Abstract
Magnetic nanoparticles can be used in different areas of biology. It is therefore important to know the effects of such nanomaterials on germline cells as they may traverse the blood-testis barrier. This work aimed to evaluate the response of bull sperm after exposure to a magnetic fluid containing DMSA-coated maghemite nanoparticles (MNP-DMSA) in order to determine nanotoxicity. Bull sperm was incubated with MNP-DMSA at final concentrations of 0.06, 0.03 or 0.015 mg Fe/mL. Sperm kinetics, plasma membrane integrity and acrosome reaction were evaluated over a 4 h incubation period. The sperm cells were also evaluated by transmission electron microscopy. Exposure of bull sperm to MNP-DMSA did not affect sperm kinetics or integrity. Neither ultrastructural damage of sperm cells nor uptake of nanoparticles by the spermatozoa was observed. In conclusion, MNP-DMSA does not affect sperm function or structure under the conditions tested.
Introduction
In recent years, nanomaterials have been used in numerous applications in daily life and healthcare [Citation1–4]. However, their potential toxicity presents a real concern [Citation5,Citation6]. Some studies have already demonstrated that nanoparticles (NPs) can easily pass through physiological barriers, such as the blood–brain [Citation7] and blood–testis barriers [Citation8,Citation9], consequently exposing male germ cells to toxic/undesirable effects [Citation10]. In fact, some NPs have already demonstrated toxicity to sperm cells. For instance, orally administered silver NPs delayed the onset of puberty, decreased adult sperm production and modified reproductive parameters, with reduced acrosome and plasma membrane integrity [Citation11] and had a slight negative influence on sperm morphology in rats [Citation12]. Furthermore, intravenously-administered silver NPs resulted in lower sperm cell counts together with a higher number of abnormal forms and DNA damage in rats [Citation13]. Zinc oxide nanoparticles, when orally administered for 12 weeks in rats [Citation14], or for 35 d in adult mice [Citation15], caused a significant decrease in the epidydimal sperm number, motility, and percentage of normal and viable sperm.
Nanoparticles also present many interesting applications in the reproductive biotechnology area. For instance, it is possible to use super paramagnetic iron oxide NPs to improve sperm selection processes [Citation16] and remove damaged spermatozoa (under a magnetic field) without impairing the fertility potential of the remaining unbound spermatozoa [Citation17,Citation18]. Odhiambo et al. [Citation17] reported the use of magnetic NPs to remove abnormal spermatozoa from bovine semen, which resulted in improved sperm fertility after artificial insemination. Gold NPs were similarly used in the pre-selection of spermatozoa carrying the X- or Y-chromosome for sperm sexing processes [Citation19,Citation20]. Another application, aimed at producing transgenic embryos, involved the use of magnetic nanoparticles containing exogenous DNA, which was transferred into the spermatozoa plasma membrane and ultimately delivered into the oocyte through in vitro fertilisation [Citation21].
The use of NPs in reproductive biotechnology is clearly promising. However, there are many different types of NPs and it is therefore important to investigate their potential individual nanotoxicity to sperm cells. In fact, assessing the toxicity of NPs in the male germline in vitro has long been of interest [Citation22]. The in vitro effects of gold NPs on human sperm have been reported with severe morphological defects and deleterious effects on sperm motility reported [Citation23,Citation24]. Gold NPs can also compromise the condensation and stabilisation states of gametic nuclear chromatin, with chromatin decondensation observed in mice epidydimal sperm in vitro [Citation25]. In bovine sperm, the toxicity of gold [Citation26,Citation27] and iron oxide [Citation28,Citation29] NPs have been evaluated. While gold NPs showed high toxicity to sperm motility and structure [Citation26,Citation27], iron oxide NPs showed no toxic effects to sperm cells [Citation28]. Makhluf et al. [Citation28] reported that PVA-coated magnetite NPs are able to penetrate sperm cells spontaneously without affecting their motility or ability to fertilise the egg. Furthermore, the aforementioned NPs did not alter the motility, acrosome reaction or structure of bovine sperm cells [Citation28].
Iron oxide NPs have especially interesting applications because of their magnetic properties, which allow their use in sperm separation or selection. Therefore, this work aimed to evaluate the toxicity of DMSA-coated maghemite nanoparticles (MNP-DMSA) to bull sperm in vitro.
