly joint meeting was held, and treatment decisions for all patients were made after group discussion. In addition, procedure-related findings and outcomes were also discussed. The following topics were decided by consensus, including patient selection, pre-operative imaging evaluation, perioperative management, technique, and follow-up strategy after the establishment of the MDT programme.
Patient selection and imaging evaluation
Patients considered for CRS/HIPEC were patients with PC from colorectal cancer, PMP, ovarian cancer, gastric cancer, peritoneal mesothelioma, and sarcoma. All candidates were referred to the MDT for further discussion (), if they met one of the following criteria: (1) CRS/HIPEC was initially planned for primary advanced PC; (2) CRS/HIPEC was considered after primary chemotherapy; (3) CRS/HIPEC was considered for salvage treatment after recurrence; or (4) palliative HIPEC to control ascites.
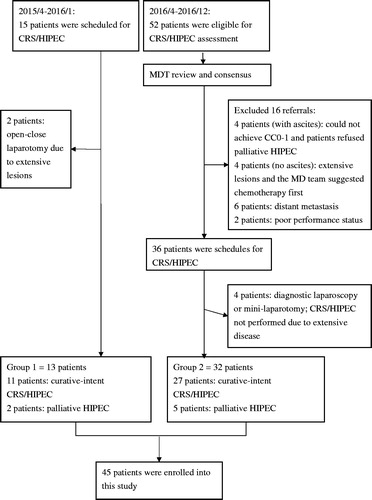
Figure 1. Study population. Group 1: from April 2015 to January 2016, 13 patients were treated before the MDT model had been implemented. Group 2: from April 2016 to December 2016, 52 patients were referred to our MDT programme, of whom 32 were treated. CC: completeness of cytoreduction score; CRS/HIPEC: cytoreductive surgery with hyperthermic intraperitoneal chemotherapy; MDT: multi-disciplinary teamwork; PC: peritoneal carcinomatosis.
All subjects underwent multidetector computed tomography (CT) for the detection of peritoneal tumours before surgery, except for one subject who received magnetic resonance imaging (MRI) for preoperative screening. The median interval between the imaging examinations and surgery was 19 days. The preoperative CT peritoneal cancer index (PCI) was scored according to the Sugarbaker classification [Citation15] by a single radiologist who was blinded to the clinical information and surgical PCI. In cases who were clinically suspected of having extra-abdominal metastases, 18-fluoro-2-deoxy-D-glucose positron emission tomography-CT was also performed.
The exclusion criteria were as follows: (1) Eastern Cooperative Oncology Group (ECOG) performance status ≥3; (2) age >80 years; (3) extensive tumour burden for which complete CRS could not be performed for curative intent; (4) unresectable extra-peritoneal metastasis.
Perioperative management of CRS/HIPEC
All patients visited the anaesthesiologist and case manager for counselling before the operation. Pre-operative bowel preparation was necessary, and all patients received perioperative antibiotic prophylaxis. After the operation, the patients received parenteral nutrition, which was changed to enteral nutrition when the physician noted normoactive bowel sounds and removed the nasogastric tube if the daily drainage was less than 100 ml.
After the procedure, the patients were admitted to general wards or the surgical intensive care unit (SICU). The criteria for SICU admission were as follows: (1) age ≥70 years; (2) extensive surgery with CRS lasting for ≥6 h; (3) intraoperative blood loss ≥1000 ml with a large blood transfusion (at least 5 units of red blood cells intraoperatively) [Citation16]. The patients admitted to the SICU were cared for by the MDT intensive care specialist.
CRS/HIPEC technique and treatment plan
The MDT programme members performed the CRS/HIPEC procedures. Diagnostic laparoscopy or mini-laparotomy was used as the assessment tool if the results of imaging studies were inconclusive as to whether CRS could be completed successfully, including cases of suspicious liver hilum involvement, extensive small-bowel obstruction or when major vessels were involved. PCI was calculated before CRS was performed in all cases. Patients with extensive tumours underwent midline laparotomy, whereas patients with limited PC underwent a laparoscopic procedure. After CRS, completeness of cytoreduction scores were assessed [Citation17].
