Abstract
Objectives: This study investigated the correlation between the peritoneal carcinomatosis index (PCI) and patient outcome depending on the tumour type.
Background: Peritoneal surface malignancy (PSM) treatment depends on tumour type. Mucinous PSM (m-PSM) is associated with a better prognosis than non-mucinous PSM (nm-PSM). The PCI’s predictive ability has not yet been evaluated.
Methods: We analysed 123 patients with PSM treated with cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) between 2008 and 2015. The m-PSM group (n = 75) included patients with appendiceal cancer (n = 15), colorectal cancer (n = 21), or low-grade appendiceal mucinous neoplasm (n = 39); the nm-PSM group (n = 48) included patients with gastric (n = 18) or colorectal (n = 30) cancer. The PCI’s predictive ability was evaluated by multiple Cox-proportional hazard regression analysis and Kaplan–Meier curves.
Results: The 5-year survival and PCI were higher in m-PSM patients (67.0%; 20.5 ± 12.1) than in nm-PSM patients (32.6%; p = 0.013; 8.9 ± 6.0; p < 0.001). Colorectal nm-PSM patients with PCI ≥16 had a worse 2-year survival (25.0%) vs. patients with PCI <16 (79.1%; log rank = 0.009), but no significant effect was observed in patients with m-PSM (66.7% vs. 68.1%; p = 0.935). Underlying disease (HR 5.666–16.240), BMI (HR 1.109), and PCI (HR 1.068) significantly influenced overall survival in all patients.
Conclusions: PCI is prognostic in nm-PSM, but not in m-PSM. CRS and HIPEC may benefit not only patients with low PCI, but also those with high PCI and m-PSM.
Introduction
Peritoneal surface malignancies (PSMs) are divided into primary and secondary PSM that differ in malignant potential and prognosis depending on their origin. Primary PSMs, like malignant peritoneal mesothelioma, are rare, with an estimated incidence of 0.5–3 per million per year [Citation1]. Most commonly, secondary PSMs are derived from ovarian, gastric, or colorectal cancer, or appendiceal neoplasms. The majority of secondary PSMs manifest as a tumour recurrence and reflect disseminated disease. For cancer patients who develop peritoneal metastases, palliative chemotherapy is usually the only treatment offered, resulting in a poor outcome.
The key factor for improving survival in patients with PSM was the development in recent decades of cytoreductive surgery (CRS) and intraperitoneal (IPC) or hyperthermic intraperitoneal chemotherapy (HIPEC). These techniques were mainly established by Paul Sugarbaker more than 20 years ago and are now performed worldwide.
To quantify the degree of peritoneal tumour seeding and to facilitate communication, the Peritoneal Cancer Index (PCI) is used [Citation2]. Important major factors, like the completeness of cytoreduction (CC score) in patients with PSM of colorectal cancer (CRC) or the extent of peritoneal seeding in patients with low-grade appendiceal neoplasms (LAMN), CRC, or gastric cancer (GC), are of the utmost significance for long-term survival [Citation3–6]. To reach CCR0/1 for a patient with a high PCI, extensive surgery is necessary, which is associated with a high rate of severe complications.
The high morbidity and mortality of these extensive surgical procedures in patients with advanced oncologic diseases led to the development of a threshold, i.e., a PCI score, which helps in the decision as to whether a cytoreductive procedure is of benefit for the patient or not. The PCI score was designed for PSM of solid organ tumours, e.g., gastric, ovarian or colorectal cancer.
However, PSM can be classified on the basis of histologic assessment of the tumour specimen. The mucinous type of PSM is mainly observed in patients with LAMN, appendix carcinoma or CRC. Ten to fifteen percent of patients with CRC have mucinous adenocarcinoma (MC), which is characterised by abundant mucous secretion comprising at least 50% of the tumour volume [Citation7]. The prognostic and predictive implications of MC, as well as the clinical implications for treatment and follow-up of patients with the mucinous form of CRC, are currently subject to debate.