Material and methods
Iron oxide nanoparticles
The MNP-DMSA were manufactured in the Chemistry Laboratory of Goiás University as previously described [Citation30]. Briefly, maghemite nanoparticles were synthesised by mixing ferric and ferrous chloride aqueous solutions (2:1 molar ratio) with concentrated ammonia aqueous solution under vigorous stirring. A DMSA aqueous solution (0.3 mol/l) was subsequently added to the magnetic suspension at a molar ratio DMSA/Fe of 11%. NaCl was added to the suspension to reach a final salinity concentration of 0.9% wt/v. The pH was adjusted to 7.2–7.4. The MNP-DMSA concentration was 16.5 × 1016 nanoparticles/mL, with an average diameter of 5.3 nm and 17 mg/mL Fe concentration. Transmission electron microscopy analysis (Jeol 1011 TEM, Tokyo, Japan) estimated an average nanoparticle diameter of 8.1 nm. The zeta potential value of particles in the acidic precursor magnetic suspension was +40 mV. After functionalisation with DMSA and pH adjustment to 7.2, the zeta potential of MNPs decreased to −47.7 mV. The zeta potential was obtained from electrophoretic mobility (μe) measurements performed by phase analysis light scattering using ZetaSizer Nano ZS equipment (Malvern Instruments, Malvern, UK).
Semen dilution medium SP-TALP (0.1 M NaCl; 25 mM NaHCO3; 3.1 mM KCl; 0.29 mM NaH2PO4; 10 mM HEPES medium; 0.01 mg/mL Phenol Red; 0.028 M sodium lactate; 2.1 mM CaCl22H2O; 0.39 mM MgCl26H2O; 1 mM sodium pyruvate; 0.075 mg/mL amikacin; 0.6% BSA; 0.01% penicillin/streptomycin) was used to incubate sperm with MNP-DMSA. In order to stabilise the nanoparticles, preventing aggregation and maintaining the hydrodynamic diameter close to the initial solution, the SP-TALP medium original BSA concentration was corrected to a final concentration of 1.5% (SP-TALP+) as previously established by our group [Data not published]. In each repetition, 7 μL MNP-DMSA was diluted in 933 μL SP-TALP + to obtain a final Fe concentration of 0.12 mg/mL (MNP-DMSA work solution).
Sperm preparation
Frozen semen samples (0.5 mL) were acquired from a breeding centre (ABS Pecplan, Uberaba, Minas Gerais, Brazil) and maintained in liquid nitrogen −196 °C until required. Twenty-seven samples from three bulls with proven fertility were used.
The experiment was repeated nine times. In each repetition, three frozen semen straws from the same bull were thawed in water at 37.5 °C. Live sperm was separated from each semen sample by Percoll gradient [Citation31]. Live sperm pellets, formed from each Percoll sample, were pooled together, subsequently washed carefully in SP-TALP + medium and finally resuspended in 420 μL of SP-TALP+ (sperm work solution – SWS). A sample of 10 μL was taken from the SWS to evaluate the sperm concentration using a Neubauer chamber.
In vitro exposure of sperm to the MNP-DMSA
The experiment consisted of three treatments groups (with increasing Fe concentrations) and a control group (CO) without nanoparticles. Treatment groups contained 0.015 (G0.015), 0.03 (G0.03) or 0.06 (G0.06) mg Fe/mL. To form the treatment groups, the MNP-DMSA work solution was mixed with SP-TALP + medium according to the desired final concentration () and 100 μL of the resulting mixture added to 100 μL of the SWS. The CO consisted of mixing 100 μL of the SWS and 100 μL of SP-TALP + medium. All groups were subsequently maintained in a water bath at 37.5 °C throughout the 4 h evaluation period.
Table 1. Volumes of SWS, MNP-DMSA work solution and SP-TALP + medium used to form each group.
Sperm motility pattern using CASA
Sperm movement was analysed using the computer assisted sperm analysis (CASA) software (Ultimate 12-Ivos Hamilton Thorne, Beverly, MA) adjusted for bull semen. The evaluations were performed at: zero hour (when the groups were formed); every 15 min over 2 h, and at the third and fourth hour of incubation.