After CRS, HIPEC was delivered using the closed method with a Performer™ HT (RanD Biotech, Medolla, Italy). The perfusate was mixed with normal saline and pentastarch (Haes-steril, 60 mg/ml, Meda, Sweden) 10% (3:1) at a dose of 2 l/m2 of body surface. The chemotherapy was delivered after an intra-abdominal temperature of 43 °C was reached. The temperature of HIPEC was 41–43 °C. The duration of HIPEC was 60–90 min. The temperature was recorded every 5 min according to the routine anaesthesia protocol. In all patients, urine output was monitored and maintained at 1 ml/kg/15 min during the HIPEC procedure. If urine output decreased, further hydration and furosemide were given. The medical oncologist chose the HIPEC regimen, which included mitomycin, oxaliplatin, cisplatin, paclitaxel, and doxorubicin according to the type of cancer [Citation6,Citation18–21].
Follow-up strategy
Data on the patients’ demographics, operative details, postoperative mortality, morbidity and pathologic results were collected according to standard operating protocols by the case manager and evaluated by the members of the MDT. Pre-operative symptoms were defined as pain with opioid control, ascites with fullness discomfort, and obstruction with constipation and vomiting. Postoperative complications were classified using a previously validated system, the National Cancer Institute Common Terminology Criteria for Adverse Events (NCI-CTCAE) version 4.0 [Citation22]. Mortality was defined as any death occurring during 30 days after surgery or during hospitalisation. Follow-up CT/MRI was performed 3–6 months after surgery or if symptoms recurred. Survival and disease recurrence were documented from medical records.
Statistical analysis
Descriptive statistics were reported as mean ± SD, median with minimum and maximum, or frequency with percentage as appropriate. Patients who underwent CRS/HIPEC before the MDT model had been established were defined as group 1, and those who underwent CRS/HIPEC after the MDT model had been implemented were defined as group 2. Differences in surgical outcomes and postoperative adverse events between two groups were examined using Fisher’s exact test for categorical variables and the Wilcoxon rank sum test for continuous variables. All calculations were performed using SPSS statistical software (IBM SPSS Statistics for Windows, version 22.0, IBM Corp., Armonk, NY). All tests were two sided, and a p values <0.05 was considered to be statistically significant.
Results
Patients
The patient enrolment flowchart is shown in . From April 2015 to December 2016, a total of 67 patients were referred for the assessment and management of PC. Before the MDT had been established, 11 patients underwent curative CRS/HIPEC and two patients underwent palliative CRS/HIPEC (group 1). After the MDT model had been implemented, 52 patients were evaluated by MDT, of whom 20 were deemed not suitable to undergo the procedure. Of the remaining 32 patients, 27 underwent curative CRS/HIPEC and five received palliative CRS/HIPEC (group 2). These 45 patients (13 in group 1 and 32 in group 2) were enrolled into this study for analysis, and their clinical and demographic data are summarised in .
Table 1. Demographic and clinical data of the patients who underwent CRS/HIPEC.
Surgical procedures
Among the 45 patients, CRS was mainly performed by laparotomy (n = 42), and only three patients with limited PC underwent laparoscopy. Twenty patients had bowel resection (five in group 1 and 15 in group 2), and six patients required stoma creation.
Operative outcomes
Differences in surgical outcomes between group 1 and group 2 are shown in . The highest peri-HIPEC body temperature in group 2 was significantly lower than that in group 1 (36.8 °C vs. 37.5 °C, p < 0.001).
Table 2. Surgical outcomes in group 1 and group 2.