This study analysed the outcome of PSM by its mucinous or non-mucinous cell type and evaluated the predictive ability of PCI for patient survival for these subgroups.
Methods
This retrospective study of prospectively collected data included all consecutive patients who were treated by CRS and HIPEC between January 2008 and August 2015 at Campus Mitte, Charité, Berlin, Germany. Patients with peritoneal mesothelioma, ovarian cancer, or cancer of unknown origin and with repeated CRS and HIPEC procedures were excluded from the analysis. Patients with signet cell carcinoma as well as those who had undergone operations with the intention of tumour debulking were also excluded. The median follow-up was 20.7 months.
Routine preoperative examination was performed in every patient; CT scans of the chest, abdomen and pelvis, and information about tumour markers (CEA, CA19–9, and CA 125) were obtained. A surgical procedure was recommended for all patients without evidence of extraperitoneal metastases if complete cytoreduction (CC) was deemed feasible by the operating surgeon. The extent of peritoneal involvement was assessed through the Peritoneal Cancer Index (PCI) [Citation2]. The assessment took place directly after explorative laparotomy and just before cytoreductive procedures and was performed by B.R. for every patient. The PCI score was simultaneously recorded and calculated by an assistant. Five patients (4 cases of CRC and 1 of gastric cancer [GC]) were classified with a PCI score of 0 because of histopathological proof of peritoneal malignancies at one stage of their treatment (either during palliative chemotherapy or at initial tumour resection) that was not present during the CRS and HIPEC procedure.
Definitive CRS was carried out to achieve complete cytoreduction using, but not limited to, the following procedures: exploratory laparotomy, abdominal wall resection, abdominal and pelvic lymphadenectomy, appendectomy, cholecystectomy, bilateral adnexectomy with hysterectomy, cytoreductive surgery and biopsy of peritoneal implants, enterolysis, and ureterolysis. The peritonectomy procedures included diaphragmatic, parietal, and pelvic peritonectomy and omentectomy. Resection of hollow viscus and/or organs was performed if they could not be cleared of disease or were affected by the primary cancer. Every effort was made to avoid extensive small bowel resection and/or ostomy formation to preserve quality of life. Complete cytoreduction was defined as nodules less than 2.5 mm in size (CC = 1) or the absence of visible tumour nodules (CC = 0). Cytoreduction was followed by immediate HIPEC. HIPEC protocols differed according to different tumour entities: patients with GC received intraperitoneal (i.p.) cisplatin and mitomycin, while patients with CRC, appendix carcinoma and LAMN were bidirectionally treated with oxaliplatin i.p. and fluorouracil and leucovorin intravenously. HIPEC was delivered for 60 min in nearly all cases (98.4%) and through a closed circulation system in 81.3% of the patients. An open circulation system was used for the remaining 18.7% of patients.
Patients were divided into two groups regarding the mucinous type of the histopathology collected in the CRS and HIPEC procedure. The histopathological characteristic of mucinous PM is the existence of intracellular mucin. Mucinous tumour cells are found mainly in the periphery of the mucinous deposits, typically with very low tumour cell content. In contrast, the tumour cells in non-mucinous PM are arranged in solid nests, surrounded by desmoplastic stroma, and have a significantly higher cell content. The first group was defined by the presence of a mucinous peritoneal surface malignancy (m-PSM) including LAMN, mucinous neoplasm (MN) of the appendix, or mucinous colorectal cancer. MN of the appendix is characterised by neoplastic villous or flat adenomatous changes of the epithelium associated with distension of the appendix lumen with secreted mucin. Cases of MN were retrospectively classified according to the 2010 World Health Organisation classification, which recognises three distinct categories: mucinous adenoma, LAMN and appendiceal mucinous adenocarcinoma [Citation8]. If a mucinous adenoma was perforated, it was reclassified as LAMN. The second group was defined as non-mucinous peritoneal surface malignancies (nm-PSM), including PSMs of gastric and colorectal origin. Comparison between the groups was made using the following parameters: age, sex, body mass index (BMI), PCI score, CC score, tumour grading, length of operation, length of hospital and intensive care unit (ICU) stays, ASA classification, comorbidities, extent of resection, and number and type of anastomoses.