In order to analyse the motility patterns during the incubation period with or without MNP-DMSA, semen samples from each group were homogenised and aliquots of 10 μL were removed and placed into specific counting chambers (Leja® standard count, SC 20.01.08.B, 20 microns, Nieuw-Vennep, Netherlands). CASA provided the percentage of total motility (MOT) and progressive motility (PMOT). Other motility patterns, such as mean velocity – VAP (μm/s), linear velocity – VSL (μm/s), curvilinear velocity – VCL (μm/s), mean lateral head amplitude – ALH (μm), frequency of head displacement – BCF (Hz), straightness coefficient – STR (%), linearity coefficient – LIN (%), were also measured.
Assessment of plasma membrane and acrosome integrity
A combination of fluorescein isothiocyanate conjugated with Arachis hypogea agglutinin (FITC-PNA) and propidium iodide (PI) was used to simultaneously assess plasma membrane and acrosome integrity. To obtain 1 mL of dye work solution, 20 μL of FITC-PNA stock solution (1 mg FITC-PNA, 1 mL PBS), 10 μL of PI stock solution (10 mg PI, 10 mL saline solution), 10 μL of 10% formaldehyde and 960 μL of 3% sodium citrate solution were mixed. A fresh dye work solution was made in each experiment replication and maintained in the dark.
A 10 μL aliquot from each group was mixed with 30 μL of dye work solution at zero hour (when the groups were formed), and at the second and fourth hour of incubation. Samples were evaluated by epifluorescence and phase contrast microscopy using wavelength filter 494/517 excitation/emission (Axiophot Zeiss, College Station, TX). A total of 200 sperm cells were analysed at each time point per group and classified according to the fluorescence emitted by each probe. Sperm were classified into three categories: 1) Viable sperm cells, considering only cells with an intact plasma membrane and intact acrosome (represented as a percentage of the total cells counted), 2) Non-viable sperm cells, considering sperm cells with a damaged membrane with or without an acrosome reaction (represented as a percentage of the total sperm cells counted), and 3) Sperm cells with an intact membrane, but with a reacted acrosome (represented as a percentage of the cells with intact membrane counted).
Sperm ultrastructure and evaluation of nanoparticle uptake
Transmission electron microscopy was used to evaluate the effects on sperm ultrastructure after exposure to the MNP-DMSA, together with the possible internalisation of these nanoparticles. At the end of the 4 h incubation period, the remaining sperm solution from each group was fixed separately in Karnovsky solution (2.5% glutaraldehyde, 2% paraformaldehyde in 0.1 M sodium cacodylate buffer, pH 7.2) in the refrigerator for 24 h. Samples were subsequently post-fixed in a solution containing 1% osmium tetroxide, 0.8% potassium ferricyanide and 5 mM CaCl2, contrasted in 0.5% uranyl acetate, dehydrated in increasing acetone concentrations and embedded in Spurr resin. Semi-thin sections were stained with toluidine blue to localise the sperm. Ultra-thin sections were examined using a Jeol 1011 transmission electron microscope (Jeol, Tokyo, Japan).
Statistical analysis
Data were subjected to analysis of variance (ANOVA) and Tukey’s test at a 5% significance level. The analyses were performed separately for each seminal characteristic evaluated. Statistical analyses were performed using Statview 5.0 for Windows (SAS Institute, Cary, NC).
Results
Sperm kinetics using CASA system
The average sperm concentration used in treated and control groups was 13.44 ± 3.12 × 106 spermatozoa/mL. The mean and standard deviation values of sperm motility and progressive motility of all groups over the incubation time are shown in and . Both parameters demonstrated a significant reduction (p < 0.05) over the incubation period for all treatments and the control. However, there was no significant difference (p > 0.05) between the control group and treated groups at any time point.
Figure 1. Motility (A) and progressive motility (B) of bull sperm in the control and treated groups during the incubation period for 240 min. CO – control group; G0.015: Group treated with MNP-DMSA at the concentration of 0.015 mg Fe/mL; G0.03: Group treated with MNP-DMSA at the concentration of 0.03 mg Fe/mL; G0.06: Group treated with MNP-DMSA at the concentration of 0.06 mg Fe/mL.
