Abstract
Rationale: Hyperthermic isolated lung Perfusion (ILuP) is used to deliver high-dose chemotherapy to pulmonary metastases while sparing systemic toxicity. Accurate leakage monitoring is however necessary. This study aimed to verify the accuracy of radionuclide leakage monitoring in patients undergoing ILuP, by comparing this method with serial blood sampling.
Methods: A total of 15 consecutive ILuP procedures were performed on eleven patients affected by lung metastases from soft tissue sarcoma. After establishing isolated perfusion, erythrocytes of systemic blood (SB) were labelled with 0.2 MBq/kg of 99mTc. The baseline SB counting rate (CR) was assessed using a γ-probe. Subsequently, erythrocytes of the circuit blood (CB) were labelled with 2 Mbq/kg of 99mTc. Radioactivity leakage factor (RLF) was continuously measured using a formula, accounting for CR, systemic/circuit activity ratio and total/systemic volume ratio. The TNF-α concentration in SB and CB was measured by enzymelinked immunosorbent assay (ELISA) throughout the procedure.
Results: RLF averaged 2.3 ± 1.5%, while the systemic/circuit TNF-α ratio was 0.05 ± 0.12%. These two indices were strictly correlated in all of the procedures (average Rvalue 0.88 ± 0.07). RLF exceeded 5% during three of 15 procedures, prompting the application of compensatory manoeuvres. ELISA confirmed a marked increase in systemic TNF-α levels in these patients (2.6 ± 3.5 ng/ml). Conversely, patients whose RLF did not exceed the 5% threshold presented a mean TNF-α of 0.02 ± 0.005 ng/ml (p < 0.01).
Conclusions: In patients submitted to ILuP, RLF monitoring is feasible and accurate. Moreover, it grants immediate results, permitting for the adoption of corrective manoeuvres for leakage, thus minimising toxicity.
Introduction
Hyperthermic isolated perfusion is a surgical procedure employed to deliver high doses of active drug to a target organ or area. Its aim is to treat malignancies within a single region, while avoiding the toxicity related to the systemic administration of supra-maximal drug doses [Citation1]. In isolated perfusion, the effects of hyperthermia can be synergistic with the drug’s cytotoxic action by reducing the oxygen concentration within the tumour lesion [Citation2], as well as by enhancing the tumour-specific immune response [Citation3].
Since its introduction in locally advanced melanoma [Citation4], isolated perfusion has been paired with drug leakage monitoring; this pairing has allowed for identifying and limiting drug spill-over from the isolated circuit into the systemic circulation [Citation5–7]. More recently, the introduction of recombinant human TNF-α (TNF-α) has further emphasised the need for prompt leakage recognition because even modest cytokine shunting to the whole-body circulation can result in severe complications, including but not limited to prolonged hypotension, renal damage, rhabdomyolysis and bone marrow failure [Citation8–11]. TNF-α toxicity, can occur for leakage ratios as low as 1%, in addition to its life-threatening nature, contributes to prolonging patients’ hospitalisation and increasing overall costs [Citation11,Citation12].
In this context, leakage monitoring with blood sampling might be unsuited for toxicity prevention because the results of TNF-α blood concentrations can only be obtained, in the best-case scenario, after a 20-min delay [Citation13,Citation14]. Conversely, using a radiopharmaceutically based technique permits the immediate application of compensatory manoeuvres [Citation13]. Owing to these advantageous characteristics, the radiopharmaceutical evaluation of systemic leakage in isolated limb perfusion has been extensively validated [Citation7,Citation13–16].
Over the years, isolated perfusion has been increasingly used; its applications have been tested in clinical studies, including in neo-adjuvant use [Citation17], rare tumour types [Citation18] and non-limb settings [Citation19].
Moreover, isolated perfusion has been extended to treat multiple and unresectable lung metastases, using diverse chemotherapy protocols [Citation6,Citation20–22] or TNF-α [Citation23–25]. However, a validation of real-time leakage monitoring in the lung perfusion has been lacking until today.