Clinical data were collected during follow-up visits and no patient was lost to follow-up. The study was approved by the ethics committee of the Charité (EA1/009/16). All statistical analyses were performed using either SPSS 23.0 (International Business Machines Corporation, Armonk, NY) or Prism 6.0 (Graphpad Software, Inc., La Jolla, CA). Continuous descriptive data are given as mean and standard deviation. Categorical data are given as frequencies and proportions. Univariate analysis of time to event data was performed using log-rank tests to compare several groups. Univariate results were visualised by Kaplan–Meier curves. To identify independent risk factors and protective factors in addition to group comparisons, a multiple Cox-proportional hazard regression analysis was applied. Variables of underlying disease, sex, age, BMI, tumour grading, operation time, comorbidities, ASA classification, PCI, CC, time from diagnosis to CRS and HIPEC, and number of anastomoses were included in the model. Backward stepwise variable selection with the Akaike information criterion (AIC) method was used. A p value less than 0.05 was defined as significant.
Results
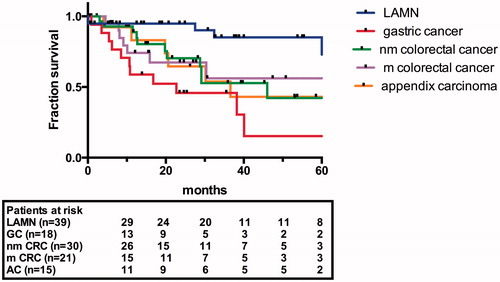
In total, 123 patients met the inclusion criteria and were divided into the non-mucinous PSM (nm-PSM) group (n = 48) or the mucinous PSM (m-PSM) group (n = 75). The nm-PSM group (median survival 29.1 months; 5-year survival 32.6%) included 18 patients with GC with a median survival of 22.7 months and 30 patients with CRC with a median survival of 46.0 months. The m-PSM group (median survival not reached; 5-year survival 67.8%) included 39 patients with LAMN, 21 patients with CRC, and 19 patients with appendiceal carcinoma (AC) with a median survival of 36.6 months. The survival curves are shown in .
Patient demographics (), surgical procedure details, and postoperative course () showed differences in tumour origin and grading, operation time (472.7 ± 202.7 vs. 360.8 ± 114.7 min), PCI (20.5 ± 12.1 vs. 8.9 ± 6.0), and extent of peritonectomy in the m-PSM compared with the nm-PSM group. Perioperative mortality was 3.3% (4/123) in total, occurred in 2/75 patients (2.6%) in the m-PSM group, and was related to fulminant pulmonary embolism in both cases. Two out of 48 (4.2%) patients in the nm-PSM group died due to intestinal fistula/perforation, with sepsis and multi-organ failure in both cases.
Table 1. Patient demographics from mucinous and non-mucinous peritoneal malignancies.
Table 2. Operation and postoperative course demographics from mucinous and non-mucinous peritoneal malignancies.
Cox regression analysis revealed differences between the groups and is shown in . Underlying disease (HR 5.666–16.240; p < 0.001–0.013), BMI (HR 1.109; p = 0.014), and PCI score (HR 1.068; p < 0.001) showed a significant correlation with patient survival in all patients. The additional analysis of mucinous groups, excluding underlying disease, showed the effects of BMI (HR 1.157; p = 0.017) and PCI (HR 1.089; p = 0.015) for nm-PSM and tumour grading (HR 1.695; p = 0.018) in the m-PSM group. The correlation for the PCI score did not reach the level of significance in the m-PSM group (HR 1.048; p = 0.091).
Table 3. Cox regression model for patient’s death.