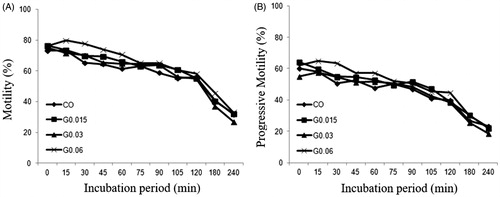
Table 2. Motility and progressive motility (mean ± SD) of bull sperm in the control and treated groups during the incubation period for 240 min.
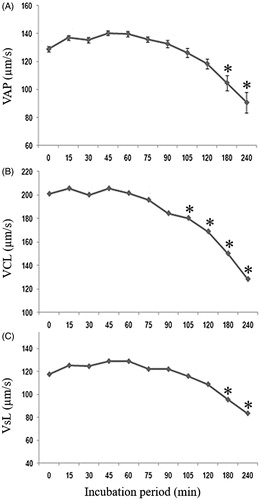
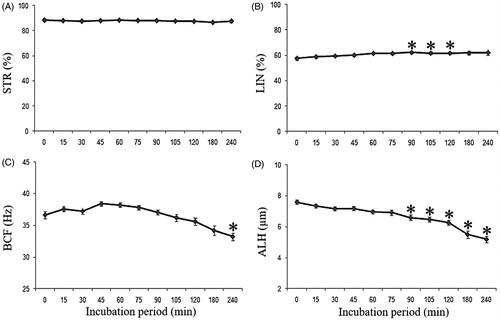
The sperm movement pattern evaluated by the CASA system (STR, LIN, BCF, ALH, VCL, VSL and VAP,) was also unaffected by incubation with MNP-DMSA (p > 0.05). Therefore, data from all groups, including CO, were grouped to evaluate the sperm movement pattern over the 4 h incubation period. shows the influence of incubation time on the qualitative characteristics of sperm movement (STR, LIN, BCF and ALH). There were no differences in STR during the incubation (p > 0.05). LIN showed small differences (p < 0.05), with no significant difference between the beginning and end of incubation. BCF and ALH presented a significant reduction (p < 0.05) during the incubation, respectively, on 240 min and from 90 min on. The influence of incubation on velocity parameters is shown in . All velocity parameters were significantly reduced (p < 0.05) on the third and fourth hours of incubation.
Figure 2. Qualitative parameters of sperm movement (mean ± SEM) during the incubation period. (A) STR – straightness coefficient (%), (B) LIN – linearity coefficient (%), (C) BCF – frequency of head displacement (Hz) and (D) ALH – lateral head amplitude (μm). *Indicates significant difference from time 0 (p < 0.05).
Plasmatic membrane integrity and acrosome status
The presence of widely agglomerated particles near the sperm head region in treated groups was noted during the plasma membrane integrity and acrosome status evaluation. As this was not observed in the control group, and the only difference between the control group and the other groups was the presence of nanoparticles in the medium, it is supposed that the clusters were composed of maghemite nanoparticles.
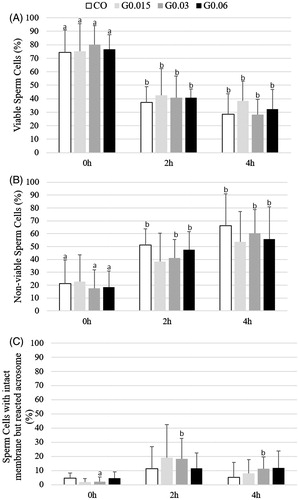
The percentage of sperm cells in each previously described category was not affected by the MNP-DMSA treatment (p > 0.05), as shown in . After 2 h of incubation, the percentage of viable sperm cells () in all groups was significantly lower in comparison to the beginning of incubation (zero hour). There was a significant increase in the number of non-viable sperm cells () in CO, G0.03 and G0.06 groups after 2 h of incubation. The percentage of sperm cells with an intact membrane and reacted acrosome significantly increased after 2 h of incubation in G0.03 only ().
Figure 4. Percentages (media ± SD) of (A) viable sperm cells, (B) non-viable sperm cells and (C) sperm cells with intact membrane and reacted acrosome during the incubation period with or without MNP-DMSA. a,bDifferent letters indicate significant difference among times in the same group (p < 0.05). CO – control group; G0.015: Group treated with MNP-DMSA at the concentration of 0.015 mg Fe/mL; G0.03: Group treated with MNP-DMSA at the concentration of 0.03 mg Fe/mL; G0.06: Group treated with MNP-DMSA at the concentration of 0.06 mg Fe/mL.
