Abstract
Purpose: To evaluate the contribution of the thermal dose parameters during regional hyperthermia (HT) treatment to the clinical outcomes in patients with cervical carcinoma (CC) who received chemoradiotherapy (CRT) plus HT.
Materials and methods: Data from a multicentre randomised clinical trial of concurrent CRT + HT vs. CRT alone were used to evaluate the efficacy and safety of this combination therapy in the CC patients. The intrarectal temperatures of patients undergoing HT were recorded. The complete thermal data of 47 (92%) of the 51 patients in the CRT + HT group were available for the thermal analysis. Thus, 47 patients who received CRT + HT were included in the present study.
Results: Among the patients who received CRT + HT, a higher CEM43T90 (≥1 min) value (a thermal dose parameter) was significantly associated with better local relapse-free survival in both univariate (p = 0.024) and multivariate (p = 0.0097) analyses. The disease-free survival of the patients with higher CEM43T90 (≥1 min) values tended to be better in comparison to patients with lower CEM43T90 (<1 min) value (p = 0.071). A complete response tended to be associated with the CEM43T90 (p = 0.056). Disease-free survival, local relapse-free survival and complete response rate for patients with higher CEM43T90 (≥1) were significantly better than those for patients with CRT alone (p = 0.036, p = 0.036 and p = 0.048).
Conclusions: Dose-effect relationships between thermal dose parameters and clinical outcomes were confirmed in the CC patients treated with a combination of CRT + HT. This study also confirmed that HT with lower CEM43T90 is insufficient to achieve a significant hyperthermic sensitisation to CRT.
Introduction
Concurrent cisplatin-based chemoradiotherapy (CRT) has been shown to improve overall survival (OS) in comparison to radiotherapy (RT) alone in patients with locally advanced cancer of the uterus cervix cervical carcinoma (CC). However, the prognosis of the disease is not satisfactory [Citation1–3]. Thus, there is further room for improving the clinical outcomes of these patients. Hyperthermia (HT) is known to be cytotoxic to cancer cells and acts as a radiosensitizer and chemosensitizer [Citation4–9]. The thermal sensitising effects with RT include the following reasons; radiation therapy-resistant tumour cells that are hypoxic, of low pH, nutritionally deprived, and in the S-phase are more sensitive to HT [Citation10]. In 2000, a Dutch group reported the results of phase III study comparing RT alone and RT plus HT in the treatment of malignant pelvic tumours. In the study, significant improvements were observed in the OS and tumour complete response (CR) rates of patients with CC of the uterus who received RT + HT [Citation11]. In the experimental setting, substantial radiosensitisation was observed at temperatures of ≥41 °C [Citation12–19]. The results of a meta-analysis of the data from four phase III studies in which of HT was used to treat advanced and recurrent breast cancer revealed that the local tumour control rates were significantly better when tumours exhibited a higher intra-tumour temperature [Citation20]. The thermal dose parameters were also correlated with the therapeutic outcomes in patients with several types of deep-seated tumour who were treated with RT + HT [Citation21–24].
Recently, the results of a Japanese multicentre randomised clinical trial of concurrent CRT + HT vs. CRT alone, which was performed to evaluate the efficacy and safety of CRT + HT in patients with locally advanced CC, were reported [Citation25]. In the randomised trial, CRT + HT resulted in a significantly improved CR rate in comparison to CRT; however, the survival-related outcomes such as local relapse-free survival (LRFS), disease-free survival (DFS) and OS did not differ to a statistically significant extent [Citation25]. To our knowledge, there are no specific reports evaluating the dose-effect relationship of HT in CC patients treated with definitive CRT + HT, and only one report for preoperative CRT + HT has investigated this relationship in CC patients [Citation26]. In this context, we hypothesised that there may be a positive dose-effect relationship between thermal dose parameters and clinical outcomes in CC patients treated with definitive CRT + HT. The purpose of this study was to assess the thermal dose parameters of regional HT and to explore the potential contribution of regional HT using the data from a Japanese multicentre randomised clinical trial of concurrent CRT + HT vs. CRT alone in patients with locally advanced CC.