A subgroup analysis of patients with CRC, which was the only group containing patients with both m-PSM and nm-PSM, showed a significant difference in PCI score of 15.5 ± 13.0 in patients with m-PSM vs. 9.1 ± 6.1 in patients with nm-PSM (p = 0.023), although there was no significant difference in patient survival (p = 0.960). Patients with nm-PSM who showed PCI scores ≥16 demonstrated inferior 2-year patient survival (25.0%) compared with patients with PCI scores <16 (79.1%; p = 0.009). In contrast, patient survival was not different between the PCI subgroups in the m-PSM group (66.7% vs. 68.1%; p = 0.935) (). Patient demographics and tumour details are shown in and .
Figure 2. Patient survival comparing patients with PCI <16 and PCI ≥16; a: patients with non-mucinous PSM; b: patients with mucinous PSM.
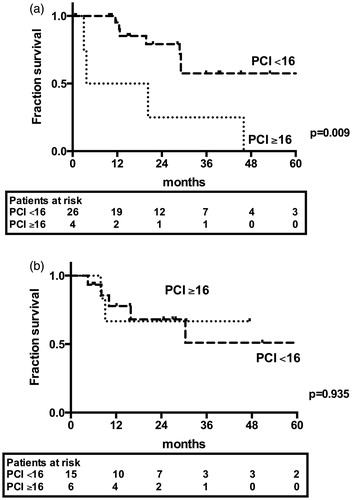
Table 4. Patient demographics from non-mucinous colorectal cancer.
Table 5. Patient demographics from mucinous colorectal cancer.
Discussion
To our knowledge, this is the first cross-disease study to examine the effect of histological mucinous-state on patient survival and to propose PCI as an important prognostic factor for non-mucinous peritoneal malignancies. PCI score was assessed to determine the intra-abdominal tumour spread and intensity, which has been shown to have a major influence on completeness of cytoreduction and patient survival.
Mucinous carcinoma in colorectal cancer
Mucinous cancer has been identified as a distinct colorectal subtype with different molecular, genetic and pathological characteristics. Mucinous cancer is more frequently associated with high levels of microsatellite instability, BRAF/p53/16 mutations, and downregulation of adhesion molecules [Citation9]. There is evidence that these differences in tumour biology are responsible for poor responses to systemic chemotherapy, resulting in significantly worse patient survival [Citation10]. Razenberg et al. [Citation11] showed in a large nationwide population-based study an incidence of 22% of mucinous carcinoma in patients with peritoneal carcinomatosis from CRC and a superior median survival of 13 months compared with adenocarcinoma (8.9 months) and signet cell carcinoma (7.1 months). The incidence in our preselected study population was 41.2%. We could not identify any significant difference in patient survival, comparing a 5-year survival of 42.2% in the nm-PSM group with 56.1% in the m-PSM group (p = 0.960). da Silva and Sugarbaker [Citation12] showed that, at a PCI >20, lymph node positivity, but not mucinous type, influenced patient survival. van Oudheusden et al. [Citation13] recently published poor outcomes of CRS and HIPEC in a small patient group with peritoneal carcinomatosis of signet cell colorectal cancer with a median survival of 14 months. These publications indicate that the histopathological state of PSMs is of increasing interest.
In our analysis, patients suffering from colorectal carcinoma were the only patient group that provided an opportunity to compare mucinous with non-mucinous PSM within the same underlying disease although the groups were rather small (30 vs. 21 patients).
Enlarging the horizon from this limited data about patients with PSM and the mucinous subtype of CRC to patients with advanced stage CRC, several studies, including two randomised trials investigating patients with metastatic CRC, have demonstrated that MC disease subtype, compared with other types of CRC, is a poor prognostic factor; median overall survival rates varied between 8.0 and 14.0 months for patients with metastatic MC, compared with 17.9–23.4 months for those with metastatic AC [Citation10,Citation14,Citation15].
Risk factors (BMI, grading)
There is limited evidence regarding the predictive ability of BMI on long-term survival in patients with PSM. Polanco et al. [Citation16] demonstrated no difference in perioperative mortality and overall survival, but an increased risk for perioperative renal and pulmonary complications for obese patients treated with CRS and HIPEC for mucinous appendiceal neoplasm. Our analysis confirmed increasing BMI as a negative predictor of overall survival, as shown by Tran et al. [Citation17] in women with recurrent ovarian cancer treated with CRS.