Sperm ultrastructure and nanoparticle uptake
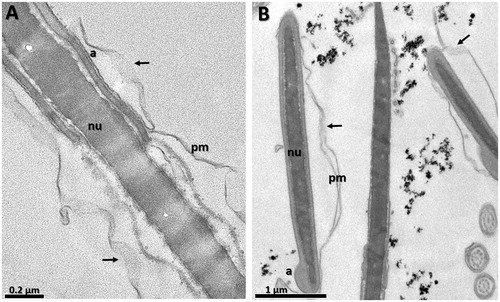
Nanoparticle clusters close to the head of the sperm cells were observed in all treated groups (). Nanoparticles were not observed inside the sperm cytoplasm, which indicates that maghemite nanoparticles coated with DMSA are not internalised by sperm cells, even after 4 h of incubation. Furthermore, all groups showed a number of sperm cells wih a disrupted plasma membrane (), including the control group, which indicates that incubation with MNP-DMSA for 4 h does not affect sperm ultrastructure.
Figure 5. Transmission electron micrographies of bull spermatozoa after 4 h of incubation. (A) CO – control group, (B) G0.015: Group treated with MNP-DMSA at the concentration of 0.015 mg Fe/mL, (C) G0.03: Group treated with MNP-DMSA at the concentration of 0.03 mg Fe/mL and (D) G0.06: Group treated with MNP-DMSA at the concentration of 0.06 mg Fe/mL. Note the presence of MNP-DMSA clusters close to the head of the sperm cells in all treated groups (arrows). Nanoparticles were not observed inside the sperm cytoplasm. pm: plasmatic membrane, a: acrosome, nu: nucleus, h: head, t: tail.
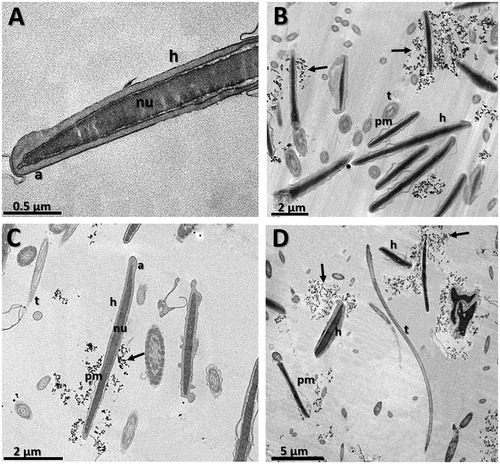
Figure 6. Transmission electron micrographies of bull spermatozoa after 4 h of incubation. (A) CO – control group, (B) G0.03: Group treated with MNP-DMSA at the concentration of 0.03 mg Fe/mL. Arrows indicate disruption in plasma membrane in both groups. pm: plasmatic membrane, a: acrosome, nu: nucleus.
Discussion and conclusion
This is the first study to evaluate the effect of MNP-DMSA at three different concentrations (0.015, 0.03 and 0.06 mg Fe/mL) on bull spermatozoa in vitro over a 4 h incubation period. Few studies have been performed to demonstrate the potential toxicity of nanoparticles in semen in vitro [Citation9], for review see [Citation32], despite numerous application proposals [Citation16,Citation17,Citation19,Citation20,Citation33].
Our data showed that MNP-DMSA does not affect the motility or the progressive motility of bovine sperm cells for up to 4 h of incubation at any of the concentrations used. Similar results were reported using PVA-coated magnetite nanoparticles on bull sperm [Citation28]. Incubation of human semen with silver [Citation24] or gold [Citation24,Citation26] NPs also did not significantly influence sperm motility or viability after 60 and 120 min of incubation. In contrast, gold NPs caused toxic effects on sperm motility and structure in bovine sperm [Citation26,Citation27] in vitro. In vivo, maghemite NPs intraperitoneally injected in mice triggered a dose-dependent decrease in sperm motility [Citation34]. Similarly, orally-administered zinc oxide nanoparticles caused a significant decrease in motility, sperm counts, and percentage of normal and live sperm in mice epididymal semen [Citation14,Citation15].