Leakage monitoring during lung perfusion could in fact be more complicated, compared with the limb scenario. The lung is in fact perfused by a dual circulation; drug delivery is performed through the pulmonary system, but a shunt between pulmonary and bronchial vessels could occur. Moreover, fluid could spill from the lung into the pleural cavity, where reuptake from the pleural venous and lymphatic vessels could occur. Fluid dynamics could in addition be altered by the vasoactive effects of both hyperthermia and TNF-α [Citation25]. In this context, adequate clamping of the pulmonary veins might not be sufficient to prevent drug leakage.
The current study aimed thus to evaluate the feasibility and reliability of radioisotope leakage monitoring during hyperthermic isolated lung perfusion (ILuP). To this purpose, we compared actual drug leakage, obtained by serial blood sampling and representing the “gold standard” of leakage quantification, with estimated blood shunting, obtained in real time by radio pharmaceutically based techniques. Moreover, to clarify the possible underlying mechanisms (bronchial vs. pleural circulation-based shunt), we evaluated the role of pleural fluid re-uptake in determining systemic leakage.
Materials and methods
The present study describes a retrospective analysis of prospectively acquired data; the study was approved by the institutional review board (Comitato etico regionale regione liguria). The study was registered in the European clinical trials database under the following number: 2012–001286-33. All of the subjects signed an informed consent form, including the utilisation of anonymized bio-material and data for research purposes. All of the experiments involving human subjects were performed in accordance with the principles of the declaration of Helsinki and its later amendments.
Patient population
Eleven patients (6 women, mean age 56 ± 21 years old, age range 19–81), affected by multiple pulmonary localizations from soft-tissue or osseous sarcoma, consecutively underwent a total of 15 ILuP procedures (4 patients were treated bilaterally, with a 30 day interval between surgeries).
The inclusion criteria were: 1) primary sarcoma eradicated or amenable to control; 2) metastatic disease limited to the lungs; 3) cardiac and lung functional reserve consistent with the planned intervention; 4) adequate haematologic, hepatic and renal function; 5) multiple (more than 3 nodules) lung metastases and 6) no other effective systemic treatment available, by collegial decision within the framework of the local multidisciplinary disease management team.
The exclusion criteria were: 1) pericardial or pleural effusions; 2) concurrent infection; or 3) hepatic or renal insufficiency. Previous thoracotomies and metastasectomies were not considered as a exclusion criteria. Pulmonary function was evaluated by arterial gas assessment, spirometry, DLCO and lung perfusion scans with 99mTc-labelled macro-aggregates. Cardiac function was evaluated by echocardiography. Further non-pulmonary disease localizations were excluded using CT, MR and whole body 18 F-FDG PET/CT scans on a case-by-case basis.
Surgical and perfusion techniques
In all cases, ILuP was performed before metastasectomy so as not to compromise pulmonary blood flow. After an antero-lateral thoracotomy at the fifth intercostal space, the pericardium was opened, to expose the pulmonary vessels and left atrium. Intravenous heparin was administered (5000 IU) before pulmonary artery (PA) clamping. A satinsky clamp was positioned across the left atrium to collect the blood flow from the involved pulmonary veins. Polypropylene purse-string sutures were placed in both the PA and pulmonary veins. Both the PA and the homolateral pulmonary vein system were cannulated and connected to the perfusion circuit. This procedure included a dedicated device (Performer-LRT, RanD, Medolla, Italy) and a paediatric oxygenator (Lilliput 2, Dideco, Mirandola, Italy), which were able to control blood oxygenation and temperature at the same time. The pre-heated fluid was pushed through the heat exchanger using a peristaltic pump, while a second pump delivered the warmed/oxygenated blood to the lung. Venous blood from the lung returned to the reservoir by gravity. The perfusion circuit integrated a filtration system to ensure possible blood clot removal. The circuit was primed with a mixture of saline (600 ml) and human albumin (50 ml), supplemented with 5000 IU of sodium heparin. The temperatures of the perfusion fluid were monitored at the oxygenator, as well as at the lung inlet and outlet, by thermistor probes (Mon-a-Therm, Covidien LLC, Mansfield, MA). The flow rate was adjusted within a 300–600 ml/min range to maintain physiological values of perfusion pressure. When the lung’s outlet fluid temperature reached 39 °C and no sign of leakage was present, TNF-α (Beromun®, Boehringer Ingelheim GmbH, Ingelheim/Rhein, Germany) was infused into an inlet port on the PA cannula at the standard dose of 1 mg. Thirty min after TNF-α injection, with the fluid temperature having reached 40 °C, melphalan (Alkeran®, Glaxo SmithKline Manufacturing spa, S. Polo di Torrile, Italy) was injected into the circuit at the standard dose of 30 mg. Subsequently, the perfusion was maintained for 60 min. At the end of perfusion, washout was performed with 4 litres of 0.9% saline solution at 37 °C. The flow rate was adjusted to maintain a zero fluid balance, as indicated by the electronic weight/volume control system. The isolated lung was ventilated during perfusion and the perfusate was oxygenated to prevent pulmonary vasoconstriction as a result of hypoxia. The circulating blood volume was restored to maintain optimal hemodynamic conditions.