Materials and methods
Patients
In the present study, we performed additional analyses of the thermal parameters in the data from a prospective, multicentre, randomised, parallel-group study. The study was conducted at five Japanese institutions by the Japanese Society of Hyperthermic Oncology (JASHO) group between September 2001 and March 2015. It aimed to evaluate the effectiveness of whole-pelvic HT using deep regional heating as well as standard CRT in the treatment of locally advanced CC. The study investigated the clinical response and survival of patients who were treated with cisplatin-based CRT vs. those who received CRT with HT (CRT + HT) [Citation25]. The study protocol was approved by the institutional review boards at participating institutions. All participants provided written informed consent by signing a form that was approved by the institutional review boards of each of the centres. The inclusion and exclusion criteria in the randomised trial were previously reported [Citation25]. The criteria are summarised in . In total, 101 patients with CC were enrolled in the trial; 50 patients were randomised into the CRT group and 51 were randomised into the CRT + HT group. The intrarectal temperatures of patients undergoing deep regional HT were recorded. The complete thermal data of 47 (92%) of the 51 patients in the CRT + HT group were available for the thermal analysis. Thus, 47 patients were included in the current study. The complete thermal data of the four remaining patients in the CRT + HT group were not available; they were therefore excluded from the study. Baseline characteristics and treatment of 47 patients in the CRT + HT group are listed in . Detailed characteristics and treatment of 50 patients in the CRT group have been described previously [Citation25].
Table 1. The inclusion and exclusion criteria.
Table 2. The patient characteristics and treatments.
Chemoradiotherapy
Chemoradiotherapy was performed according to a previously described protocol [Citation25]. In brief, whole-pelvis RT using 6- or 10-MV high-energy linear accelerators was performed, and an additional dose was given to the parametrium with central shielding. RT was delivered with anteroposterior and posteroanterior parallel-opposed ports or four-field box in fractions of 1.8–2 Gy daily, five days per week. Additionally, patients also received high-dose rate intracavitary brachytherapy, which was administered at point A (5–6 Gy per session once per week). The patients were also treated weekly with cisplatin (30–40 mg/m2 for 3–5 cycles).
Hyperthermia and thermal parameters
In all cases, HT treatment consisted of 8 MHz radiofrequency-capacitive regional HT using a Thermotron RF-8 (Yamamoto Vinita Co., Osaka, Japan). The physical features of this instrument and the thermal distribution in a phantom model and the human body have been reported previously [Citation27–29]. The median duration of heating in one heating session was 50 min (30–50). The number of heating sessions ranged from 2 to 5 (median, 5) (). Regional whole-pelvis HT was administered on a weekly basis concurrent with cisplatin-CRT, but not with brachytherapy. Successive treatments were administered in the following order: cisplatin, RT and HT. External RT was performed less than 1 h after the administration of cisplatin. Deep regional HT was applied within 30 min of external RT during an external beam RT course. The three therapeutic modalities (cisplatin, RT and HT) were performed at the same hospital in every patient.
We directly measured the intrarectal temperature using a 4-point microthermocouple sensor (Yamamoto Vinita, LAS-4, Osaka, Japan). The sensor was inserted into the rectum at the level of the uterus during each HT treatment. Intrarectal temperature measurement was performed because vaginal or uteral insertion is more complex and less well tolerated by patients. In addition, a previous study that compared intra-tumour and intra-luminal temperatures during deep regional HT demonstrated that distributions of intrarectal temperature showed a similar pattern as intra-tumour and intra-vaginal temperatures in CC patients [Citation30]. The temperature was automatically recorded each minute during HT. The thermal parameters of the cumulative equivalent min at 43 °C for the T90 (CEM43T90), T90, T25 and T50 were obtained on the basis of the intrarectal temperatures during all of the HT sessions. The Tx is an index temperature, which indicates when the temperature was reached or exceeded by x% of the intrarectal measurement points. The median Tx was defined as the median value of Tx during each HT session. The thermal dose parameter of CEM43T90 has been used extensively (and successfully) in clinical trials to assess the efficacy of heating [Citation19,Citation31–33]. It represents the thermal isoeffect dose expressed in cumulative equivalent min at a reference temperature of 43 °C based on the low end of the temperature distribution (T90). The CEM43T90 was calculated from the time-temperature data using the following formula:
When the temperature is higher than 43 °C, R = 0.5. When the temperature is lower than 43 °C, R = 0.25. In this formula, ti is the time interval of the ith sample (ti = 1.0 min). The temperature exceeding the temperature at 90% of the intrarectal measurement points during the ith minute is designated as T90i. The CEM43T90 is then used to convert each T90i into an equivalent time at 43 °C. These equivalent times are added together over the entire treatment duration of n min.