Among the patients treated with curative intent in group 1 (n = 11), five patients (45.5%) had CC0 CRS and five patients (45.5%) had CC1 CRS. One patient (9.1%) had only CC3 resection because of extensive peritoneal lesions. In group 2 (n = 27), 18 patients (66.7%) had CC0 CRS and six patients (22.2%) had CC1 CRS. Three patients (11.1%) received CC2 resection only due to bulky tumours with pancreatic head deep invasion, multiple tumours with liver surface and parenchymal invasion, and a tumour behind the stomach with deep invasion, respectively.
Four patients (30.7%) in group 1 developed major complications (grade ≥3). With regards to all major adverse events, there were two cases of acute kidney injury, one of severe hypoalbuminemia (1.8 g/dl), one bowel perforation, one pulmonary embolism, and one fascial dehiscence. Among them, two patients suffered from two episodes of major complications. One patient (7.7%) died post-operatively because of pulmonary embolism. After the MDT programme had been implemented (group 2), four patients (12.4%) developed major complications, including one with pancreatic duct leakage, two with fascial dehiscence, and one with aspiration pneumonia. Two of these patients (6.3%) died post-operatively because of peritonitis secondary to pancreatic duct leakage and aspiration pneumonia-related respiratory failure.
Two patients who received a cisplatin regimen for HIPEC suffered from grade-3 acute kidney injury in group 1. The perioperative hydration protocol was modified after the MDT programme had been implemented, after which no further renal injuries were recorded.
Risk factors for major complications
shows the associations between clinical variables and major complications. The patients who presented with abdominal pain before CRS/HIPEC had a higher risk of developing major complications (33.3% vs. 4.2%, p = 0.017).
Table 3. Risk analysis for major post-operative complications of CRS/HIPEC.
Oncological outcomes
Up to 31 January 2017, the median follow-up period was 6.5 months. Of the 38 patients who underwent potentially curative CRS/HIPEC, 26 (68.4%) had no evidence of disease and eight (21.1%) were alive with disease. Of the eight patients who were alive with disease, the median progression-free period was 2.5 months (range 1.4–9.8 months). Six patients had distant recurrence, and two patients had peritoneal recurrence including uterine sarcoma and recurrent colon cancer, respectively. With regards to distant metastasis, the liver was the most commonly involved organ (six patients).
On the other hand, of the seven patients who received palliative CRS/HIPEC, one died after 30 days and two had progressive peritoneal tumours but no recurrence of ascites. The other four patients maintained stable disease. The duration of peritoneal disease control of these six patients ranged from 2.3 to 19.0 months (median 6.2 months).
Discussion
In this study, we report our preliminary experience of an MDT programme for CRS/HIPEC in Taiwan. After the MDT programme had been implemented, the complete CRS rate increased and the major post-operative complication rate decreased.
The learning curve of CRS/HIPEC is often assessed using incomplete CRS (CC2–3), open-close laparotomy, grade 3–5 morbidity, and procedure-related mortality and reflects patient selection and treatment expertise [Citation7,Citation8]. In general, the rate of incomplete CRS and open–close surgery is high after implementing a CRS/HIPEC programme, reportedly due to the inappropriate enrolment of patients with extensive disease [Citation8]. In a mentor institution, the rate of CC0–1 can be higher than 80% due to strict selection criteria and higher level of surgical experience [Citation5,Citation7,Citation11]. In the current study, the rate of CC0–1 after the MDT programme had been implemented (group 2) did not differ from the pre-MDT period (group 1); however, the rate of CC0 increased. This implies improvements in patient selection, treatment planning and surgical procedures after the MDT programme had been implemented. In addition, there were three cases of incomplete CRS in group 2 due to tumour invasion to visceral organs and a difficult surgical location. Even though diagnostic laparoscopy or minilaparotomy can prevent a large wound in open-close laparotomy [Citation23], diagnostic laparoscopy still has limitations. These three malignant lesions were missed by preoperative imaging, and their location behind major organs meant that they could not be assessed by diagnostic laparoscopy.