In our study, the incubation period was found to influence sperm motility and progressive motility in all groups, which decreased significantly after two hours of incubation and continued to decline to the fourth hour. A time dependent decrease in sperm motility is expected. This evaluation can even be used for assessing semen quality, which becomes critical if motility values fall below 15% after the third hour of incubation [Citation35]. In our study, the percentages of mobile sperm at the third and fourth hours of incubation were around 40 and 30%, respectively, in all groups including the control group.
In fact, it was noted that sperm cells from all treatment groups presented motility parameters compatible with hyperactive bovine sperm [Citation36], as shown by the values found for VCL, VAP, VSL, ALH and LIN. Hyperactivation motility is a flagellar phenomenon, even though it is often measured by changes in the movement of the sperm head, and may aid in sperm penetration of the zona pellucida [Citation37]. It was shown that precocious hyper activation could be induced in mouse spermatozoa by incubation in medium containing BSA [Citation38]. Therefore, it is possible that the changes in motility parameters observed in this study were due to the addition of BSA in the medium, necessary to stabilise MNP-DMSA as described previously.
Moreover, incubation with MNP-DMSA did not affect the percentage of sperm cells with an intact membrane and reacted acrosome. Since neither the motility nor the percentage of viable cells were affected by the treatments, it is suggested that MNP-DMSA does not affect sperm fertilising capability, which in fact was proved by other studies. For example, the fertilising capability of swine sperm cell was not affected after incubation with iron oxide nanoparticles [Citation21]. The authors used magnetic nanoparticles to improve sperm-mediated gene transfer to produce transgenic porcine embryos. It was found that “magnetofected” boar spermatozoa could fertilise oocytes in vitro. In bulls, incubation of spermatozoa with PVA-coated magnetite nanoparticles did not affect the ability of sperm cells to undergo acrosome reaction [Citation28], which is a parameter used to predict fertilising capability [Citation39].
Nanoparticles that aggregated near the sperm heads, shown by TEM and noticed during epifluorescence analysis, were also reported by other authors for magnetite nanoparticles in bull sperm [Citation28] and for gold nanoparticles in human sperm [Citation23]. In other cell types, there is a tendency of anionic NPs to agglomerate around the nucleus after internalisation by the cell [Citation40]. Although in the present study the NPs were not internalised by the sperm cells, NPs agglomeration in the sperm head region might have something to do with the presence of the nucleus at this site.
In this work, nanoparticles were not observed within the sperm cell, which indicates that MNP-DMSA are not internalised. In contrast, PVA-coated magnetite nanoparticles were internalised by bull sperm cells [Citation28]. The authors [Citation28] suggest that the PVA coating promoted a non-specific endocytosis-based nanoparticle uptake by the cells. It is possible that DMSA does not have the same potential to promote this uptake. In contrast, internalisation of DMSA-coated maghemite nanoparticles has been reported in macrophages [Citation30], growing neurons [Citation40] and fibroblasts [Citation41]. It is supposed that nanoparticle uptake into cells may be affected by not only the coating or the cell type, but also by the nanoparticle properties, such as superficial charge, hydrodynamic size, shape and superficial area [Citation29,Citation42].
Similar to PVA-coated magnetite nanoparticles, MNP-DMSA did not affect sperm ultrastructure [Citation28]. In contrast, incubation with uncoated gold nanoparticles promoted fragmentation of human sperm cells [Citation23]. The absence of coating in gold nanoparticles could have caused the damaging effects, since metallic nanoparticles without a biocompatible coating may have a toxic effect on biological structures [Citation43]. Although in the present study some sperm cells presented a disrupted plasma membrane in all groups, it is quite expected because samples for TEM analysis were taken after 4 h of incubation, and at this time 50–60% of the sperm were non-viable cells (cells with a damaged membrane) as shown by the plasma membrane and acrosome integrity assessment ().