The evaluation of the treatment outcomes
A complete response (CR) was achieved when no tumour was detected in a physical examination or by magnetic resonance imaging with negative cytology or a negative biopsy result for at least one month after treatment. OS was defined as all-cause death as an outcome event. DFS was all-cause death, local relapse or distant metastasis as outcome events. LRFS was local relapse as an outcome event. In the patients with non-CR of the tumour, the day of the local relapse was defined as the day of the completion of study treatments for the DFS and LRFS. A modification of the Radiation Therapy Oncology Group (RTOG) morbidity scale was used to score patient acute toxicity [Citation34]. The patients were followed up at one month intervals during the first year and at two month intervals thereafter.
Statistical analysis
The LRFS, DFS, and OS rates were calculated using the Kaplan–Meier method. The statistical significance of the differences between the actuarial curves was assessed using the log-rank test. Univariate analyses were performed using certain factors to identify the prognostic factors for LRFS, DFS and OS. Multivariable Cox proportional hazards models were used to investigate the survival outcomes. The Mann–Whitney U-test was used to evaluate differences in the CEM43T90 values of the patients in the CR and non-CR groups. The CR rates and the frequency of acute-phase treatment-related toxicities between the groups of the patients with higher CEM43T90 (≥1 min) and lower CEM43T90 (<1 min) were analysed using Fisher’s exact test. A p < 0.05 was considered significant for all statistical tests.
Results
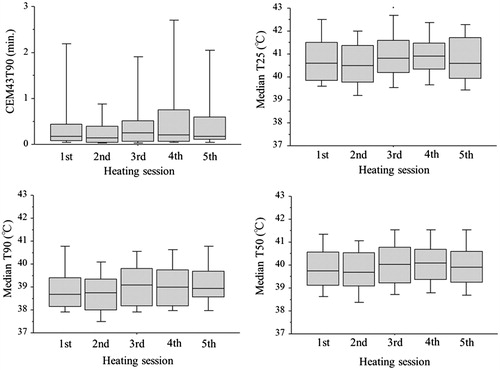
shows the thermal parameters in each of the HT treatment sessions for the 47 patients who received CRT + HT. Throughout the entire treatment course, the CEM43T90 ranged from 0.1 to 46.6 min (median, 1.1 min), the median T25 ranged from 39.0 to 43.0 °C (median, 40.6 °C), the median T90 ranged from 37.7 to 42.2 °C (median, 38.9 °C) and the median T50 ranged from 38.4 to 42.4 °C (39.9 °C).
Figure 1. The thermal parameters (CEM43T90, median T25, median T90 and median T50) in each of the HT treatment sessions.
The five-year LRFS rate of the 47 patients was 81%. Among these patients, a univariate analysis showed that higher CEM43T90 (≥1 min) values over the whole course of treatment and histology of squamous cell carcinoma (SqCC) were significantly associated with better LRFS (p = 0.024 and p = 0.034) ( and . In subsequent multivariate analyses, higher CEM43T90 (≥1 min) and SqCC were both significantly associated with a better prognosis (p = 0.0097 and p = 0.0046) (). LRFS rates for the 26 patients with a higher CEM43T90 (≥1) and the 50 patients treated with CRT alone were significantly different (p = 0.036), whereas LRFS rates for the 21 patients with a lower CEM43T90 (<1) and the 50 patients treated with CRT alone were not different (.
