Abstract
Introduction: Cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) have been found to prolong survival in patients with peritoneal disease but is associated with significant morbidity. We evaluate the perioperative complications and the association with the chemotherapy agent used for HIPEC.
Methods: Retrospective analysis of a prospectively collected database of CRS–HIPEC cases between April 2001 and February 2016 was performed. Patients were stratified by the chemotherapy used, and perioperative complications were compared.
Results: Out of 214 CRS–HIPEC cases, 113 procedures used Mitomycin-C(MMC), 92 used cisplatin, 8 used oxaliplatin and the HIPEC regimen for one procedure was not recorded and excluded. 94 patients (44%) suffered low-grade complications (grade I–II), and 49 patients (23%) suffered high-grade complications (grade III–V). The frequency of low-grade complications for the cisplain, oxaliplatin and MMC groups were 49%, 50% and 40%, respectively, whereas that of high-grade complications were 24%, 50% and 20%, respectively. HIPEC with platinum agents was associated with a higher rate of acute renal impairment (ARI) compared to MMC (32% and 62% for cisplatin and oxaliplatin vs. 5.6% for MMC), whereas grade IV ARI requiring dialysis occurred only in the cisplatin group (5.6%). HIPEC with oxaliplatin was associated with higher rates of post-operative bleeding (25% vs. 1.1% and 0.88%). Rates of other complications did not differ significantly between the groups receiving different HIPEC regimens.
Conclusions: The overall complication rates do not significantly differ after HIPEC with MMC and platinum based agents. Renal impairment tends to be more common and of greater severity when a platinum agent is used, whereas oxaliplatin is associated with significant post-operative bleeding.
Keywords:
Introduction
Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy (CRS/HIPEC) have been shown to achieve good long-term survival in treating peritoneal metastases from colorectal [Citation1], ovarian [Citation2], appendiceal [Citation3], mesothelioma [Citation4] and primary peritoneal neoplasms. This increase in survival times may be at the expense of considerable perioperative morbidity and mortality although patients are able to return to baseline or improved quality of life by 6–12 months [Citation5].
Major morbidity in CRS/HIPEC varies widely and has been reported to be between 12% and 67.6% [Citation6,Citation7], with a median of 31% reported from a review of several studies in the literature [Citation8]. Rates of perioperative mortality vary from 0% to 9% [Citation9,Citation10], with a median of 4% [Citation8]. The high morbidity rate has been attributed to the steep learning curve associated with the procedure, although in several high volume centres, the complication rate remains high and is comparable to major gastrointestinal surgery, as reported in a recent systemic review [Citation11].
CRS aims to remove all macroscopic disease [Citation12], and is combined with HIPEC, which targets the microscopic disease [Citation13,Citation14]. Most centres use mitomycin C (MMC) or oxaliplatin for colorectal and appendiceal peritoneal metastases, and cisplatin for ovarian and gastric neoplasms and mesotheliomas [Citation11]. The high complication rates quoted are often a function of the extensive surgery, coupled with the complications associated with the HIPEC that may have an additive effect.
Commonly reported complications with HIPEC include nephrotoxicity and neutropenia. The former is particularly seen with the use of cisplatin [Citation12–15]. In addition, the use of cisplatin as compared to MMC was associated with acute renal impairment (ARI) in a reported multivariate model [Citation15]. The incidence of neutropenia in patients who have undergone CRS/HIPEC with MMC has been reported to be as high as 40% [Citation16,Citation17], but a direct comparison with cisplatin is lacking. Evidence suggests that the incidence of neutropenia is associated with the degree of systemic redistribution of MMC [Citation16], which has previously been related to extent of peritonectomy [Citation17].
In this article, we aim to investigate if the agent used for HIPEC affects the complication rates of patients undergoing CRS/HIPEC. In doing so, we hope to be able to address the specific drug-related morbidity, and modify our approach in the peri-operative management for at-risk patients, in order to reduce the morbidity of CRS/HIPEC. In addition, the knowledge gained from this study would enable specific treatment-directed pre-operative counselling of patients.
Methods
A prospectively maintained, Institutional Review Board-approved database of all patients who underwent CRS/HIPEC for peritoneal-based malignancies at a single institution from April 2001 through to February 2016 was retrospectively reviewed in this SingHealth Ethics Board-approved study. Demographics including age, gender, race and tumour type were included in the database and are reported.
