Abstract
Purpose: Capillary regression is commonly observed in response to disuse muscle atrophy. Heat stress is known to alleviate muscle atrophy, while effect of heat exposure on capillary adaptation following disuse atrophy is not defined. Here, we examined the effect of heat treatment on capillarisation and the associated signalling in slow-oxidative soleus and fast-glycolytic plantaris muscles following Achilles tendon ablation (tenotomy).
Materials and methods: Male Wistar rats were assigned into control (CON), control with heat stress (CON + HEAT), tenotomy (TEN) and tenotomy with heat stress (TEN + HEAT) groups. Tenotomy was induced for 8 days in TEN and TEN + HEAT groups. Heat stress was maintained at 40.5–41.5 °C, 30 min for 7 days.
Results: Tenotomy resulted in reduction of capillary-to-fibre ratio, decreased VEGFR-2 and increased TSP-1 in soleus muscle, whereas VEGF protein expression remained unaffected. Tenotomy had no effect on capillary distribution and angiogenic signalling in plantaris muscle. These results were concomitant with larger reduction of cross-sectional area (CSA) in MHC type I and II myofibres of soleus compared to plantaris muscles. Interestingly, heat stress increased VEGFR-2 and attenuated TSP-1 protein expression in tenotomised soleus, but not plantaris muscles. Additionally, CSA of both type I and type II myofibres was greater in tenotomised soleus than plantaris muscles after heat treatment.
Conclusions: Heat stress mitigated effect of tenotomy-induced capillary regression in a fibre-type-specific response, in part, by shifting the balance between angiogenic and angiostatic regulators. These results suggest beneficial effect of heat treatment for maintaining microcirculation in disuse muscle atrophy.
Introduction
Microcirculation of skeletal muscle plays an important role in the delivery of oxygen and substrates to cells as well as removal of metabolites by diffusion. Skeletal muscle capillary network adapts in response to alteration of neuromuscular activity in order to meet tissue functional and metabolic demands. During adult life, angiogenesis is initiated in response to tissue repair or pathologic conditions [Citation1]. Several studies have shown that endurance training induces muscle angiogenesis, while disuse muscle atrophy leads to capillary regression [Citation2]. Regarding skeletal muscle atrophy, capillarisation impairment results in inadequate supply of oxygen to the muscle, reduced aerobic capacity to generate ATP, and consequently leading to physical disability and decreased quality of life.
Capillary regression has been established in several muscle atrophy models [Citation3,Citation4]. In addition, alterations in capillarity are fibre type specific [Citation5]. Accordingly, the capillary-to-fibre ratio (C/F ratio) of rat slow-twitch (oxidative, myosin heavy chain type I (MHC I)) soleus muscle following 5 and 9 days of hindlimb unloading-induced muscle atrophy decreased by 17% and 23%, respectively [Citation5]. On the other hand, the C/F ratio started to decrease at 10 days and decreased by 52% in mouse fast-twitch (glycolytic, MHC type II) gastrocnemius muscle after 30-day denervation [Citation3].
The balance of muscle capillarisation is tightly regulated by pro-angiogenic and anti-angiogenic factors [Citation1]. Vascular endothelial growth factor (VEGF) has been proposed to be the most potent angiogenic regulator that increases vascular permeability and angiogenesis [Citation6]. Many studies have demonstrated that VEGF is capable of initiating angiogenic signal in skeletal muscle [Citation1,Citation7]. For example, in vivo overexpression of VEGF by muscle-derived stem cells transplanted into dystrophic skeletal muscle increased angiogenesis and enhanced muscle regeneration [Citation7]. Inversely, isolated satellite cells from ageing and muscular dystrophic mice showed lower VEGF expression and reduced capacity to promote angiogenesis in vitro [Citation8]. Although VEGF comprises several isoforms (VEGF-A to VEGF-D), VEGF-A plays a more pivotal role in skeletal muscle angiogenesis [Citation9]. The pro-angiogenic effects of VEGF-A are primarily mediated through VEGF receptor-2 (VEGFR-2, KDR/Flk-1), which is predominantly expressed on endothelial cells, leading to regulation of endothelial cell survival, proliferation and migration, as well as production of new vessels from the existing framework [Citation10].
Thrombospondin-1 (TSP-1) is an anti-angiogenic regulator and is a matrix glycoprotein produced by a variety of cells including endothelial cells that appears to be associated with capillary regression [Citation11]. The binding of TSP-1 through its CD36 receptor can inhibit the activation of VEGF-A signalling cascade [Citation11]. Previously, administration of the TSP-1 mimetic (ABT-510) resulted in diminished skeletal muscle capillarity [Citation12]. Moreover, an impairment in TSP-1 expression leads to delayed wound healing process [Citation13]. Accumulating lines of evidence demonstrate that TSP-1 plays a crucial role in the regulation of the angiogenic response during physiological and pathophysiological conditions [Citation12].
Currently, vascular targeted therapy has been prescribed to rehabilitate skeletal muscle abnormalities, including muscular dystrophy and muscle atrophy. Although several pharmacological agents such as tadalafil and sildenafil (phosphodiesterase 5 inhibitor) have been shown to increase blood flow to muscle tissue [Citation14,Citation15], these drugs may have profound side effects. Therefore, non-pharmacological therapeutic strategies should be considered as an alternative approach to handle these clinical problems. Interestingly, previous reports have demonstrated that an induction of heat-shock proteins (HSPs) by heat stress could regulate endothelial cells and vascularisation in various tissue pathologies [Citation16,Citation17]. In addition, heat stress preserved muscle mass in tenotomised slow-twitch soleus to a greater extent than fast-twitch plantaris muscles [Citation18]. Nevertheless, the effect of heat stress on capillary adaptation in disuse muscle atrophy in both muscle fibre types is not well defined.