Figure 2. (a) The LRFS for the 26 cases with CEM43T90 ≥ 1 min was significantly better than LRFS for the 21 cases with CEM43T90 < 1 min (p = 0.024) and the 50 CRT alone cases (p = 0.036). The CEM43T90 < 1 min cases had LRFS indistinguishable from the CRT alone cases (p = 0.80). At five years the LRFS for the CEM43T90 ≥ 1 min, CEM43T90 < 1 min, and CRT alone cases were 92%, 65%, and 71% respectively. (b) The DFS for the cases with CEM43T90 ≥ 1 min was significantly better than DFS for the CRT alone cases (p = 0.036), but did not achieve statistical significance when compared with the CEM43T90 < 1 min cases (p = 0.071). The CEM43T90 < 1 min cases had DFS indistinguishable from the CRT alone cases (p = 0.89). At five years the DFS for the CEM43T90 ≥ 1 min, CEM43T90 < 1 min, and CRT alone cases were 81%, 60%, and 61% respectively.
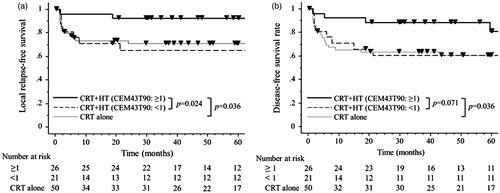
Table 3. The factors predicting survival in the 47 patients treated with definitive CRT plus regional HT.
The five-year DFS rate of the 47 patients was 73%. A univariate analysis revealed that higher CEM43T90 (≥1 min) values tended to predict better DFS (p = 0.071) (). DFS rates for the 26 patients with a higher CEM43 °CT90 (≥1) and the 50 patients treated with CRT alone were significantly different (p = 0.036), whereas DFS rates for the 21 patients with a lower CEM43°CT90 (<1) and the 50 patients treated with CRT alone were not different (.
The OS rate in the 47 patients was 65%. The OS rates of the 26 patients with higher CEM43T90 (≥1 min) values and the 21 patients with lower CEM43T90 (<1 min) values did not differ to a statistically significant extent. There was also no significant difference between for the OS rates between the 26 patients with a higher CEM43T90 (≥1) and the 50 patients treated with CRT alone.
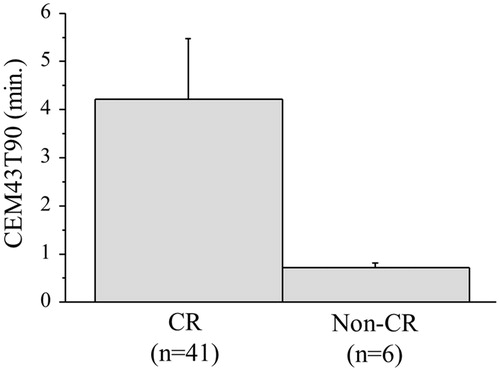
After the completion of the protocol treatment, a CR was achieved in 41 of the 47 patients (87%). A CR tended to be associated with the CEM43T90; the median CEM43T90 value was 1.3 min in 41 patients with a CR; in contrast, the median value in the 6 patients with a non-CR was 0.7 min (p = 0.056) (. A CR was recognised in 25 (96%) of the 26 patients with higher CEM43T90 (≥1 min) values and 16 (76%) of the 21 patients with lower CEM43T90 (<1 min) (p = 0.076). CR rates for the patients with a higher CEM43°CT90 (≥1) and the patients treated with CRT alone were significantly different (p = 0.048), whereas CR rates for the patients with a lower CEM43°CT90 (<1) and the patients treated with CRT alone were not different.
Figure 3. The CR rate tended to be correlated with the CEM43T90 value (p = 0.056). The error bars show the standard error.
The incidence of acute-phase toxicities and the RTOG grades of the CEM43T90 (≥1 min) and CEM43T90 (<1 min) were similar (). There were no significant differences between the two groups in the incidence of any grade 3–4 acute-phase toxicities. The incidence of late-phase toxicities was also similar between the two groups. Late toxicities in the patients treated with CRT + HT with CEM43T90 (≥1 min) were as follows: ileus in 2 (8%) patients, vesico-vaginal fistula in one (4%) patient and leg oedema in one (4%) patient. The findings in the patients treated with CRT + HT with CEM43T90 (<1 min) were as follows: ileus in 2 (10%) patients, ureter stenosis in one (5%) patient and leg oedema in one (5%) patient.