CRS/HIPEC is an aggressive treatment modality, often associated with high-grade complications. These complications have been reported to be associated with factors, such as comorbidities (diabetes mellitus, hypertension, smoking, cardiac disease), performance status, tumour involvement, perioperative systemic temperature control, treatment-related variables (chemotherapy regimens, surgical method, the number of resected organs) and surgical proficiency [Citation24,Citation25]. A high HIPEC carrier solution temperature can increase the risk of hyperthermia, leading to unstable hemodynamic status, metabolic problems and a challenge for anaesthesiologists [Citation25]. In our series, better control of the peri-HIPEC temperature in group 2 compared to group 1 (36.8 °C vs. 37.5 °C, p < 0.001) lowered the risk of developing hyperthermia. Interestingly, we found that the patients who presented with pain before CRS/HIPEC had a significantly higher complication rate (p = 0.017). In addition, the patients with abdominal pain had a higher mean preoperative PCI score than those without abdominal pain, although the difference was not statistically significant (17.1 vs. 12.0, respectively, p = 0.064, data not shown). A higher PCI score has been associated with surgical difficulty [Citation5]; however, we did not find any associations between factors such as comorbidities, PCI, chemotherapy agents or post-operative condition with the development of complications. This may be due to the small sample size, and further large-scale studies are warranted to validate our findings.
Patients with diffuse PC often suffer from massive ascites, which can lead to pseudo-obstruction, poor appetite, and poor nutrition. These symptoms can be temporarily relieved by repeated paracentesis and diuresis. However, repeated ascites tapping causes patient discomfort, and intra-abdominal adhesion limits the efficacy of these procedures. In theory, HIPEC can eradicate cancer cells on the peritoneal surface to prevent the re-accumulation of ascites [Citation26]. Randle et al. reported that CRS/HIPEC was effective in controlling ascites in 93% of their patients for 3 months, even in those with CC2–3 resection [Citation27]. Valle et al. reported excellent ascites resolution in 83% of their patients with malignant ascites after laparoscopic HIPEC [Citation26]. In this study, our patients had a 100% 3-month ascites-free rate after receiving CRS/HIPEC. Good control of ascites can improve the quality of life, nutrition and provide further opportunities for treatment.
Developing high-quality MDT care for patients with PC is a challenge in a new CRS/HIPEC centre. However, teamwork including the cooperation of medical professionals and organisation with experienced members can ensure high-quality diagnoses, treatment, and care [Citation28]. A new institution can achieve encouraging outcomes and a low complication rate if the staffs receive training and mentoring from experienced hospitals [Citation10,Citation11]. One new centre in Ireland even achieved no major complications or cases of mortality using this approach [Citation11]. In addition, an MDT framework can be helpful in improving outcomes with regards to decision-making, technical factors, patient care and in shortening the learning curve [Citation28–30]. In our preliminary experience, the major complication rate was 30.7% in group 1 which then decreased to 12.4% in group 2. Our MDT programme was established after two key members completed a training course held by the European Society of Surgical Oncology, and we now hold regular meetings to discuss cases, new developments in the field, identify and analyse problems and design practical standard operating protocols [Citation29]. Subsequently, we have achieved improvements in the complete CRS rate and a reduction in the major complication rate. Furthermore, we encourage the team members to participate in international training courses to improve their surgical skill and perioperative care.
The limitations of this study are the short follow-up period and small sample size. Nevertheless, our preliminary experience suggests that an MDT framework can be established even without the hands-on training provided by pioneer institutions despite the lack of advanced surgical experience such as with the extensive peritonectomy procedure for patients with PMP. We therefore recommend further participation in advanced training courses and more prompt consultation with international experts.
Conclusions
The advantages of MDT are appropriate patient selection, risk identification, and perioperative team care. A higher complete CRS rate and improved morbidity rate were observed in group 2 compared to group 1. The establishment of an MDT model in our new CRS/HIPEC programme resulted in better performance and shorter learning curve. Further studies with a longer follow-up period are warranted to confirm our findings.