Additionally, it has been shown that the alteration of fibre-type composition, i.e., slow-to-fast transformation, during disuse atrophy occurs leading to increased muscle fatigability [Citation19]. In contrast, increased oxidative capacity through greater slow muscle fibre content has been shown to alleviate muscle dystrophy and muscle dysfunction in insulin resistance progression [Citation20]. Previously, endurance exercise-induced muscle angiogenesis played a permissive role in a slow-oxidative fibre-type promotion [Citation21]. However, it is still unclear whether heat stress could modulate fibre-type composition in soleus and plantaris muscles following 8 days of tenotomy.
Taken together, the aims of this study were to evaluate the effects of heat stress on the adaptation of capillary content, angiogenic regulators and fibre-type composition after Achilles tendon transection in rat soleus (tenotomised soleus) and plantaris (tenotomised plantaris) muscles. Both muscle types were chosen in this study because soleus represents the predominantly slow-twitch and oxidative postural muscles, whereas plantaris muscle represents predominantly fast-twitch and glycolytic muscles [Citation22]. Since tendon rupture is commonly encountered following trauma or degenerative musculoskeletal diseases [Citation23], we chose this animal model in the present study. Here, we hypothesised that heat stress could alleviate capillary regression of tenotomised muscle in different angiogenic responses between soleus and plantaris muscles. In addition, heat stress-induced capillarisation could be associated with the promotion of slow-oxidative fibre in disuse muscle atrophy.
Materials and methods
Animals
Male Wistar rats (10 weeks old) were obtained from the National Laboratory Animal Centre of Thailand, Salaya, Nakhon Pathom. All animal procedures were approved by the animal care and use guidelines established by the Ethics Committee on the Use of Experimental Animals, Faculty of Science, Mahidol University (Protocol no. MUSC58-005-320). After 1 week of acclimatisation, four groups of rats (n = 6 in each group) were randomly allocated into a control (CON), a control with heat stress (CON + HEAT), a tenotomy without heat stress (TEN) and a tenotomy with heat stress (TEN + HEAT) groups. All rats were pair-fed to eliminate confounding factors of food intake between groups. Heat stress protocol was conducted 24 h before and after tenotomy and continued for 5 consecutive days as previously described [Citation18]. Rats were terminated 48 h after exposure to the last heat stress. Thus, the animals were subjected to tenotomy for 8 days and heat stress for 7 days.
Tenotomy
Tenotomy was performed by Achilles tendon transection as previously described [Citation18]. All surgical procedures were operated under 5% isoflurane gas–oxygen mixture using SurgiVet® Classic T3TM vaporiser (VCT302; Smith medical, Waukesha, WI) and aseptic conditions. An incision in the skin from the calf down to the Achilles tendon and calcaneus bone on the right hindlimb was made. Subsequently, the distal part of the Achilles tendon was transected 3 mm in length above the insertion to the calcaneus bone without damaging the nerve and blood supply. The skin was then sutured and rats were allowed to return to the cages after they regained consciousness.
Heat treatment
Heat stress was achieved according to the protocol described previously by Selsby et al. [Citation24]. In brief, rats were anaesthetised with 5% isoflurane gas–oxygen mixture and maintained with 1.5–2.0% concentration. The core body temperatures of all rats were monitored throughout the experiment using temperature probes (YSI401; Yellow Spring, OH) inserted 4 cm into the rectum. For heat treatment, pre-warmed thermal blanket was wrapped around the body leaving the head and tail unwrapped. The core temperature was recorded every 5 min and maintained at 40.5–41.5 °C for 30 min. After removing the thermal blanket, the core temperature was further monitored until it returned to 37 °C. To prevent dehydration, each rat was orally administrated 10–15 ml of water after recovery from anaesthesia [Citation25]. None of the rats died during and after heating in the present study. Rats not receiving heat treatment (CON and TEN groups) were operated identically and their core temperatures were maintained at 37 °C.
Sample collection
Under anaesthesia using pentobarbital (100 mg/kg, i.p.), soleus and plantaris muscles were harvested, and thereafter the rats were sacrificed by cardiectomy. After trimming of excess connective tissue, muscle samples were weighted and cut in the mid-belly of the whole fibre to obtain transversal cross-sections. The upper portion was then frozen in liquid nitrogen and transferred to a −80 °C freezer for immunoblotting analysis (n = 6/group). The lower portion was placed into optimal cutting temperature Tissue-Tek® (4583; Electron Microscopy Sciences, Hatfield, PA), frozen in isopentane (M32631; Sigma, St Louis, MO) which was pre-cooled by liquid nitrogen and kept at −80 °C for immunofluorescence analysis (n = 3–5/group).
Western blot analysis
Muscle samples were homogenised (approximately 50 mg, 1:20 w/v) using ice-cold buffer containing 50 mM Tris–HCl pH 7.5, 5 mM EDTA, 1 mM PMSF, a protease inhibitor cocktail (P8340; Sigma), a phosphatase inhibitor cocktail (524625; Millipore, Temecula, CA). Muscle homogenates were centrifuged at 10 000 g for 10 min (4 °C). The supernatant was collected and immediately stored at −80 °C, and thereafter protein concentration was quantified in triplicate using bicinchoninic acid (BCA) assay kit (Thermo Scientific; Rockford, IL) as previously described [Citation18].