Table 4. Acute-phase toxicities between the CRT + HT with CEM43T90 (≥1) and CRT + HT with CEM43T90 (<1).
Discussion
The present study demonstrated that the thermal dose parameter was associated with better clinical outcomes in patients with CC of the uterus treated with CRT + HT. The strengths of the current study include the fact that it used prospectively randomised sampling data, and that its detailed treatment protocol was defined. Many clinical phase III trials have indicated that the addition of HT to RT can improve clinical outcomes, especially local control rates and CR rates, in patients with superficial tumours [Citation20,Citation35,Citation36]. However, as noted previously, meta-analyses of the thermal data from four clinical phase III trials evaluating RT, with or without HT in the treatment of advanced and recurrent breast cancer, showed that significant improvements in local control rates were only achieved in the patients in whom higher intra-tumour temperatures were achieved [Citation20]. In experimental studies using canine and feline tumours, the efficacy of CEM43 to predict tumour response or local control was confirmed during combined treatment with RT + HT [Citation37,Citation38]. Franckena et al. [Citation21] also demonstrated a dose-effect relationship of HT in CC patients who were treated with definitive RT alone + deep regional HT; intraluminally measured thermal parameters, including CEM43T90, were significantly and independently correlated with tumour control and survival. Sreenivasa et al. [Citation26] reported the positive relationships between certain thermal parameters and the tumour response in 32 patients with non-resectable CC who were treated with preoperative CRT + deep regional HT. They indicated that the CEM 43 °C values in the vagina of 24 responders were significantly higher in comparison to 6 non-responders. In the current study of definitive CRT + deep regional HT in patients with CC, a higher CEM43T90 value in the rectum was found to be significantly associated with better LRFS, and it also tended to be significantly associated with better DFS and CR rates. This study also confirmed that regional HT with lower CEM43T90 is insufficient to achieve a significant hyperthermic sensitisation to CRT.
Excessive power deposition in the subcutaneous fatty tissue, which is associated with pain or thermal burn, may limit the effectiveness of capacitive regional HT and there is also a depth limit in the skin-cooling ability of the 8 MHz RF capacitive heating device [Citation39]. Yahara et al. [Citation24] reported the thermal analyses of 75 prostate cancer patients who received RT + deep regional HT of the pelvis using the 8 MHz radiofrequency-capacitive heating device and indicated that higher CEM43T90 values in the rectum could be achieved in patients with thinner subcutaneous fat in the pelvis. In other studies of capacitive devices in which thermal analyses were performed, thinner subcutaneous fat, a good performance status, and a younger age were significant predictive factors for successful deep regional heating in the thoracic region [Citation23,Citation40]. Asian patients are considered to be relatively suitable for capacitive regional HT due to their slender constitution, whereas radiative phased array devices are more suitable for administering regional HT to Caucasian patients. The current study confirmed, based on the data from a prospective randomised trial, that higher thermal parameters significantly improved the LRFS of CC patients who were treated with definitive CRT + HT. Thus, the further development of deep regional HT methods should be promoted. At the same time, Jones et al. conducted a randomised, phase III trial in patients with superficial cancer treated with RT, with or without HT; patients received a test dose of HT and tumours deemed heatable (≥0.5 CEM43°CT90) were randomly assigned to receive additional HT versus no additional HT [Citation32]. Future randomised trials evaluating the addition of regional HT to CRT in patients with CC should be designed to select patients who are more likely to benefit from HT.