The significant influence of tumour grading on patient survival in m-PSM has to be interpreted with regard to the differences in diseases. All patients suffering from LAMN showed a grading of one while the 19 patients with grading three were treated due to CRC. LAMN vs. CRC was itself a predictive factor in Cox regression analysis of the full cohort. In the subgroup analysis, underlying disease as a factor was eliminated. Nevertheless, poor differentiation is known to be a negative predictive factor for patient survival in CRC [Citation18].
General
The analysis of different underlying diseases can be challenging, owing to the variability in patient survival, e.g., 5-year survival of 72.9% for patients with LAMN compared with 15.3% for patients with GC. Our study included a highly preselected cohort using the curative approach for CRS and HIPEC as inclusion criteria. Patients with palliative CRS and HIPEC or CC scores of 3 were excluded from this analysis, which led to a CC 0–1 ratio of 85.3% and 97.9% in the m-PSM and nm-PSM groups, respectively. These exclusions might have contributed to the fact that the CC score did not reach significance in any Cox regression model, which seems contrary to the existing data, but this also is likely due to the limited sample size (only 9.8% had CC >1 cytoreductions). Nevertheless, PCI score was a significant factor for survival only in the nm-PSM group, indicating its specific importance in this subgroup of patients. The different mean PCI scores between the nm-PSM (8.9 ± 6.04) and m-PSM (20.5 ± 12.07; p < 0.001) groups seems to confirm this hypothesis in our study population. The only disease-independent analysis between both groups was performed in patients with CRC, and it demonstrated the predictive value of PCI for patient survival in nm-PSM patients, with a 2-year survival of 25.0% in patients with PCI ≥16 compared with 79.1% (p = 0.009) in patients with PCI <16. However, this predictor was not significant in the m-PSM group (66.7% vs. 68.1%; p = 0.935). A recent study published by Goere et al. [Citation19] proposed the cut-off of survival benefit for patients at PCI scores of 17, while other large retrospective studies demonstrated that a PCI higher than 16 or 20 was associated with worse patient survival [Citation12,Citation20]. This predictor showed no value in the m-PSM group. Another cross-disease study recently showed a PCI cut-off of 15 in 201 patients [Citation21].
We showed that patients with a high PCI score of mucinous PSM did not have inferior survival and therefore should not be excluded from CRS and HIPEC due to a higher score per se. PCI was not predictive in mucinous peritoneal surface malignancies, although subgroup analysis of various diseases were weakened due to sample size. Recently developed scoring systems, like the Peritoneal Surface Disease Severity Score [Citation22], aim to include and combine predictive factors like clinical symptoms, PCI, and pathological evaluation of the primary tumour and might be helpful in certain diseases.
In conclusion, we showed that PCI was a significant factor for patient survival in our study cohort, with greater influence in patients with non-mucinous PSM. The dogma of conducting CRS with the goal of complete cytoreduction (CC 0–1) is of major importance and represents the fundamental point in deciding whether CRS and HIPEC could benefit the patient or not. Patients with mucinous PSM in whom complete cytoreduction seems possible should be considered for CRS and HIPEC, even when the PCI is higher than proposed cut offs.
Disclosure statement
The authors have no financial relationships to disclose; no grants, equipment or drugs were supplied by outside parties.
The authors have no conflicts of interest to disclose.
References
- Yan TD, Welch L, Black D, Sugarbaker PH. (2007). A systematic review on the efficacy of cytoreductive surgery combined with perioperative intraperitoneal chemotherapy for diffuse malignancy peritoneal mesothelioma. Ann Oncol 18:827–34.
- Jacquet P, Sugarbaker PH. (1996). Clinical research methodologies in diagnosis and staging of patients with peritoneal carcinomatosis. Cancer Treat Res 82:359–74.
- Elias D, Gilly F, Quenet F, et al. (2010). Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 36:456–62.
- Chua TC, Moran BJ, Sugarbaker PH, et al. (2012). Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30:2449–56.