Denatured protein samples were separated on SDS–polyacrylamide gel (10–12.5% separating gel) at room temperature (RT) and blotted onto PVDF blotting membrane (IPVH00010; Immobilon®-P, Millipore). Membranes were blocked with 5% fat-free milk in Tris-buffered saline (TBS) plus Tween-20® (20 mM Tris, pH 7.5, 150 mM NaCl and 0.1% Tween-20®) at RT for 90 min and then incubated overnight (4 °C) with the following primary antibody: 1:5000 anti-GAPDH (ABS16; Millipore), 1:1000 anti-TSP-1 (14778; Cell Signaling Technology, Beverly, MA), 1:4000 anti-VEGF-A (ABS82; Millipore, Cambridge, CA) and 1:1000 anti-VEGFR-2 (9698; Cell Signaling Technology). The membranes were then washed with TBS plus Tween-20® buffer and incubated at RT for 90 min with 1:10 000 goat anti-mouse IgG peroxidase conjugate (31430; Thermo Scientific) or 1:7000 goat anti-rabbit IgG conjugated horseradish peroxidase (AP132P; Millipore) secondary antibodies. Protein bands were detected by enhanced chemiluminescence (ECL) (WBLUR0100; Millipore) and exposed to UltraCruz Autoradiography film (STCSC-201696; Santa Cruz Biotechnology, Santa Cruz, CA). Band intensity was quantified with ImageJ software version 1.44o (NIH; National Institutes of Health, Bethesda, MD). The expression levels of TSP-1, VEGF-A and VEGFR-2 proteins were individually normalised to GAPDH.
Immunohistochemistry
Muscle sections, 10 μm thickness, were allowed to air-dry for 15 min and rehydrated with 1× PBS (137 mM NaCl, 2.7 mM KCl, 8 mM Na2HPO4, 1.46 mM KH2PO4, pH 7.4) as previously described [Citation18]. Only PECAM-1 (MAB1393; Millipore) staining was pre-fixed with ice-cold methanol for 10 min. All sections were incubated with 10% normal goat serum (PCN5000; Invitrogen, Rockford, IL) for 1 h in moist chamber at RT so as to block non-specific staining. To visualise capillaries, 1:50 primary mouse anti-PECAM-1 in PBS was applied to the sections in moist chamber at RT for 1 h, subsequently incubated overnight at 4 °C. However, the following primary antibodies, including 1:200 rabbit anti-laminin antibody (L9393; Sigma), 1:2000 rabbit anti-fast MHC antibody (ab-91506; Abcam), 1:2000 mouse anti-slow MHC antibody (ab-11083; Abcam) in PBS, were incubated at RT for 2 h. Thereafter, the sections were incubated in 1:1000 ChromeoTM 488 goat anti-rabbit (ab60314; Abcam) and 1:500 goat anti-mouse Alexa Fluor 568 (A11004; Invitrogen) secondary antibodies for 1.5 h in dark moist chamber. Fast MHC, slow MHC and laminin-stained sections were post-fixed with 4% paraformaldehyde for 10 min. Sections were then mounted with VECTASHIELD® Mounting Medium containing DAPI (H-1200; Vector Laboratories, Burlingame, CA). Immunofluorescence images were captured with digital camera (DP 73, Olympus, Tokyo, Japan) under a fluorescence microscope (BX 53; Olympus) and the qualitative image analysis was determined using ImageJ software version 1.44o (NIH). The fibre cross-sectional area (CSA) and fibre-type composition were measured from four-to-six images at magnification 100×. The C/F ratio was determined by counting capillaries and myofibres on three-to-five images from each muscle section at magnification 200×. An average of 55–103 muscle fibres and 80–388 capillaries were measured within each counted area.
Statistical analysis
Data are presented as mean ± standard error of the mean (SEM). Normal distribution and homogeneity of variance were determined using Shapiro–Wilk test and Levene’s test, respectively. One-way analysis of variance (ANOVA) with Newman–Keuls post hoc test was used to determine the difference between treatment conditions. In case of homogeneity of variance was not assumed, data were analysed by the Kruskal–Wallis test. If statistical significance was detected, Dunn’s test was used to indicate significant differences with the significant level at p < 0.01 and p < 0.05. Statistical analysis was performed using SPSS version 17.0 (SPSS Inc., Chicago, IL).
Results
Heat stress alleviates tenotomy-induced myofibre atrophy predominantly in soleus muscle but had no effect on MHC type I and II content in both soleus and plantaris muscles
There was no statistical difference in the initial body weight among groups. After treatment, however, the final body weight increased by 9.5%, 11.5%, 8.4% and 5.5% in CON, CON + HEAT, TEN and TEN + HEAT groups, respectively (). In addition, heat stress alone had no effect on the wet weights of both soleus and plantaris muscles compared to the CON group. However, by day 8 after tenotomy, both absolute muscle wet weight and muscle weight relative to body weight of soleus and plantaris were significantly (p < 0.01) reduced when compared to CON and CON + HEAT groups. Of note, tenotomy-induced atrophy in soleus muscle (absolute and relative wet weights were decreased from CON group by 50.1% and 45.7%, respectively) was more pronounced than that of plantaris muscle (absolute and relative wet weights were decreased from CON group by 14.3% and 10.3%, respectively). On the other hand, heat treatment in the tenotomised rats significantly (p < 0.05) increased the absolute and relative muscle weights of soleus by 28.6% and 26.3%, respectively, whereas such increases in the plantaris muscle were only 3.9% and 6%, respectively (). These results indicate that heat treatment partially rescued muscle loss after tenotomy, predominantly in soleus muscle.