Several prognostic factors have been reported in CC patients treated with CRT. A large population-based study recently showed that a histological finding of adenocarcinoma was still an independently associated with a worse prognosis in CC patients treated with concurrent CRT [Citation41]. On the other hand, Rose et al. [Citation42] reported, using the data from the Gynaecologic Oncology Group trials, that non-squamous cell carcinoma was associated with similar progression-free and OS to squamous cell carcinomas of the cervix when patients were treated with CRT using cisplatin. Parker et al. [Citation43] performed a retrospective analysis and demonstrated that the tumour size was a significant predictor of OS after CRT in CC patients. Nugent et al. reviewed 118 patients with locally advanced CC who were treated with definitive RT and weekly cisplatin (40 mg/m2). They found that nearly 70% of the patients successfully completed six scheduled cycles of cisplatin, and that the completion of 5 or 6 cycles of cisplatin concurrently with RT was significantly correlated with PFS and OS [Citation44]. In the current study, neither the tumour size nor the total dose of cisplatin was significant predictors of LRFS; however, the CEM43T90 value and the tumour histology were significant predictors of LRFS in both the univariate and the multivariate analyses. Thus, the CEM43T90 value might be an independent factor that predicted LRFS in patients treated with definitive CRT plus regional HT.
The present study was associated with several limitations. First, the complete thermal data were not available for four of the patients in the CRT + HT group in the JASHO randomised trial, and these patients were excluded from the analysis. Although the remaining 47 patients with complete thermal data could be properly analysed, the missing data might have influenced the results of the current study. Second, the small study population of the present study made it difficult to detect significance. The application of regional HT as a treatment for malignancies is currently limited throughout the world. Our findings should be validated in future phase III studies or meta-analyses involving larger study populations.
In summary, this is the first report to assess the dose-effect relationship between thermal dose parameters and efficacy of definitive CRT + regional HT in patients with locally advanced CC. The previously reported results of the prospective, multicentre, randomised, parallel-group study conducted at five Japanese institutions by the JASHO group indicated the only significant benefit of adding regional HT to CRT was an improvement of the CR rate [Citation25]. In the current study, we demonstrated that the addition of regional HT to CRT could improve the clinical outcomes in patients with higher CEM43T90 values. Our results provide justification for further evaluations of this treatment strategy. The results also indicate the importance of the accurate selection of treatable patients with higher thermal parameters and that further benefits may be achieved by progress in deep regional heating methods.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Keys HM, Bundy BN, Stehman FB, et al. (1999). Cisplatin, radiation, and adjuvant hysterectomy compared with radiation and adjuvant hysterectomy for bulky stage IB cervical carcinoma. N Engl J Med 340:1154–61.
- Morris M, Eifel PJ, Lu J, et al. (1999). Pelvic radiation with concurrent chemotherapy compared with pelvic and para-aortic radiation for high-risk cervical cancer. N Engl J Med 340:1137–43.
- Rose PG, Bundy BN, Watkins EB, et al. (1999). Concurrent cisplatin-based radiotherapy and chemotherapy for locally advanced cervical cancer. N Engl J Med 340:1144–53.
- Datta NR, Rogers S, Klingbiel D, et al. (2016). Hyperthermia and radiotherapy with or without chemotherapy in locally advanced cervical cancer: a systematic review with conventional and network meta-analyses. Int J Hyperthermia 32:809–21.
- Datta NR, Puric E, Klingbiel D, et al. (2016). Hyperthermia and radiation therapy in locoregional recurrent breast cancers: a systematic review and meta-analysis. Int J Radiat Oncol Biol Phys 94:1073–87.
- Cihoric N, Tsikkinis A, van Rhoon G, et al. (2015). Hyperthermia-related clinical trials on cancer treatment within the ClinicalTrials.gov registry. Int J Hyperthermia 31:609–14.
- Longo TA, Gopalakrishna A, Tsivian M, et al. (2016). A systematic review of regional hyperthermia therapy in bladder cancer. Int J Hyperthermia 32:381–9.
- Sousa A, Pineiro I, Rodriguez S, et al. (2016). Recirculant hyperthermic IntraVEsical chemotherapy (HIVEC) in intermediate-high-risk non-muscle-invasive bladder cancer. Int. J Hyperthermia 32:374–80.
- Datta NR, Rogers S, Ordonez SG, et al. (2016). Hyperthermia and radiotherapy in the management of head and neck cancers: a systematic review and meta-analysis. Int J Hyperthermia 32:31–40.
- Jones EL, Samulski TV, Leonard RP, Dewhirst MW. (2003). Hyperthermia, 4th ed. Philadelphia: Lippincott Williams & Wilkins,.