Metastasectomy was then performed according to standard techniques, the basic commitment being to spare as much lung parenchyma as possible.
Leakage monitoring
Systemic leakage from the circuit was monitored with a radioisotope technique, according to a modified version of the method proposed by Kristoffersen et al. [Citation26]. Briefly, thyroid blockade was performed with potassium perchlorate (400 mg), administered orally 2 h before the intervention, to avoid the uptake and subsequent redistribution of free pertechnetate. Soon after circuit setup and before drug administration, a gamma scintillation probe (NucleoProbe, NucleoMed, Rome, Italy) was securely fixed over the temporal artery and set in a specific ILuP mode (energy window: 140 KeV ±10%, time of single reading: 10 s). Red blood cell labelling was performed with an in vivo technique: a single-dose vial of stannous pyrophosphate (Angiocis, Iba Molecular, Paris, France) was reconstructed and injected into the systemic circulation; 20–30min later, a dose of 200 KBq per kg of body weight of 99mTc was injected. Counts were then continuously measured; once a steady state was reached, the baseline counting-rate (bCR) was recorded. Thereafter, RBCs within the isolated circuit were labelled with a ten-fold dose of the same radioisotope, utilising the same in vivo technique. ILuP was then started and executed as described above. In five patients, blood samples in ethylene diamine tetra acetic acid were separately collected at the procedure end from both the isolated circuit and the systemic blood; its content was centrifuged (500 g for 10 min) to separate RBCs from plasma. Labeling efficiency (i.e. the ratio between RBC activity and total activity) was in all cases greater than 95%.
Percent radioactive leakage factor (RLF) was defined by the following formula:
where “bCR” represents the baseline CR over the temporal artery, before isotope injection into the isolated circuit; “dcCR” is the actual measurement during ILuP, corrected for the physical 99mTc decay (decay-corrected CR); sysA and perfA correspond to the injected activities (in MBq) into the systemic and perfusion circuits, respectively and “Total Volume” refers to the estimated total blood volume in litres, calculated according to the standard formulation (Weight in kg x X/1000, with X = 75 for men and X = 65 for women), while “Systemic Volume” refers to the difference between Total Volume and the known volume within the perfusion circuit.
Readings from the probe were continuously monitored by a nuclear medicine specialist throughout the procedure to detect any sudden changes in the 10 s counting rate. Data were then inserted into a spreadsheet on a handheld device every 5 min, which used the above-described formula to calculate RLF. Leakage of 10% was set as the maximum acceptable limit [Citation11,Citation17]: if RLF exceeded this value for more than two consecutive readings, ILuP was halted. To prevent this occurrence, compensatory manoeuvres, including clamp revision and flow rate adjustment, were started whenever RLF exceeded 5% on two consecutive readings [Citation13,Citation14].
Determination of TNF-α levels
Samples from the perfusate and peripheral blood were collected before and during ILuP at 3, 15, 30, 60 and 90 min, respectively. Subsequently, venous blood samples were obtained at 1, 3 and 24 h after the end of the surgical procedure. Quotas from pleural fluids were also collected at the end of the procedure. Samples were centrifuged (10 min at 2000 rpm) to allow for separation and either the perfusate or the serum was collected. Quantitative determination of total TNF-α (either administered or autologous) in the perfusate and serum was performed using a commercially available ELISA Kit (Invitrogen Corp, Camarillo, CA) according to the manufacturer’s instructions. Samples from the perfusate were properly diluted to obtain values in the range of the TNF-α standard curve (0–1000 pg/ml).