Patient selection
Patients considered for CRS/HIPEC had to be of Eastern Cooperative Group (ECOG) performance status 0 or 1, with no distant metastases. All patients were recommended for CRS/HIPEC after evaluation in a multidisciplinary tumour board. The extent of disease of the abdomen and pelvis was examined on computed tomography (CT) or magnetic resonance imaging (MRI) scans, and the absence of extra-abdominal disease was determined either via CT scans of the thorax or positron emission tomography (PET)-CT scans.
CRS and HIPEC
CRS/HIPEC proceeded according to previously published technique [Citation18]. The extent of disease was documented according to the peritoneal cancer index (PCI) [Citation12]. Complete cytoreduction was attempted whenever possible, and the extent of cytoreduction was recorded by the completeness of cytoreduction (CC) score [Citation14]. Chemotherapy was infused via a hyperthermia pump (Belmont) into a closed abdomen at a target temperature of 41 °C for 60 min. The chemotherapeutic agent used was determined by the patient’s medical oncologist on the basis of malignancy type, typically MMC for appendiceal and colorectal cancer (CRC), and cisplatin for ovarian cancer and mesothelioma. In our institution, MMC was given at a dose of 10 mg/body surface area (BSA) and cisplatin was given at a dose of 40 mg/BSA. Oxalipatin was used in a small number of patients (n = 8) at the initial period of the institution’s peritoneal malignancy programme (PMP), but ceased after a HIPEC protocol with MMC and cisplatin was established.
Post-operative care and evaluation of complications
Post-operatively, patients were transferred to either the surgical intensive care unit (SICU) or the high-dependency unit, with drains and invasive monitoring lines in situ. Prior to December 2012, all patients were planned for early post-operative intra-peritoneal chemotherapy (EPIC) with 5-FU or paclitaxel for gastrointestinal and ovarian cancers, respectively. The receipt and duration of EPIC (0–5 days) depended on the absence or presence of surgical complications, and haematological and biochemical derangements. However, EPIC was discontinued from December 2012 onwards as there was insufficient evidence to support the efficacy of EPIC, and some of our patients suffered resultant morbidity from persistent intra-abdominal collections. The remaining 110 patients in this cohort only received HIPEC.
Serum full blood counts, coagulation panel and urea and creatinine were monitored on post-operative days 1, 3 and 5. Appropriate transfusion of blood and blood products was administered at the discretion of the primary physician after correlation with the risk of further bleeding. Retrospective data were collected for absolute neutrophil levels measured within the first 2 weeks after CRS/HIPEC, neutropenia was defined as levels mild (<1.5 × 109/L), moderate (< 1.0 × 109/L) or severe (<0.5 × 109/L). If the renal parameters were elevated or showed an uprising trend, appropriate treatment including hydration and renal replacement therapy were initiated. Daily renal panels were done for such patients until renal function normalised or until discharge. Renal impairment was graded according to the NCI-CTCAE version 3.0 criteria [Citation19]. Grade 1 includes patients with creatinine 1.5–2 times above baseline, grade 2 includes patients with creatinine 2–3 times above baseline, grade 3 includes patients with creatinine >3 × baseline, whereas grade 4 includes patients who required dialysis (renal failure).
Documentation of other peri-operative complications and hospitalisation duration was also performed. Post-operative complications were categorised according to the Clavien-Dindo classification, with major complications defined as Clavien III and IV [Citation20].
All patients were followed up at the outpatient unit at 1 week after discharge from the hospital, 3-monthly for 1 year, and 6-monthly subsequently, with imaging and biochemical studies. Patients were also followed up by their medical oncologists and received adjuvant systemic chemotherapy as necessary.
Study parameters
For purposes of comparison, patients were divided into three groups based on the chemotherapy agent used for HIPEC (MMC, cisplatin or oxaliplatin). Thirteen patients underwent more than one CRS/HIPEC procedure during the study period. Data were analysed at the CRS/HIPEC level in order to increase the generalisability of results to include patients who undergo multiple procedures. In these instances, listed patient characteristics are representative of the patient’s state at the time of each included operation.
Statistical analysis
Continuous variables were expressed as mean ± 1 SD (normally distributed data) or medians with interquartile ranges (nonparametric data) and categorical data as proportions throughout the article.
Clinical variables or surgical outcomes and grouping status were compared using chi-square, Fisher’s exact or independent-samples t-tests, as appropriate. Multiple testing correction was performed using the Holm–Bonferroni method. p < 0.05 was considered statistically significant. All statistical analyses were performed using R 3.2.3 [Citation21].