- van der Zee J, Gonzalez Gonzalez D, van Rhoon GC, et al. (2000). Comparison of radiotherapy alone with radiotherapy plus hyperthermia in locally advanced pelvic tumours: a prospective, randomised, multicentre trial. Dutch Deep Hyperthermia Group. Lancet 355:1119–25.
- Ben-Hur E, Elkind MM. (1974). Thermally enhanced radioresponse of cultured Chinese hamster cells: damage and repair of single-stranded DNA and a DNA complex. Radiat Res 59:484–95.
- Krawczyk PM, Eppink B, Essers J, et al. (2011). Mild hyperthermia inhibits homologous recombination, induces BRCA2 degradation, and sensitizes cancer cells to poly (ADP-ribose) polymerase-1 inhibition. Proc Natl Acad Sci U S A 108:9851–6.
- Issels R, Kampmann E, Kanaar R, Lindner LH. (2016). Hallmarks of hyperthermia in driving the future of clinical hyperthermia as targeted therapy: translation into clinical application. Int J Hyperthermia 32:89–95.
- Oei AL, Vriend LE, Crezee J, et al. (2015). Effects of hyperthermia on DNA repair pathways: one treatment to inhibit them all. Radiat Oncol 10:165
- Horsman MR, Overgaard J. (2007). Hyperthermia: a potent enhancer of radiotherapy. Clin Oncol (R Coll Radiol) 19:418–26.
- Dewey WC, Hopwood LE, Sapareto SA, Gerweck LE. (1977). Cellular responses to combinations of hyperthermia and radiation. Radiology 123:463–74.
- Sapareto SA, Hopwood LE, Dewey WC. (1978). Combined effects of X irradiation and hyperthermia on CHO cells for various temperatures and orders of application. Radiat Res 73:221–33.
- van Rhoon GC. (2016). Is CEM43 still a relevant thermal dose parameter for hyperthermia treatment monitoring? Int J Hyperthermia 32:50–62.
- Sherar M, Liu FF, Pintilie M, et al. (1997). Relationship between thermal dose and outcome in thermoradiotherapy treatments for superficial recurrences of breast cancer: data from a phase III trial. Int J Radiat Oncol Biol Phys 39:371–80.
- Franckena M, Fatehi D, de Bruijne M, et al. (2009). Hyperthermia dose-effect relationship in 420 patients with cervical cancer treated with combined radiotherapy and hyperthermia. Eur J Cancer 45:1969–78.
- Rau B, Wust P, Tilly W, et al. (2000). Preoperative radiochemotherapy in locally advanced or recurrent rectal cancer: regional radiofrequency hyperthermia correlates with clinical parameters. Int J Radiat Oncol Biol Phys 48:381–91.
- Ohguri T, Imada H, Yahara K, et al. (2009). Radiotherapy with 8-MHz radiofrequency-capacitive regional hyperthermia for stage III non-small-cell lung cancer: the radiofrequency-output power correlates with the intraesophageal temperature and clinical outcomes. Int. J Radiat Oncol Biol Phys 73:128–35.
- Yahara K, Ohguri T, Yamaguchi S, et al. (2015). Definitive radiotherapy plus regional hyperthermia for high-risk and very high-risk prostate carcinoma: thermal parameters correlated with biochemical relapse-free survival. Int J Hyperthermia 31:600–8.
- Harima Y, Ohguri T, Imada H, et al. (2016). A multicentre randomised clinical trial of chemoradiotherapy plus hyperthermia versus chemoradiotherapy alone in patients with locally advanced cervical cancer. Int J Hyperthermia 32:801–8.
- Sreenivasa G, Hildebrandt B, Kummel S, et al. (2006). Radiochemotherapy combined with regional pelvic hyperthermia induces high response and resectability rates in patients with nonresectable cervical cancer > or = FIGO IIB “bulky”. Int J Radiat Oncol Biol Phys 66:1159–67.
- Song CW, Rhee JG, Lee CK, Levitt SH. (1986). Capacitive heating of phantom and human tumors with an 8 MHz radiofrequency applicator (Thermotron RF-8). Int J Radiat Oncol Biol Phys 12:365–72.