Table 1. Effects of tenotomy and heat stress on body weight and wet weight of soleus and plantaris muscles.
To analyse the alteration of fibre-type composition in both soleus and plantaris muscles, we performed immunofluorescence staining using specific antibodies for MHC type I and II isoforms. The results showed that there were no significant differences in the percentage of MHC type I, type II and hybrid fibre numbers among CON, CON + HEAT, TEN or TEN + HEAT groups in both soleus and plantaris muscles ( and ). Analysis of CSA of MHC type I, type II and hybrid fibres further revealed that there were no differences of fibre CSA in both CON and CON + HEAT groups in either muscle. In parallel with muscle wet weight, tenotomy reduced muscle CSA in MHC type I, type II and hybrid fibres to a higher degree in soleus than in plantaris muscles. Heat treatment, however, partially rescued tenotomy-induced CSA reduction of MHC type I and type II myofibres in soleus muscle by 33.1% and 18.3%, respectively, while only 2.5% and 9.6%, increases in fibre CSA of MHC type I and type II, respectively, of the tenotomised plantaris muscle were noted. These results demonstrate that heat stress mitigated tenotomy-induced atrophy in both MHC type I and II myofibres predominantly in soleus muscle ( and ).
Figure 1. Effects of tenotomy and heat stress on muscle fibre cross-sectional area (CSA) and fibre-type composition of soleus muscle. (A) Immunofluorescence staining of myosin heavy chain (MHC) type I (red) and MHC type II (green), DAPI (blue) and merged (right panel) images in control (CON), control and heat treatment (CON + HEAT), tenotomy (TEN) and tenotomy combined with heat stress (TEN + HEAT) groups. Scale bar =100 μm. (B) Mean CSA of MHC type I, II and hybrid fibres (a total of 2860 fibres from CON, 1901 fibres from CON + HEAT, 2459 fibres from TEN and 2015 fibres from TEN + HEAT). (C) Percentage of fibre-type composition (n = 3–4 rats/group); *p < 0.05 vs. CON within each muscle fibre type; and #p < 0.05 vs. CON + HEAT within each muscle fibre type (one-way ANOVA with Newman–Keuls post hoc test).
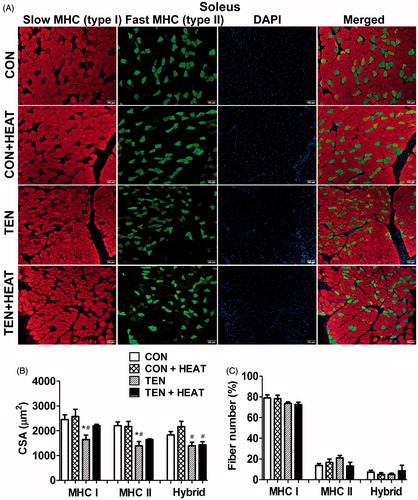
Figure 2. Effects of tenotomy and heat stress on muscle fibre cross-sectional area (CSA) and fibre-type composition of plantaris muscle. (A) Immunofluorescence staining of myosin heavy chain (MHC) type I (red) and MHC type II (green), DAPI (blue) and merged (right panel) images in CON, CON + HEAT, TEN and TEN + HEAT groups. Scale bar =100 μm. (B) Mean CSA of MHC type I, II and hybrid fibres (a total of 2759 fibres from CON, 1735 fibres from CON + HEAT, 2425 fibres from TEN and 2442 fibres from TEN + HEAT). (C) Percentage of fibre-type composition (n = 4 rats/group; one-way ANOVA with Newman–Keuls post hoc test).
Heat stress enhances capillarisation in soleus but not in plantaris muscles after tenotomy
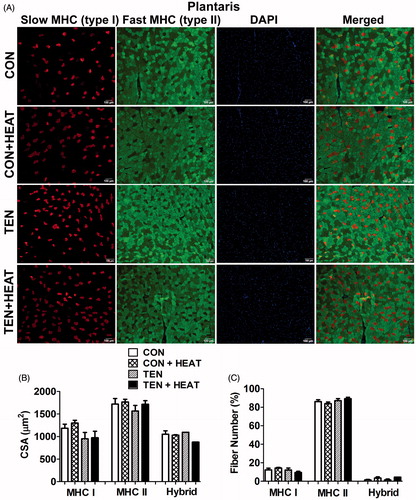
The capillary-to-fibre ratio (C/F) was determined using immunofluorescence for PECAM-1, which determines the endothelial marker, and counterstained with laminin to visualise muscle fibre structure. The C/F ratio was not significantly different between CON and CON + HEAT groups in both muscles examined. In response to tenotomy, however, the C/F ratio in soleus muscle was significantly (p < 0.05) lower than that of CON and CON + HEAT groups, while this ratio was preserved in plantaris muscle (). Interestingly, heat treatment enhanced capillarisation in soleus muscle, which is illustrated by a 24.3% increase in the C/F ratio in TEN + HEAT compared with TEN group. Nevertheless, there was no apparent difference in C/F ratio of plantaris muscle in either TEN or TEN + HEAT groups ().