Acknowledgements
We are grateful to the Prof. Ying-Huang Tsai, the Superintendent of Chang Gung Memorial Hospital, Chiayi, and the members of the Peritoneal Malignancy program of our Cancer Centre: Wen-Shih Huang (proctologist, chief), Meng-Chiao Hsieh, Chih-Jung Chen (proctologists), Chao-Yu Chen (gynaeco-oncologist, vice chief), Ting-Yao Wang (medical oncologist), Tzu-Hao Huang (general surgeon), Yu-San Liao, Li-Wen Lee (radiologists), Wen-Tzu Liao, Chung-Ming Yu (anaesthesiologists), Ming-Shian Lu (thoracic surgeon), Che-Chia Chang (pulmonary and critical care), Tzu-Ting Liao (case manager), Tzu-Ting Lin (business manager), and Yu-Fen Chen (secretary).
We also thank the follow educational courses: the ESSO Advanced Course on the Management of HIPEC after CRS 10th–12th March 2016 in Hamburg and Perioperative Management in CRS and HIPEC of Peritoneal Malignancy 25th–30th November 2016 in Berlin.
We thank ATS Medical Editing and Review Solutions for language editing.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Spratt JS, Adcock RA, Muskovin M, et al. (1980). Clinical delivery system for intraperitoneal hyperthermic chemotherapy. Cancer Res 40:256–60.
- Yan TD, Stuart OA, Yoo D, Sugarbaker PH. (2006). Perioperative intraperitoneal chemotherapy for peritoneal surface malignancy. J Transl Med 4:17.
- Verwaal VJ, Bruin S, Boot H, et al. (2008). 8-year follow-up of randomized trial: cytoreduction and hyperthermic intraperitoneal chemotherapy versus systemic chemotherapy in patients with peritoneal carcinomatosis of colorectal cancer. Ann Surg Oncol 15:2426–32.
- Mirnezami R, Mehta AM, Chandrakumaran K, et al. (2014). Cytoreductive surgery in combination with hyperthermic intraperitoneal chemotherapy improves survival in patients with colorectal peritoneal metastases compared with systemic chemotherapy alone. Br J Cancer 111:1500.
- Chua TC, Moran BJ, Sugarbaker PH, et al. (2012). Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30:2449–56.
- D'Hondt V, Goffin F, Roca L, et al. (2016). Interval cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in first-line treatment for advanced ovarian carcinoma: a feasibility study. Int J Gynecol Cancer 26:912–17.
- Kusamura S, Baratti D, Virzi S, et al. (2013). Learning curve for cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in peritoneal surface malignancies: analysis of two centres. J Surg Oncol 107:312–19.
- Smeenk RM, Verwaal VJ, Zoetmulder FA. (2007). Learning curve of combined modality treatment in peritoneal surface disease. Br J Surg 94:1408–14.
- Tan G, Chia C, Kumar M, et al. (2017). 201 consecutive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in a single Asian tertiary centre. Int J Hyperthermia 33:288–94.
- Kuijpers AM, Hauptmann M, Aalbers AG, et al. (2016). Cytoreduction and hyperthermic intraperitoneal chemotherapy: the learning curve reassessed. Eur J Surg Oncol 42:244–50.
- Chang KH, Kazanowski M, Staunton O, et al. (2017). Mentored experience of establishing a national peritoneal malignancy programme – experience of first 50 operative cases. Eur J Surg Oncol 43:395–400.
- Kang LY, Mok KT, Liu SI, et al. (2013). Intraoperative hyperthermic intraperitoneal chemotherapy as adjuvant chemotherapy for advanced gastric cancer patients with serosal invasion. J Chin Med Assoc 76:425–31.