- Hiraoka M, Jo S, Akuta K, et al. (1987). Radiofrequency capacitive hyperthermia for deep-seated tumors. I. Studies on thermometry. Cancer 60:121–7.
- Abe M, Hiraoka M, Takahashi M, et al. (1986). Multi-institutional studies on hyperthermia using an 8-MHz radiofrequency capacitive heating device (Thermotron RF-8) in combination with radiation for cancer therapy. Cancer 58:1589–95.
- Fatehi D, van der Zee J, Notenboom A, van Rhoon GC. (2007). Comparison of intratumor and intraluminal temperatures during locoregional deep hyperthermia of pelvic tumors. Strahlenther Onkol 183:479–86.
- Oleson JR, Samulski TV, Leopold KA, et al. (1993). Sensitivity of hyperthermia trial outcomes to temperature and time: implications for thermal goals of treatment. Int J Radiat Oncol Biol Phys 25:289–97.
- Jones EL, Oleson JR, Prosnitz LR, et al. (2005). Randomized trial of hyperthermia and radiation for superficial tumors. J Clin Oncol 23:3079–85.
- Dewhirst MW, Viglianti BL, Lora-Michiels M, et al. (2003). Basic principles of thermal dosimetry and thermal thresholds for tissue damage from hyperthermia. Int J Hyperthermia 19:267–94.
- Trotti A, Byhardt R, Stetz J, et al. (2000). Common toxicity criteria: version 2.0. an improved reference for grading the acute effects of cancer treatment: impact on radiotherapy. Int J Radiat Oncol Biol Phys 47:13–47.
- Triantopoulou S, Efstathopoulos E, Platoni K, et al. (2013). Radiotherapy in conjunction with superficial and intracavitary hyperthermia for the treatment of solid tumors: survival and thermal parameters. Clin Transl Oncol 15:95–105.
- Overgaard J, Gonzalez Gonzalez D, Hulshof MC, et al. (1995). Randomised trial of hyperthermia as adjuvant to radiotherapy for recurrent or metastatic malignant melanoma. European Society for Hyperthermic Oncology. Lancet 345:540–3.
- Dewhirst MW, Sim DA, Sapareto S, Connor WG. (1984). Importance of minimum tumor temperature in determining early and long-term responses of spontaneous canine and feline tumors to heat and radiation. Cancer Res 44:43–50.
- Thrall DE, LaRue SM, Yu D, et al. (2005). Thermal dose is related to duration of local control in canine sarcomas treated with thermoradiotherapy. Clin Cancer Res 11:5206–14.
- Kroeze H, van de Kamer JB, de Leeuw AA, et al. (2003). Treatment planning for capacitive regional hyperthermia. Int J Hyperthermia 19:58–73.
- Ohguri T, Yahara K, Moon SD, et al. (2011). Deep regional hyperthermia for the whole thoracic region using 8 MHz radiofrequency-capacitive heating device: relationship between the radiofrequency-output power and the intra-oesophageal temperature and predictive factors for a good heating in 59 patients. Int J Hyperthermia 27:20–6.
- Lee JY, Kim YT, Kim S, et al. (2015). Prognosis of cervical cancer in the era of concurrent chemoradiation from national database in Korea: a comparison between squamous cell carcinoma and adenocarcinoma. PLoS One 10:e0144887.
- Rose PG, Java JJ, Whitney CW, et al. (2014). Locally advanced adenocarcinoma and adenosquamous carcinomas of the cervix compared to squamous cell carcinomas of the cervix in gynecologic oncology group trials of cisplatin-based chemoradiation. Gynecol Oncol 135:208–12.
- Parker K, Gallop-Evans E, Hanna L, Adams M. (2009). Five years' experience treating locally advanced cervical cancer with concurrent chemoradiotherapy and high-dose-rate brachytherapy: results from a single institution. Int J Radiat Oncol Biol Phys 74:140–6.
- Nugent EK, Case AS, Hoff JT, et al. (2010). Chemoradiation in locally advanced cervical carcinoma: an analysis of cisplatin dosing and other clinical prognostic factors. Gynecol Oncol 116:438–41.