Figure 3. Effects of tenotomy and heat stress on capillary-to-fibre ratio of soleus and plantaris muscles. (A) Immunofluorescence staining of PECAM-1 (red) and laminin (green) in CON, CON + HEAT, TEN and TEN + HEAT of soleus (left panel) and plantaris (right panel) muscles. Scale bar = 50 μm. (B) Capillary-to-fibre ratio (n = 3–5 rats/group); *p < 0.05 vs. CON within each muscle; and #p < 0.05 vs. CON + HEAT within each muscle (one-way ANOVA with Newman–Keuls post hoc test).
Differential effects of heat stress on angiogenic adaptive regulators in soleus and plantaris muscles after tenotomy
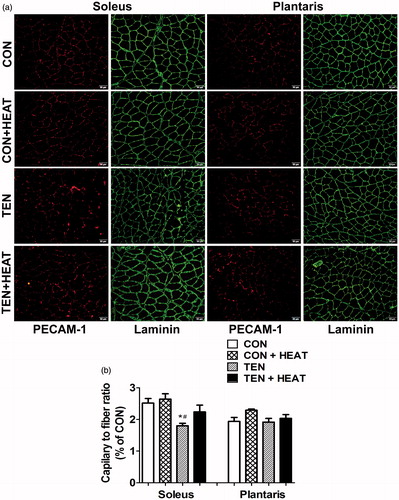
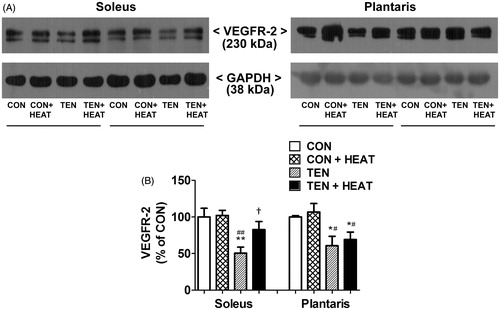
To demonstrate the mechanism by which heat stress modulates capillary adaptation following tenotomy, the expression of VEGF-A, VEGFR-2 and TSP-1 proteins was examined using Western blot analysis. Our results revealed that neither tenotomy nor heat stress affected VEGF-A protein expression in either soleus or plantaris muscles (). Additionally, heat stress failed to affect the expression of VEGFR-2 protein in both intact muscles. On the other hand, tenotomy resulted in a significant (p < 0.01) decrease in the protein expression of VEGFR-2 in soleus muscle compared with CON and CON + HEAT groups. Similarly, VEGFR-2 protein expression was significantly (p < 0.05) lower in the tenotomised plantaris muscle compared to CON and CON + HEAT groups. Heat stress attenuated this effect only in soleus muscle; thus, VEGFR-2 protein expression was significantly (p < 0.05) increased in TEN + HEAT compared with TEN groups, whereas it had virtually no effect in plantaris muscle ().
Figure 4. Effects of tenotomy and heat stress on VEGF-A protein expression of soleus and plantaris muscles. (A) Representative VEGF-A protein expression evaluated by Western blotting. (B) Quantified data of VEGF-A. VEGF-A band density was normalised to GAPDH (n = 6 rats/group; one-way ANOVA with Newman–Keuls post hoc test).
Figure 5. Effects of tenotomy and heat stress on VEGFR-2 protein expression of soleus and plantaris muscles. (A) Representative VEGFR-2 protein expression evaluated by Western blotting. (B) Quantified data of VEGFR-2. VEGFR-2 band density was normalised to GAPDH (n = 6 rats/group); **,*p < 0.01, 0.05 vs. CON within each muscle; ##,#p < 0.01, 0.05 vs. CON + HEAT within each muscle; and †p < 0.05 vs. TEN within each muscle (one-way ANOVA with Newman–Keuls post hoc test).
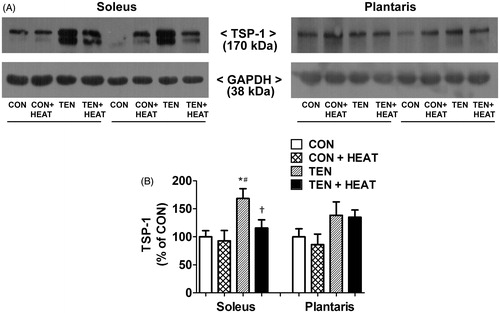
In parallel with pro-angiogenic regulators, TSP-1 protein expression did not differ between CON and CON + HEAT groups in either muscle. After tenotomy for 8 days, however, TSP-1 protein expression in soleus muscle was significantly (p < 0.05) increased when compared with CON and CON + HEAT groups. In contrast, tenotomy had no effect on the TSP-1 protein expression in plantaris muscle. Noteworthy, the increase in TSP-1 protein expression in tenotomised soleus was significantly (p < 0.05) attenuated by heat treatment, while this effect was not observed in tenotomised plantaris muscle ().
Figure 6. Effects of tenotomy and heat stress on TSP-1 protein expression of soleus and plantaris muscles. (A) Representative TSP-1 protein expression evaluated by Western blotting. (B) Quantified data of TSP-1. TSP-1 band density was normalised to GAPDH (n = 6 rats/group); *p < 0.05 vs. CON within each muscle; #p < 0.05 vs. CON + HEAT within each muscle; and †p < 0.05 vs. TEN within each muscle (one-way ANOVA with Newman–Keuls post hoc test).