Post-operative follow-up
After ILuP and metastasectomy, patients were transferred to the intensive care unit, where they were observed for the next 24 h for possible signs of TNF-α related toxicity.
Arterial blood pressure, HCO3-, lactate, arterial gases, potassium, glucose and creatinine were monitored to identify possible shock syndrome and consequent metabolic acidosis.
Fluid infusion and dopamine were used to treat hypotension whenever needed. Similarly, the onset of metabolic acidosis and lung oedema was treated according to the current intensive care unit procedures.
After discharge, the patients were followed up with monthly visits, which include a complete physical examination, blood work-up, pulmonary function testing, chest X-ray and thoracic CT, as required. Chronic pulmonary toxicity was assessed by CT scan and pulmonary function tests before ILuP and 3, 6 and 9 months after treatment. Forced vital capacity, forced expiratory volume in 1 s and diffusion lung capacity for carbon monoxide were measured.
Statistical analysis
All of the data are reported as the mean ± standard deviation. The area under the curve (AUC) of the TNF-α concentration was computed for each patient as the sum of the timex concentration products, divided by the procedure duration in minutes. Differences between groups were tested using one-way analysis of variance, with intergroup comparison afforded using Bonferroni’s test. Differences between repeated samplings were investigated using a generalised linear model for repeated samples. Regression analysis was performed by the least squares method. A probability value of p < 0.05 was considered significant. Statistical analyses were performed using a software application (SPSS, v. 21.0, IBM, Armonk, NY).
Results
RLF levels
In all of the patients, RLF steadily increased until minute 50 (4.2 ± 1.1%) and then slowly descended, reaching a mean value of 3.2 ± 2.4% at the end of the procedure (p < 0.05, ). The overall mean was 2.3 ± 1.5%. However, its behaviour was largely heterogeneous because the 5% threshold was surpassed in three of 15 cases (20%). In these patients, defined as “heavy leakers” (HL), RLF showed a biphasic curve with two peaks at minutes 40 (6.8 ± 0.7%, p < 0.01 vs. the other patients) and 65 (6.9 ± 2.1% p < 0.01 vs. the remaining patients), respectively, before dropping to 4.2 ± 3.7% at minute 90 (). In all of these patients, as soon as the 5% threshold was exceeded, the surgical team started compensatory procedures within two minutes.
Figure 1. Mean actual and estimated TNF-α leakage. Top panel illustrates (log scale) the fluctuation in systemic/circuit TNF-α ratio; note how this value is inconspicuous in “low” leakers. In the bottom panel is depicted the radioisotope estimation of circuit-to-systemic leakage.
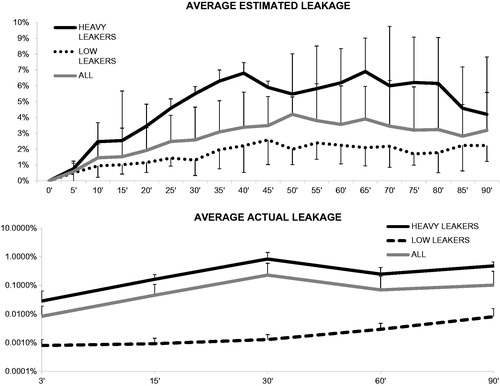
In contrast, the remaining 12 of 15 patients, defined as “low leakers” (LL), RLF remained steadily low during the whole perfusion period, averaging 1.6 ± 0.5%; therefore, compensatory manoeuvres were not required ().
TNF-α levels in the circuit and in the peripheral blood
In the whole population, the maximum TNF-α concentration in the isolated circuit reached its peak (1966 ± 846 ng/ml) at minute 3 after ILuP start () and slowly dwindled thereafter, reaching a mean value of 701 ± 375 ng/ml at the end of the 90 min (p < 0.01). No differences were noted between HL and LL at any time point, documenting that lesion exposure to the therapeutic agent was similar in the two groups.
Figure 2. Circuit and systemic TNF-α levels. Mean circuit levels (top panel) tended to progressively decrease during the ILuP procedure, as the drug was delivered to the target tissue. Systemic levels (depicted in a logarithmic scale) showed a differential behaviour in “heavy” and “low” leakers: in the former, corrective interventions could counter the initial drug shunt, while in the latter, the levels were consistently low throughout the procedure.