In summary, MNP-DMSA did not affect the motility, viability or the ultrastructure of bull spermatozoa, and there is no evidence that they can be internalised by the sperm cells. It is possible to conclude that the direct exposure of bull sperm cells to MNP-DMSA did not cause toxic effects on sperm cell function or structure. The results of this study suggest that MNP-DMSA could be used to improve in vitro semen technologies, such as sperm cell separation methods, although additional studies are necessary to prove it. In the same way, if these nanoparticles were to be used for any in vivo application, it is unlikely that they would negatively impact the sperm cells, even if they can cross the blood-testis barrier, but again further studies are needed to investigate this point.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Sekhon BS. (2014). Nanotechnology in agri-food production: an overview. Nanotechnol Sci 7:31–53.
- Seal S, Karn B. (2014). Safety aspects of nanotechnology based activity. Safety Sci 63:217–25.
- Thiesen B, Jordan A. (2008). Clinical applications of magnetic nanoparticles for hyperthermia. Int J Hyperthermia 24:467–74.
- Kaur P, Aliru ML, Chadha AS, et al. (2016). Hyperthermia using nanoparticles-promises and pitfalls. Int J Hyperthermia 32:76–88.
- Foote RH. (1999). Fertility of rabbit sperm exposed in vitro to cadmium and lead. Reprod Toxicol 13:443–9.
- Kozissnik B, Bohorquez AC, Dobson J, et al. (2013). Magnetic fluid hyperthermia: advances, challenges, and opportunity. Int J Hyperthermia 29:706–14.
- Wang J, Chen Y, Chen BA, et al. (2010). Pharmacokinetic parameters and tissue distribution of magnetic Fe3O4 nanoparticles in mice. Int J Nanomedicine 5:861–6.
- Hussain SM, Braydich-Stolle LK, Schrand AM, et al. (2009). Toxicity evaluation for safe use of nanomaterials: recent achievements and technical challenges. Adv Mater 21:1549–59.
- Lan Z, Yang WX. (2012). Nanoparticles and spermatogenesis: how do nanoparticles affect spermatogenesis and penetrate the blood–testis barrier. Nanomedicine 7:579–96.
- McAuliffe ME, Perry MJ. (2007). Are nanoparticles potential male reproductive toxicants? A literature review. Nanotoxicology 1:204–10.
- Mathias FT, Romano RM, Kizys MM, et al. (2015). Daily exposure to silver nanoparticles during prepubertal development decreases adult sperm and reproductive parameters. Nanotoxicology 9:64–70.
- Lafuente D, Garcia T, Blanco J, et al. (2016). Effects of oral exposure to silver nanoparticles on the sperm of rats. Reprod Toxicol 60:133–9.
- Gromadzka-Ostrowska J, Dziendzikowska K, Lankoff A, et al. (2012). Silver nanoparticles effects on epididymal sperm in rats. Toxicol Lett 214:251–8.
- Hussein MM, Ali HA, Saadeldin IM, et al. (2016). Querectin alleviates zinc oxide nanoreprotoxicity in male albino rats. J Biochem Mol Toxicol 30:489–96.
- Talebi AR, Khorsandi L, Moridian M. (2013). The effect of zinc oxide nanoparticles on mouse spermatogenesis. J Assist Reprod Genet 30:1203–9.
- Farini VL, Camaño CV, Ybarra G, et al. (2016). Improvement of bovine semen quality by removal of membrane-damaged sperm cells with DNA aptamers and magnetic nanoparticles. J Biotechnol 229:33–41.
- Odhiambo JF, DeJarnette JM, Geary TW, et al. (2014). Increased conception rates in beef cattle inseminated with nanopurified bull semen. Biol Reprod 91:97.
- Feugang J, Liao S, Crenshaw M, et al. (2015). Lectin-functionalized magnetic iron oxide nanoparticles for reproductive improvement. JFIV Reprod Med Genet 3:17–9.
- Barchanski A, Taylor U, Klein S, et al. (2011). Golden perspective: application of laser-generated gold nanoparticle conjugates in reproductive biology. Reprod Domest Anim 46:42–52.
- Rath D, Barcikowski S, de Graaf S, et al. (2013). Sex selection of sperm in farm animals: status report and developmental prospects. Reproduction 145:R15–30.
- Kim TS, Lee SH, Gang GT, et al. (2010). Exogenous DNA uptake of boar spermatozoa by a magnetic nanoparticle vector system. Reprod Domest Anim 45:201–6.
- Braydich-Stolle L, Hussain S, Schlager JJ, et al. (2005). In vitro cytotoxicity of nanoparticles in mammalian germline stem cells. Toxicol Sci 88:412–19.