Discussion
The main purpose of the present study was to investigate the effect of whole body heat stress on the modulation of intramuscular capillarisation following tenotomy in slow-dominant soleus and fast-dominant plantaris muscles. The significant finding demonstrated that heat stress retarded the tenotomy-induced capillary regression that preferentially occurred in soleus but not plantaris muscles. This effect was associated with a shift in the balance between pro-angiogenic and anti-angiogenic regulators.
Differential responses of tenotomy on capillary adaptation, angiogenic regulatory molecule expression and muscle atrophy between soleus and plantaris muscles
We demonstrate that tenotomy induced a reduction in intramuscular capillary supply, especially in the soleus muscle, as measured by C/F ratio, which is widely used to assess muscle capillary quantitation [Citation26]. Muscle disuse capillary adaptation was prominently observed by a reduction in the C/F ratio (1.4-fold) in slow-oxidative soleus muscle compared with the control, while capillary distribution was preserved in the fast-glycolytic plantaris muscle. These results are in agreement with previous studies [Citation4,Citation5]. However, there was no alteration of VEGF-A protein level in either soleus or plantaris muscles by day 8 of tenotomy compared to the control muscle. This is consistent with a study showing that VEGF-A protein expression is unaffected by hindlimb unloading for 7 days [Citation5]. Our results could be explained as in previous studies that the adaptation of mature capillaries was independent of VEGF expression [Citation11,Citation27]. In a parallel finding, several studies also reported that there was no change in VEGF expression during muscle atrophy-induced capillary regression. In contrast, other angiogenic factors were more affected under this condition [Citation11,Citation28]. In skeletal muscle, the pro-angiogenic action of VEGF on endothelial cells is mainly mediated by binding to the VEGFR-2 [Citation29]. Roudier et al. [Citation5] showed that VEGFR-2 protein expression in soleus muscle was attenuated, while VEGF-A protein was not changed in rats subjected to hindlimb unloading for 7 days. This report is in line with the present study, which shows that tenotomy significantly down-regulated VEGFR-2 protein expression by 2- and 1.6-fold in soleus and plantaris muscles, respectively, compared to control muscles.
It is well documented that microcirculatory regulation is fine-tuned by the balance between pro- and anti-angiogenic factors. We, therefore, investigated protein expression of TSP-1. In the present study, TSP-1 protein expression was significantly increased in tenotomised soleus muscle, but not in plantaris muscle. Olfert et al. [Citation11] proposed that an elevation of angiostatic factors such as TSP-1 plays a pivotal role in the initiation of capillary regression, in which TSP-1 interferes with the binding of VEGF to its receptor [Citation30,Citation31]. Although the level of VEGF was not changed, TSP-1 binding to CD36 receptor leads to suppression of the phosphorylation of VEGFR-2 and inhibition of the canonical signal pathway of VEGF [Citation30]. Altogether, tenotomy-induced muscle capillary regression within the soleus muscle could be attributable to the reduction of VEGFR-2 and increased TSP-1 protein expression. In contrast, tenotomy did not affect TSP-1 protein level in plantaris muscle, which correlates well with the maintenance of capillary content in this muscle. Collectively, muscle capillary regression after 8-day tenotomy was fibre type specific.
Regarding tenotomy-induced muscle atrophy, 8-day tenotomy resulted in a higher muscle mass reduction in slow-oxidative soleus muscle compared to fast-glycolytic plantaris muscle, which is consistent with our previous studies [Citation18,Citation32]. Furthermore, soleus muscle fibre CSA was reduced in both MHC type I and II, while only MHC type I CSA in plantaris muscle was decreased following tenotomy. Previous studies explained that the capillary supply to a muscle fibre is positively correlated with the individual CSA [Citation33,Citation34]. Hence, the higher atrophic response represented by reduced fibre CSA in the soleus after tenotomy is partly due to the loss of capillaries that can impair delivery of oxygen and substrates to the tenotomised soleus muscle.
Heat stress modulates capillary adaptation and muscle atrophic responses in soleus and plantaris muscles following tenotomy
The major finding in the present study is that heat stress prevented tenotomy-induced capillary regression in soleus muscle compared to the muscles with no heat treatment. Concomitantly, exposure to heat resulted in a significant increase in expression of VEGFR-2 and a decrease in expression of TSP-1 protein in tenotomised soleus muscle compared with the untreated group. In contrast, such changes were not found in the plantaris muscle. Taken together, heat stress attenuated the microvessel reduction following tenotomy, at least in part, through shifting the balance between positive and negative angiogenic factors in the soleus muscle. Accordingly, heat stress-enhanced capillary redistribution that enhances blood flow to the working muscle could contribute to the increased fibre CSA observed in tenotomised soleus muscle.
Additionally, degradation of extracellular matrix (ECM) by matrix metalloproteinases (MMPs) and their inhibitors, tissue inhibitors of metalloproteinases (TIMPs), have been known to play an important role in skeletal muscle angiogenesis [Citation35]. Since the ECM degradation provides space to the endothelial cells to migrate and liberate VEGF within the ECM [Citation1]. Moreover, TIMP-2 has been shown to be a potent regulator of anti-angiogenic activity in vitro and in vivo [Citation2]. The binding of TIMP-2 to the α3β1 integrin receptor on the endothelial cells results in inactivation and dephosphorylation of VEGFR-2 binding to VEGF [Citation2]. Our recent study showed that heat stress decreased TIMP-2 expression in tenotomised soleus muscle, with no change in MMP-2 activity leading to an increase in the ratio of MMP-2:TIMP-2 [Citation18]. However, these results were less affected in tenotomised plantaris muscle. Therefore, attenuation of TIMP-2 expression by heat stress may lead to increased activity of MMP-2, contributing to enhanced ECM degradation and subsequently to increased muscle angiogenesis in the soleus muscle.