Results
Baseline characteristics of study population
Between April 2001 and February 2016, 201 patients underwent a total of 214 CRS–HIPEC ( and ). Eleven patients underwent CRS–HIPEC twice and an additional one patient had CRS–HIPEC thrice. 113 (53%) procedures used MMC, 92 (43%) used cisplatin, 8 (4%) used oxaliplatin, and a final procedure did not have the HIPEC regimen recorded and was not included in this study.
Figure 1. Flow diagram depicting distribution of study population by histology and chemotherapy administered.
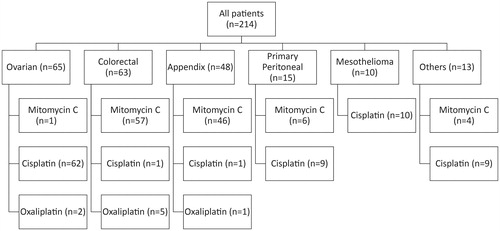
Table 1. Demographics.
The median follow-up of all patients was 18 months (IQR: 6–27 months).
CRS/HIPEC details
There were minimal differences in CRS/HIPEC procedures between the three groups, with significant differences reflecting the primary tumour origin ( and ). Information on the HIPEC regimens is included in .
Table 2. Details of CRS/HIPEC/EPIC regime.
Table 3. Grade of perioperative complications.
Perioperative complications
There was no overall difference in the MMC, cisplatin and oxalipatin groups respectively in terms of intraoperative blood loss, intraoperative blood transfusions, post-operative blood transfusions, SICU stay or total hospital stay ().
Overall, 94 patients suffered low-grade complications (grade I–II), and 49 patients suffered high-grade complications (grade III–V). In the cisplatin group, there were 45 (49%) low-grade and 22 (24%) high-grade complications, whereas in the MMC group, there were 45 (40%) low-grade and 23 (20%) high-grade complications, and in the oxaliplatin group 4 (50%) low grade, and 4 (50%) high grade ().
Within the MCC group, the most common complications were that of respiratory (17%), intra-abdominal collections (8.8%), anastomotic leak (4.4%), wound infection (7.2%), ileus (6.2%) and ARI (5.6%). With the cisplatin group, the most common complications were that of ARI (36%), respiratory (14%), intra-abdominal collections (11%) and wound infections (5.4%). The oxaliplatin group of patients had the most respiratory complications (75%; p < 0.01), followed by ARI (62%), bleeding (25%), wound infection (12%) and intra-abdominal collections (12%). However the majority of these complications did not significantly differ (). The significant differences were seen in the rate of respiratory and bleeding complications, with the use of oxaliplatin compared to cisplatin and MMC, and in the rates of ARI with the use of platinum agents compared to MMC (36% and 62% for cisplatin and oxaliplatin vs. 5.6% for MMC). Grade IV ARI requiring dialysis was only seen in the cisplatin group (5.6%). Rates of other complications including neutropenia (1%, 0%, 2%) did not differ significantly between HIPEC regimen ().
Table 4. Details of perioperative complications.
Discussion
Despite multiple studies that show that CRS/HIPEC confers survival benefits for selected patients with peritoneal metastases, many physicians are still reluctant to consider the procedure for their patients in view of its associated morbidity. In particular, oncologists are reluctant to refer their patients with peritoneal disease for consideration of the procedure because of the fear that patients may be significantly debilitated after the procedure that may preclude adjuvant chemotherapy. In particular, patients who develop long-standing renal impairment post-CRS/HIPEC may have limited adjuvant and future chemotherapeutic options. These are all valid considerations and are addressed in this paper.
In line with standard practice by our medical oncologists, patients treated with MMC were typically had appendiceal (41%) or colorectal (50%) primary cancers, whilst the majority of patients treated with cisplatin were ovarian cancer primaries (67%), primary peritoneal cancers (9.8%) and peritoneal mesotheliomas (11%). The majority of the 8 patients treated with oxaliplatin had CRC (62%) as their primary cancer. Baseline demographics did not differ between the groups, but patients treated with MMC tended to have more extensive disease as indicated by the higher PCI score (15 vs. 10 vs. 11, p < 0.01), longer duration of surgery, and a higher number of procedures undergone per patient (median of 3 vs. 2 vs. 2, p = 0.02). This finding may be inflated because conversely, patients in the cisplatin group (ovarian and primary peritoneal primaries) tended to have undergone neoadjuvant chemotherapy before CRS/HIPEC, hence down-staging the disease before surgery. However, this information was not available for analysis.