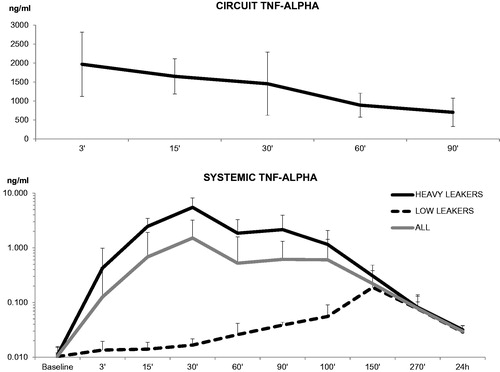
Systemic TNF-α levels showed a different behaviour, starting at 0.01 ± 0.004 ng/ml before the procedure, peaking 30 min after the ILuP start (2.3 ± 4.4 ng/ml) and slowly returning to 0.6 ± 1.3 ng/ml at minute 90 (). These levels remained elevated up to 24 h after the procedure (0.03 ± 0.008 ng/ml, p < 0.001 vs. baseline, ).
In agreement with RLF, the TNF-α peak at minute 30 was markedly higher in HL’s than in LL’s (5.5 ± 7.9 vs. 0.02 ± 0.005 ng/ml, p < 0.01, ). The effectiveness of compensatory procedures was confirmed by the subsequent decrease in systemic cytokine levels (1.8 ± 1.4 ng/ml at minute 60) in the systemic blood of HL’s. Nevertheless, TNF-α concentrations remained significantly higher in HL’s than in LL’s up to 60 min after the procedure ended (, p < 0.01) and gradually decreased during the subsequent 24 h in both groups down to 0.032 ± 0.006 ng/ml vs. 0.028 ± 0.008 ng/ml in HL’s and LL’s, respectively ().
Accuracy of RLF in TNF-α spill-over detection
To estimate its agreement with RLF, actual drug leakage (AL) was expressed as a percentage ratio of systemic to circuit TNF-α concentrations. The mean AL was 0.05 ± 0.12%. In each patient, RLF and AL were closely correlated, with R-values ranging from 0.72 to 0.96 and a median of 0.9 (, ). Remarkably, this correlation persisted even when the whole population was cumulatively considered (R index= 0.88, , p < 0.01).
Figure 3. Correlation between actual and estimated leakage. A significant correlation was detected when considering either all of the patients (top panel), “heavy” leakers (middle panel), or low leakers (bottom panel).
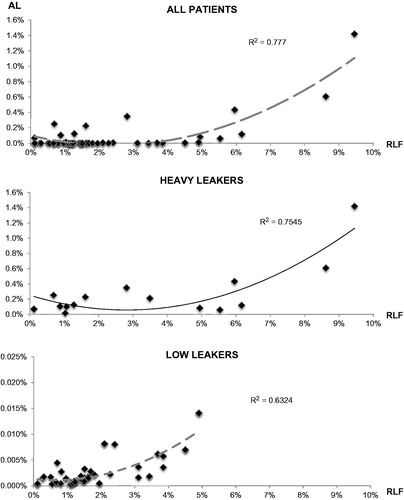
Table 1. Actual and estimated leakage in Heavy leakers (HL) and Low leakers (LL).
At the end of the procedure, the TNF-α concentration in the pleural aspirate was 458.5 ± 390.2 ng/ml and showed a direct correlation with the circuit TNF-α AUC (R = 0.64, p < 0.05, ). RLF was inversely correlated with cytokine concentrations in the pleural aspirate reservoir (R index 0.8, p < 0.01, ), while it was directly correlated with the difference between circuit/reservoir TNF-α concentrations (0.74, p < 0.01, ).
Figure 4. Role of pleural shunt. The definite integral of TNF-α levels within the circuit is mirrored by the quantity of cytokine found in the aspirate (upper panel). However, it is the efficiency of TNF-α removal from the cavity that determines the success of leakage prevention: patients with high levels of removed cytokine (middle panel) or with a smaller difference between the circuit and pleural aspirate TNF-α (bottom panel) have correspondingly lower RLF. Black dots represent “heavy leakers”.