A previous study has shown a significant relationship between capillarisation and oxidative capacity in muscle fibres [Citation36]. Under unloading conditions, a fibre-type shift from slower oxidative fibre towards faster glycolytic fibre with a decrease in proportion of type I/IIa and an increase in type IIb/IIx fibres has been reported [Citation19]. However, heat stress has been demonstrated to increase MHC type I mRNA and protein content but decrease MHC IIx expression in mouse C2C12 myoblasts [Citation37]. To our surprise, the percentage of MHC type I and type II fibres in both soleus and plantaris muscles were not changed in either tenotomy or heat treatment when compared to controls. To support this notion, Waters et al. [Citation21] proposed that the muscle fibre-type transition might be a sequential response at a later period after the capillarity alteration. To clarify the effect of heat stress on fibre-type composition in disuse muscle, further work to assess MHC isoform expression using a triple labelling for MHC type I, IIa and IIb + IIx during longer experiments is needed.
Skeletal muscle atrophy occurs in a variety of conditions and affects specific fibre types [Citation38], especially during a disuse state that demonstrates preferential loss of capillaries and muscle mass within slow-twitch oxidative fibres. In the current study, heat treatment is capable of maintaining microcirculation in a fibre-type-specific manner. This differential muscle fibre-type response can lead to design of therapeutic interventions appropriate for specific muscle disorders. It also conceivable that the application of heat treatment together with other pharmaceutical drugs, such as sildenafil or losartan, may use as an alternative adjuvant to improve intramuscular capillary network and enhance vasorelaxation capacity in muscle disorders, especially type I muscle atrophy under disuse conditions. The synergistic effect of heat stress needs to be further verified, as it may reduce needed drug doses to avoid potential side effects.
Conclusions
Our study illustrates that heat stress attenuated tenotomy-induced capillary regression in soleus, but not in plantaris muscles. The effect was in part mediating by shifting of the balance between pro-angiogenic and anti-angiogenic regulators in the muscles. These results suggest that heat treatment during disuse condition is capable of preventing muscle atrophy partially through maintaining microcirculation of the slow-dominant skeletal muscle type.
Acknowledgements
The authors gratefully thank Dr. Tossaporn Yimlamai for valuable suggestions on the initial phase of this project and for supporting the reagents. Special thanks are given to Professor Dr. Chumpol Pholpramool and Assistant Professor Dr. Christopher Fry for critical reading of this manuscript. The proof reading of this manuscript was supported by the Editorial Office, Faculty of Graduate Studies, Mahidol University. We also acknowledge the Olympus Bioimaging Center, Faculty of Science, Mahidol University, for technical and instrument supports.
Disclosure statement
This research project was supported by Srinakharinwirot University to MH. The authors alone are responsible for the content and writing of the manuscript. The authors declare no conflicts of interest.
Additional information
Funding
References
- Heissig B, Hattori K, Friedrich M, et al. (2003). Angiogenesis: vascular remodeling of the extracellular matrix involves metalloproteinases. Curr Opin Hematol 10:136–41.
- Stetler-Stevenson WG, Seo DW. (2005). TIMP-2: an endogenous inhibitor of angiogenesis. Trends Mol Med 11:97–103.
- Wagatsuma A, Osawa T. (2006). Time course of changes in angiogenesis-related factors in denervated muscle. Acta Physiol (Oxf) 187:503–9.
- Jozsa L, Kannus P, Thoring J, et al. (1990). The effect of tenotomy and immobilisation on intramuscular connective tissue. A morphometric and microscopic study in rat calf muscles. J Bone Joint Surg Br 72:293–7.
- Roudier E, Gineste C, Wazna A, et al. (2010). Angio-adaptation in unloaded skeletal muscle: new insights into an early and muscle type-specific dynamic process. J Physiol (Lond) 588:4579–91.
- Leung DW, Cachianes G, Kuang WJ, et al. (1989). Vascular endothelial growth factor is a secreted angiogenic mitogen. Science 246:1306–9.
- Deasy BM, Feduska JM, Payne TR, et al. (2009). Effect of VEGF on the regenerative capacity of muscle stem cells in dystrophic skeletal muscle. Mol Ther 17:1788–98.
- Rhoads RP, Flann KL, Cardinal TR, et al. (2013). Satellite cells isolated from aged or dystrophic muscle exhibit a reduced capacity to promote angiogenesis in vitro. Biochem Biophys Res Commun 440:399–404.
- Hellsten Y, Hoier B. (2014). Capillary growth in human skeletal muscle: physiological factors and the balance between pro-angiogenic and angiostatic factors. Biochem Soc Trans 42:1616–22.
- Carmeliet P. (2000). Mechanisms of angiogenesis and arteriogenesis. Nat Med 6:389–95.
- Olfert IM. (2016). Physiological capillary regression is not dependent on reducing VEGF expression. Microcirculation 23:146–56.
- Audet GN, Fulks D, Stricker JC, Olfert IM. (2013). Chronic delivery of a thrombospondin-1 mimetic decreases skeletal muscle capillarity in mice. PLoS One 8:e55953.