There was no overall difference in the MMC, cisplatin and oxalipatin groups respectively in terms of low-grade morbidity (40% vs. 49% vs. 50%), SICU stay (1 vs. 1 vs. 1 day) or total hospital stay (14 vs. 14 vs. 18 days). An increased rate of high-grade morbidity (50% vs. 20, 24%) in the oxaliplatin group may possibly be due to the majority (88%) of these patients undergoing CRS/HIPEC during the start of the PMP and before the collective experience and the passage of the learning curve allowed implementation of strategies to reduce complications post-CRS/HIPEC. These strategies included the routine intra-operative insertion of chest tubes for patients undergoing sub-diaphragmatic stripping to prevent the development of post-operative pleural effusions, and imbrication of the stomach edge when a complete omentectomy, including ligation of the gastroepiploic vessels close to the edge of the greater curve of the stomach was performed, for prevention of gastric necrosis and perforation. Another major reason for the increased morbidity is likely related to the increased bleeding seen with oxaliplatin. This has previously been reported [Citation22].
There was an increased rate of ARI observed in the patients who received platinum-based agents (38% and 62% for the cisplatin and oxaliplatin groups) when compared to the MMC group (6%). However, grade 4 ARI requiring dialysis only occurred only in the cisplatin group (5.6%), and not the MMC or oxaliplatin groups. The observed association between the use of platinum based agents and ARI has previously been reported [Citation23,Citation24]. In the paper from MD Anderson Cancer Centre, patients received HIPEC with cisplatin after CRS for sarcomatosis, with 25% of patients developing ARI, but this was reduced to 0% when pre-operative hydration at greater than maintenance rate and intravenous sodium thiosulphate (STS) was administered simultaneously with HIPEC [Citation25]. In a separate paper by the French group, Amifostine was administered to reduce the incidence of ARI, and seemed to show benefit in reducing ARI in patients receiving HIPEC with cisplatin [Citation26].
Another significant morbidity associated with HIPEC is neutropenia [Citation16,Citation27]. However, this previously reported association of MMC with neutropenia was not observed in our study. This may be due to the more conservative dose of MMC used in our centre (10 mg/BSA) compared to that reported previously (32.5 mg/BSA) [Citation16].
We have recently reported on our group of ovarian cancer patients who underwent CRS and received HIPEC with cisplatin [Citation28]. Risk factors for AKI were found to be age, baseline creatinine, baseline estimated glomerular filtration rate, pre-operative albumin, number of cycles of pre-operative carboplatin, the time interval between pre-operative chemotherapy and CRS/HIPEC, and the volume of blood transfusion. This knowledge will allow for the pre-operative optimisation of renal function, tailoring of chemotherapy regimen, closer peri-operative management and careful fluid management, which we believe are measures that will aid in reducing the ARI rates.
While MMC is associated with a low risk of ARI (5.6%), the complications seen in this group of patients are probably associated with the extent of disease necessitating more extensive surgery and a longer operation times. This is reflected in the higher rates of anastomotic leak (4.4%) and ileus (6.2%), as compared to the patients who received platinum agents. In these patients with extensive disease and an estimated long surgical duration, pre-operative down-staging of disease with neoadjuvant chemotherapy may be considered whilst intra-operative factors that are important include minimisation of blood loss, consideration of a defunctioning stoma in cases of low rectal anastomoses, and inclusion of the anaesthetic team in the pre-operative briefing to ensure careful fluid management.
We acknowledge that the small sample size, and different primary malignancies in our group of CRS/HIPEC patients is the main limitation of the study. However, we attempted to account for this by using multiple testing corrections. The retrospective nature of this paper also has its inherent biases, but the data utilised were collected in a prospective fashion in order to reduce these biases.
Conclusions
The knowledge of regimen-specific complications is imperative to improve outcomes for patients undergoing CRS/HIPEC and can be reduced by the implementation of directed strategies to reduce morbidity.
Disclosure statement
All the authors have participated in the research design, analysing of data and writing of the paper. The authors have reviewed the manuscript and approved it for submission. All authors declare no conflict of interest or receive any funding for research.
References
- Kuijpers AM, Mirck B, Aalbers AG, et al. (2013). Cytoreduction and HIPEC in The Netherlands: nationwide long-term outcome following the dutch protocol. Ann Surg Oncol 20:4224–30.
- Spiliotis J, Halkia E, Lianos E, et al. (2015). Cytoreductive surgery and HIPEC in recurrent epithelial ovarian cancer: a prospective randomized phase III study. Ann Surg Oncol 22:1570–5.
- Ansari N, Chandrakumaran K, Dayal S, et al. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in 1000 patients with perforated appendiceal epithelial tumours. Eur J Surg Oncol 42:1035–41.