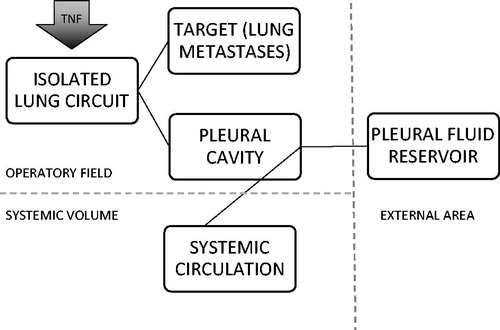
A schematic representation of the possible TNF-α pathways is depicted in .
Figure 5. Metabolic destinies of TNF-α. After injection into the isolated circuit, the cytokines can be distributed to the lung and to the lung metastases or they can leak into the pleural cavity. Thereby, they can be removed by the operator by aspiration or can be reabsorbed by the pleural venous and lymphatic circulation.
Clinical findings
In six of 15 procedures (40%), post-operative reversible edema was observed in the treated lung, which however recovered within two to three days following the intervention. No sign of pulmonary parenchyma damage was evident on CT imaging 3, 6 and 9 months after ILuP. However, forced vital capacity and forced expiratory volume decreased with respect to baseline and then slowly returned to pre-ILuP values 6 months after the intervention. Diffusing capacity of the lungs for carbon monoxide values remained lower than baseline at the 9-month follow up.
Following the intervention, two of three HL patients presented a late hypotensive syndrome, which required the intravenous infusion of fluids and the administration of inotropic drugs. A temporary rise in creatinine levels was also observed in both subjects. However, this complication was successfully resolved by supportive treatment and these two patients were normally discharged after 11 and 14 days, respectively. In contrast, no complications traceable to systemic TNF-α effects could be observed in any LL.
Discussion
Selective drug administration is a treatment modality that is potentially able to change the course of diseases in advanced stages, which are usually characterised by scarce therapeutic options [Citation22,Citation23,Citation25]. Its main tenet is the accurate isolation of the target circulatory district [Citation27] and thus requires the availability of reliable methods capable of promptly and accurately detecting any possible spill-over to the systemic circulation. This task is particularly challenging when highly cytotoxic substances are employed [Citation28–30] or when the target organ is characterised by multiple possible shunts, such as the lung [Citation31].
The present study approached this specific setting and demonstrated that radio-isotopic leakage monitoring is feasible and easy to implement and allows for the correct and timely identification of the occurrence of systemic contamination. Specifically, this conclusion is based on the correlation of RLF with evidence of TNF-α appearance in the systemic circulation, as detected by serial sampling: both methods were actually able to identify the heavy leaker subgroup. From the methodological point of view, RLF reliability was confirmed by its high predictive accuracy, which was independent of the leakage amount (whether trivial or considerable). From the pathophysiological point of view, the present study confirmed the complexity of properly isolating the lung circulation. TNF-α concentrations within the circuit over time (the area under the curve) showed a certain variation among patients; however, drug concentrations in the reservoir were proportional to the quantity circulating in the isolated circuit. This finding suggests that, being the injected cytokine dose equal in all patients, the distribution to the desired target (i.e. the lung metastases) was variable and the longer that a high TNF-α quantity circulates within the circuit, the more likely that it is to eventually spill over into the pleural space. However, pleural reuptake can be prevented by regular and accurate fluid drainage. This finding was confirmed by the inverse correlation between TNF-α concentrations in the reservoir and the leakage factor: the more fluid that was removed, the less that remained available for reuptake and subsequent diffusion into the systemic circulation.
This circuit-pleural-systemic pathway represents the most relevant difference with respect to limb perfusion, in which the sole possibility of shunting is via the venous system [Citation1,Citation4,Citation7,Citation8]. In contrast, toxic drug concentrations in the systemic circulation were only reached in the three HL subjects, in whom RLF detected relevant leakage. In all of these subjects, the prompt hazard signal offered by radionuclide measurements permitted the application of compensatory procedures, the effectiveness of which was monitored by RLF and was subsequently confirmed by the analysis of systemic blood TNF-α levels.
RLF monitoring can be achieved with overall limited costs; it requires a minimal amount of tracer with trivial radiation exposure to the patient and to the surgical personnel [Citation26]. RLF monitoring can be performed with a gamma probe and any spreadsheet-capable device. From the technical point of view, a great deal of attention must be paid to correct probe placement, which must be steadily fixed above the artery. Angular shift must be avoided to avoid the contribution of salivary gland uptake to recorded counts.