- Lin EK, Hsieh MC, Chen CH, et al. (2016). Outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for colorectal cancer with peritoneal metastasis. Medicine (Baltimore) 95:e5522.
- Lungoci C, Mironiuc AI, Muntean V, et al. (2016). Multimodality treatment strategies have changed prognosis of peritoneal metastases. World J Gastrointest Oncol 8:67–82.
- Sugarbaker PH. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in the management of gastrointestinal cancers with peritoneal metastases: progress toward a new standard of care. Cancer Treat Rev 48:42–9.
- Mogal HD, Levine EA, Fino NF, et al. (2016). Routine admission to intensive care unit after cytoreductive surgery and heated intraperitoneal chemotherapy: not always a requirement. Ann Surg Oncol 23:1486–95.
- Sugarbaker PH. (2007). Peritonectomy procedures. Cancer Treat Res 134:247–64.
- Turaga K, Levine E, Barone R, et al. (2014). Consensus guidelines from The American Society of Peritoneal Surface Malignancies on standardizing the delivery of hyperthermic intraperitoneal chemotherapy (HIPEC) in colorectal cancer patients in the United States. Ann Surg Oncol 21:1501–5.
- Spiliotis J, Halkia E, Lianos E, et al. (2015). Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 22:1570–5.
- Delotte J, Desantis M, Frigenza M, et al. (2014). Cytoreductive surgery with hyperthermic intraperitoneal chemotherapy for the treatment of endometrial cancer with peritoneal carcinomatosis. Eur J Obstet Gynecol Reprod Biol 172:111–14.
- Roviello F, Caruso S, Neri A, Marrelli D. (2013). Treatment and prevention of peritoneal carcinomatosis from gastric cancer by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: overview and rationale. Eur J Surg Oncol 39:1309–16.
- Pascual-Ramirez J, Garcia SS, Gonzalez Ruiz de la Herran F, et al. (2014). Security and efficiency of a closed-system, turbulent-flow circuit for hyperthermic intraperitoneal chemotherapy after cytoreductive ovarian surgery: perioperative outputs. Arch Gynecol Obstet 290:121–9.
- Sommariva A, Zagonel V, Rossi CR. (2012). The role of laparoscopy in peritoneal surface malignancies selected for hyperthermic intraperitoneal chemotherapy (HIPEC). Ann Surg Oncol 19:3737–44.
- Ihemelandu CU, McQuellon R, Shen P, et al. (2013). Predicting postoperative morbidity following cytoreductive surgery with hyperthermic intraperitoneal chemotherapy (CS + HIPEC) with preoperative FACT-C (Functional Assessment of Cancer Therapy) and patient-rated performance status. Ann Surg Oncol 20:3519–26.
- Webb CA, Weyker PD, Moitra VK, Raker RK. (2013). An overview of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for the anesthesiologist. Anesth Analg 116:924–31.
- Valle SJ, Alzahrani NA, Alzahrani SE, et al. (2015). Laparoscopic hyperthermic intraperitoneal chemotherapy (HIPEC) for refractory malignant ascites in patients unsuitable for cytoreductive surgery. Int J Surg 23:176–80.
- Randle RW, Swett KR, Swords DS, et al. (2014). Efficacy of cytoreductive surgery with hyperthermic intraperitoneal chemotherapy in the management of malignant ascites. Ann Surg Oncol 21:1474–9.
- Herzer KR, Rodriguez-Paz JM, Doyle PA, et al. (2009). A practical framework for patient care teams to prospectively identify and mitigate clinical hazards. Jt Comm J Qual Patient Saf 35:72–81.
- Rodriguez-Paz JM, Mark LJ, Herzer KR, et al. (2009). A novel process for introducing a new intraoperative program: a multidisciplinary paradigm for mitigating hazards and improving patient safety. Anesth Analg 108:202–10.
- Moran BJ. (2006). Decision-making and technical factors account for the learning curve in complex surgery. J Public Health (Oxf) 28:375–8.