- Yan TD, Deraco M, Baratti D, et al. (2009). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for malignant peritoneal mesothelioma: multi-institutional experience. J Clin Oncol 27:6237–42.
- Chia CS, Tan GHC, Lim C, et al. (2016). Prospective quality of life study for colorectal cancer patients with peritoneal carcinomatosis undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg Oncol 23:2905–13.
- Murphy EM, Sexton R, Moran BJ. (2007). Early results of surgery in 123 patients with Pseudomyxoma peritonei from a perforated appendiceal neoplasm. Dis Colon Rectum 50:37–42.
- Elias D, Honoré C, Ciuchendéa R, et al. (2008). Peritoneal pseudomyxoma: results of a systematic policy of complete cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Br J Surg 95:1164–71.
- Mohamed F, Moran BJ. (2009). Morbidity and mortality with cytoreductive surgery and intraperitoneal chemotherapy: the importance of a learning curve. Cancer J 15:196–9.
- Simkens GA, van Oudheusden TR, Luyer MD, et al. (2015). Serious postoperative complications affect early recurrence after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis. Ann Surg Oncol 22:2656–62.
- Newton AD, Bartlett EK, Karakousis GC, et al. (2016). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy: a review of factors contributing to morbidity and mortality. J Gastrointest Oncol 7:99–111.
- Chua TC, Yan TD, Saxena A, Morris DL. (2009). Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure? A systematic review of morbidity and mortality. Ann Surg 249:900–7.
- Sugarbaker PH. (2007). Peritonectomy procedures. Cancer Treat Res 134:247–64.
- Witkamp AJ, de Bree E, Van Goethem R, Zoetmulder FA. (2001). Rationale and techniques of intra-operative hyperthermic intraperitoneal chemotherapy. Cancer Treat Rev 27:365–74.
- Jacquet P, Sugarbaker PH. (1996). Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–74.
- Kusamura S, Baratti D, Younan R, et al. (2007). Impact of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy on systemic toxicity. Ann Surg Oncol 14:2550–8.
- Kemmel V, Mercoli H-A, Meyer N, et al. (2015). Mitomycin C pharmacokinetics as predictor of severe neutropenia in hyperthermic intraperitoneal therapy. Ann Surg Oncol 22:873–9.
- Van der Speeten K, Stuart OA, Chang D, et al. (2011). Changes induced by surgical and clinical factors in the pharmacology of intraperitoneal mitomycin C in 145 patients with peritoneal carcinomatosis. Cancer Chemother Pharmacol 68:147–56.
- Teo MCC, Tan GHC, Tham CK, et al. (2013). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy in Asian patients: 100 consecutive patients in a single institution. Ann Surg Oncol 20:2968–74.
- DCTD, NCI, NIH, DHHS. (2006). Cancer Therapy Evaluation Program, Common Terminology Criteria for Adverse Events, Version 3.0.
- Dindo D, Demartines N, Clavien P-A. (2004). Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–13.
- R Core Team (2016). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing. Available from: http://www.R-project.org/.
- Charrier G, Passot J, Peron, et al. (2016). Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy with oxaliplatin increases the risk of postoperative hemorrhagic complications: analysis of predictive factors. Ann Surg Oncol 2:2315–22.
- Hakeam AH, Breakiet M, Azzam A, et al. (2014). The incidence of cisplatin nephrotoxicity post hyperthermic intraperitoneal chemotherapy (HIPEC) and cytoreductive surgery. Ren Fail 36:1486–91.
- Hayes-Jordan A, Green H, Ludwig J, Anderson P. (2012). Toxicity of hyperthermic intraperitoneal chemotherapy (HIPEC) in pediatric patients with sarcomatosis/carcinomatosis: early experience and phase 1 results. Pediatr Blood Cancer 59:395–7.
- Green H. (2014 Jun). Perioperative renal protective treatment avoids renal toxicity in pediatric and adult patients undergoing HIPEC with cisplatin. J Pediatr Oncol 2:10–16.
- Bouhadjari N, Gabato W, Calabrese D, et al. (2016). Hyperthermic intraperitoneal chemotherapy with cisplatin: amifostine prevents acute severe renal impairment. Eur J Surg Oncol 42:219–23.
- Lambert LA, Armstrong TS, Lee JJ, et al. (2009). Incidence, risk factors, and impact of severe neutropenia after hyperthermic intraperitoneal mitomycin C. Ann Surg Oncol 16:2181–7.
- Sin E, Chia C, Tan G, et al. (2017). Acute kidney injury in ovarian cancer patients undergoing cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Int J Hyperthermia 33:690--95.