- Wiwantitkit V, Sereemaspum A, Rojanathanes R. (2009). Effect of gold nanoparticles on spermatozoa: the first world report. Fertil Steril 91:e7–8.
- Moretti E, Terzuoli G, Renieri T, et al. (2012). In vitro effect of gold and silver nanoparticles on human spermatozoa. Andrologia 45:392–6.
- Zakhidov ST, Marshak TL, Malolina EA, et al. (2010). Gold nanoparticles disturb nuclear chromatin decondensation in mouse sperm in vitro. Biochemistry 4:293–6.
- Taylor U, Barchanski A, Petersen S, et al. (2014). Gold nanoparticles interfere with sperm functionality by membrane adsorption without penetration. Nanotoxicology 8:118–27.
- Zakhidov ST, Pavliuchenkova SM, Samoĭlov AV, et al. (2013). Bovine sperm chromatin is not protected from the effects ultrasmall gold nanoparticles. Izv Akad Nauk Ser Biol 16:645–52.
- Makhluf SBD, Qasem R, Rubinstein S, et al. (2006). Loading magnetic nanoparticles into sperm cells does not affect their functionality. Langmuir 22:9480–2.
- Mahmoudi M, Simchi A, Milani AS, et al. (2009). Cell toxicity of superparamagnetic iron oxide nanoparticles. J Colloid Interface Sci 336:510–8.
- Valois CRA, Braz JM, Nunes ES, et al. (2010). The effect of DMSA-functionalized magnetic nanoparticles on transendothelial migration of monocytes in the murine lung via β2 integrin-dependent pathway. Biomaterials 21:366–74.
- Machado GM, Carvalho JO, Siqueira Filho E, et al. (2009). Effect of Percoll volume, duration and force of centrifugation, on in vitro production and sex ratio of bovine embryos. Theriogenology 71:1289–97.
- Ema M, Kobayashi N, Naya M, et al. (2010). Reproductive and developmental toxicity studies of manufactured nanomaterials. Reprod Toxicol 30:343–52.
- Vasquez ES, Feugang JM, Willard ST, et al. (2016). Bioluminescent magnetic nanoparticles as potential imaging agents for mammalian spermatozoa. J Nanobiotechnology 17:14–20.
- Nasri S, Rezai-Zarchi S, Kerishchi P, et al. (2015). The effect of iron oxide nanoparticles on sperm numbers and mobility in male mice. Zahedan J Res Med Sci 17:0–10.
- Dimitropoulos R. (1967). La signification du test de la thermo résitance dans l' appreciation de la valeur fécondante du sperm econgelé. Ann Méd Vét 4:215–24.
- Gillan L, Kroetsch T, Chis Maxwell WM, et al. (2008). Assessment of in vitro sperm characteristics in relation to fertility in dairy bulls. Anim Reprod Sci 103:201–14.
- Mortimer ST. (1997). A critical review of the physiological importance and analysis of sperm movement in mammals. Hum Reprod Update 3:403–139.
- Suarez SS, Osman RA. (1987). Initiation of hyperactivated flagellar bending in mouse sperm within the female reproductive tract. Biol Reprod 36:1191–8.
- Henkel R, Müller C, Miska W, et al. (1993). Determination of the acrosome reaction in human spermatozoa is predictive of fertilization in vitro. Hum Reprod 8:2128–32.
- Pisanic TR, Blackwell JD, Shubayev VI, et al. (2007). Nanotoxicity of iron oxide nanoparticle internalization in growing neurons. Biomaterials 28:2572–81.
- Auffan M, Decome L, Rose J, et al. (2006). In vitro interactions between DMSA-coated maghemite nanoparticles and human fibroblasts: a physicochemical and cyto-genotoxical study. Environ Sci Technol 40:4367–73.
- Auffan M, Rose J, Wiesner MR, et al. (2009). Chemical stability of metallic nanoparticles: a parameter controlling their potential cellular toxicity in vitro. Environ Pollut 157:1127–33.
- Gupta AK, Gupta M. (2005). Cytotoxicity suppression and cellular uptake enhancement of surface modified magnetic nanoparticles. Biomaterials 26:3995–4021.