- Faron M, Macovei R, Goere D, et al. (2016). Linear relationship of peritoneal cancer index and survival in patients with peritoneal metastases from colorectal cancer. Ann Surg Oncol 23:114–19.
- Sun JH, Ji ZH, Peng KW, et al. (2016). Cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy for the treatment of primary peritoneal serous carcinoma: results of a Chinese retrospective study. Int J Hyperthermia 32:289–97.
- Bosman FT, Carneiro F, Hruban RH, Theise ND. (2010). WHO classification of tumours of the digestive system. 4th ed. Geneva, Switzerland: International Agency for Research on Cancer.
- Carr NJ, McCarthy WF, Sobin LH. (1995). Epithelial noncarcinoid tumors and tumor-like lesions of the appendix. A clinicopathologic study of 184 patients with a multivariate analysis of prognostic factors. Cancer 75:757–68.
- Nitsche U, Zimmermann A, Spath C, et al. (2013). Mucinous and signet-ring cell colorectal cancers differ from classical adenocarcinomas in tumor biology and prognosis. Ann Surg 258:775–82; discussion 82–3.
- Catalano V, Loupakis F, Graziano F, et al. (2009). Mucinous histology predicts for poor response rate and overall survival of patients with colorectal cancer and treated with first-line oxaliplatin- and/or irinotecan-based chemotherapy. Br J Cancer 100:881–7.
- Razenberg LG, van Gestel YR, Lemmens VE, et al. (2015). The prognostic relevance of histological subtype in patients with peritoneal metastases from colorectal cancer: a nationwide population-based study. Clin Colorectal Cancer 14:e13–19.
- da Silva RG, Sugarbaker PH. (2006). Analysis of prognostic factors in seventy patients having a complete cytoreduction plus perioperative intraperitoneal chemotherapy for carcinomatosis from colorectal cancer. J Am Coll Surg 203:878–86.
- van Oudheusden TR, Braam HJ, Nienhuijs SW, et al. (2015). Poor outcome after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis with signet ring cell histology. J Surg Oncol 111:237–42.
- Mekenkamp LJ, Heesterbeek KJ, Koopman M, et al. (2012). Mucinous adenocarcinomas: poor prognosis in metastatic colorectal cancer. Eur J Cancer 48:501–9.
- Negri FV, Wotherspoon A, Cunningham D, et al. (2005). Mucinous histology predicts for reduced fluorouracil responsiveness and survival in advanced colorectal cancer. Ann Oncol 16:1305–10.
- Polanco PM, Sanchez AI, Ramalingam L, et al. (2014). Does obesity affect outcomes of cytoreductive surgery and hyperthermic intraperitoneal chemoperfusion for disseminated mucinous appendiceal neoplasms? Ann Surg Oncol 21:3963–9.
- Tran AQ, Cohen JG, Li AJ. (2015). Impact of obesity on secondary cytoreductive surgery and overall survival in women with recurrent ovarian cancer. Gynecol Oncol 138:263–6.
- Beaton C, Twine CP, Williams GL, Radcliffe AG. (2013). Systematic review and meta-analysis of histopathological factors influencing the risk of lymph node metastasis in early colorectal cancer. Colorectal Dis 15:788–97.
- Goere D, Souadka A, Faron M, et al. (2015). Extent of colorectal peritoneal carcinomatosis: attempt to define a threshold above which HIPEC does not offer survival benefit: a comparative study. Ann Surg Oncol 22:2958–64.
- Elias D, Blot F, El Otmany A, et al. (2001). Curative treatment of peritoneal carcinomatosis arising from colorectal cancer by complete resection and intraperitoneal chemotherapy. Cancer 92:71–6.
- Tan G, Chia C, Kumar M, et al. (2017). 201 consecutive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in a single Asian tertiary centre. Int J Hyperthermia 33:288–94.
- Pelz JO, Stojadinovic A, Nissan A, et al. (2009). Evaluation of a peritoneal surface disease severity score in patients with colon cancer with peritoneal carcinomatosis. J Surg Oncol 99:9–15.