- Agah A, Kyriakides TR, Lawler J, Bornstein P. (2002). The lack of thrombospondin-1 (TSP1) dictates the course of wound healing in double-TSP1/TSP2-null mice. Am J Pathol 161:831–9.
- Ennen JP, Verma M, Asakura A. (2013). Vascular-targeted therapies for Duchenne muscular dystrophy. Skelet Muscle 3:9.
- Rinaldi B, Donniacuo M, Sodano L, et al. (2013). Effects of sildenafil on the gastrocnemius and cardiac muscles of rats in a model of prolonged moderate exercise training. PLoS One 8:e69954.
- Li M, Fuchs S, Bose T, et al. (2014). Mild heat stress enhances angiogenesis in a co-culture system consisting of primary human osteoblasts and outgrowth endothelial cells. Tissue Eng Part C Methods 20:328–39.
- Gong B, Asimakis GK, Chen Z, et al. (2006). Whole-body hyperthermia induces up-regulation of vascular endothelial growth factor accompanied by neovascularization in cardiac tissue. Life Sci 79:1781–8.
- Hirunsai M, Srikuea R, Yimlamai T. (2015). Heat stress promotes extracellular matrix remodelling via TGF-beta1 and MMP-2/TIMP-2 modulation in tenotomised soleus and plantaris muscles. Int J Hyperthermia 31:336–48.
- Pierno S, Desaphy JF, Liantonio A, et al. (2002). Change of chloride ion channel conductance is an early event of slow-to-fast fibre type transition during unloading-induced muscle disuse. Brain 125:1510–21.
- Schiaffino S, Sandri M, Murgia M. (2007). Activity-dependent signaling pathways controlling muscle diversity and plasticity. Physiology 22:269–78.
- Waters RE, Rotevatn S, Li P, et al. (2004). Voluntary running induces fiber type-specific angiogenesis in mouse skeletal muscle. Am J Physiol Cell Physiol 287:C1342–8.
- Bloemberg D, Quadrilatero J. (2012). Rapid determination of myosin heavy chain expression in rat, mouse, and human skeletal muscle using multicolor immunofluorescence analysis. PLoS One 7:e35273.
- Jamali AA, Afshar P, Abrams RA, Lieber RL. (2000). Skeletal muscle response to tenotomy. Muscle Nerve 23:851–62.
- Selsby JT, Dodd SL. (2005). Heat treatment reduces oxidative stress and protects muscle mass during immobilization. Am J Physiol Regul Integr Comp Physiol 289:R134–9.
- Tolson JK, Roberts SM. (2005). Manipulating heat shock protein expression in laboratory animals. Methods 35:149–57.
- Kano Y, Shimegi S, Furukawa H, et al. (2002). Effects of aging on capillary number and luminal size in rat soleus and plantaris muscles. J Gerontol A Biol Sci Med Sci 57:B422–7.
- Olfert IM, Howlett RA, Tang K, et al. (2009). Muscle-specific VEGF deficiency greatly reduces exercise endurance in mice. J. Physiol (Lond.) 587:1755–67.
- Wagatsuma A. (2008). Effect of hindlimb unweighting on expression of hypoxia-inducible factor-1alpha vascular endothelial growth factor, angiopoietin, and their receptors in mouse skeletal muscle. Physiol Res 57:613–20.
- Claesson-Welsh L. (2003). Signal transduction by vascular endothelial growth factor receptors. Biochem Soc Trans 31:20–4.
- Chu LY, Ramakrishnan DP, Silverstein RL. (2013). Thrombospondin-1 modulates VEGF signaling via CD36 by recruiting SHP-1 to VEGFR2 complex in microvascular endothelial cells. Blood 122:1822–32.
- Greenaway J, Gentry PA, Feige JJ, et al. (2005). Thrombospondin and vascular endothelial growth factor are cyclically expressed in an inverse pattern during bovine ovarian follicle development. Biol Reprod 72:1071–8.
- Hirunsai M, Yimlamai T, Suksamrarn A. (2016). Effect of 20-hydroxyecdysone on proteolytic regulation in skeletal muscle atrophy. In Vivo 30:869–77.
- Wust RC, Gibbings SL, Degens H. (2009). Fiber capillary supply related to fiber size and oxidative capacity in human and rat skeletal muscle. Adv Exp Med Biol 645:75–80.
- Ballak SB, Buse-Pot T, Harding PJ, et al. (2016). Blunted angiogenesis and hypertrophy are associated with increased fatigue resistance and unchanged aerobic capacity in old overloaded mouse muscle. Age 38:39.
- Haas TL, Milkiewicz M, Davis SJ, et al. (2000). Matrix metalloproteinase activity is required for activity-induced angiogenesis in rat skeletal muscle. Am J Physiol Heart Circ Physiol 279:H1540–7.
- Mathieu-Costello O, Hepple RT. (2002). Muscle structural capacity for oxygen flux from capillary to fiber mitochondria. Exerc Sport Sci Rev 30:80–4.
- Yamaguchi T, Suzuki T, Arai H, et al. (2010). Continuous mild heat stress induces differentiation of mammalian myoblasts, shifting fiber type from fast to slow. Am J Physiol Cell Physiol 298:C140–8.
- Wang Y, Pessin JE. (2013). Mechanisms for fiber-type specificity of skeletal muscle atrophy. Curr Opin Clin Nutr Metab Care 16:243–50.