This study presented some limitations. It was a retrospective, single-centre study, including a relatively small number of patients due to the novelty of the procedure and because of the very stringent inclusion criteria. However, the correlation between RLF and actual leakage was robust on a patient-by-patient basis, in both in low and heavy leakers. The measurement of RLF in the temporal artery could be a potential source of artefacts because even minor volume changes within the cerebral blood volume could affect the counting rate. To prevent this occurrence, the probe could also be placed on the aortic bifurcation or over a common iliac artery. However, the feasibility of this alternate placement depends on the operation theatre setup because it could hinder some operation-related procedures. Finally, blood pool measurements were executed with red blood cell labelling rather than the long validated human serum albumin [Citation4]. Marked erythrocytes can present a poorer labelling yield, leading to relative inaccuracies relative to the confounding effects of the unbound tracer. However, thyroid blockade and proper probe placement minimised this potential pitfall. Moreover, the use of 99mTc allows for better photon detection, compared to high-energy 131I photons [Citation32]. Furthermore, a major advantage of this protocol is that it enables virtually every centre to reproduce the technique, especially in areas where labelled human serum albumin is not commercially available. Finally, automated software applications now allow for the connecting of the probe to a portable computer, facilitating instant RLF calculation and curve generation [Citation33]. However, they require a commercial licence and to this day this is a slightly more cumbersome set-up. The possibility of wirelessly sending data from the probe to any handheld or remote device would represent a definite improvement of such systems. In contrast, the procedure described here could maximise the diffusion of ILuP calculation because it does not require specific software applications or setup.
Conclusions
This paper represents the first report of real-time dual leakage monitoring during isolated lung perfusion in humans and it shows that the radiopharmaceutically derived values correlate with actual leakage indices. Furthermore, it demonstrates that the gap between blood collection and results readings could be sidestepped using this method. Larger multi-centre, prospective trials could be helpful in better defining of its application fields and its reliability under different drug and perfusion regimens. As regional perfusion grows and encompasses a number of previously untreatable scenarios, this technique will serve in avoiding unnecessary toxicity, thus improving clinical outcomes and patients’ quality of care.
Disclosure statement
The authors report no conflicts of interest.
This article does not contain any studies with animals performed by any of the authors.
Additional information
Funding
References
- Rosin RD. (1997). The complications of isolated limb perfusion. Surg Technol Int 6:143–50.
- Jakob J, von Rege I, Weiss C, Hohenberger P. (2012). Impact of hyperthermic isolated limb perfusion on tumour oxygenation in soft tissue sarcoma. Int J Hyperthermia 28:591–6
- Olofsson R, Lindberg E, Karlsson-Parra A, et al. (2013). Melan-A specific CD8+ T lymphocytes after hyperthermic isolated limb perfusion: a pilot study in patients with in-transit metastases of malignant melanoma. Int J Hyperthermia 29:234–8
- Creech Jr O, Ryan RF, Krementz ET. (1961). Regional chemotherapy by isolated perfusion in the treatment of melanoma of the extremities. Plast Reconstr Surg Transplant Bull 28:333–46.
- Olofsson R, Ny L, Eilard MS, et al. (2014). Isolated hepatic perfusion as a treatment for uveal melanoma liver metastases (the SCANDIUM trial): study protocol for a randomized controlled trial. Trials 15:317.
- Ratto GB, Toma S, Civalleri D, et al. (1996). Isolated lung perfusion with platinum in the treatment of pulmonary metastases from soft tissue sarcomas. J Thorac Cardiovasc Surg 112:614–22.
- Stehlin Jr JS, Clark Jr RL, Dewey WC. (1961). Continuous monitoring of leakage during regional perfusion. Arch Surg 83:943–9.
- Lienard D, Ewalenko P, Delmotte JJ, et al. (1992). High-dose recombinant tumor necrosis factor alpha in combination with interferon gamma and melphalan in isolation perfusion of the limbs for melanoma and sarcoma. J Clin Oncol 10:52–60
- Thom AK, Alexander HR, Andrich MP, et al. (1995). Cytokine levels and systemic toxicity in patients undergoing isolated limb perfusion with high-dose tumor necrosis factor, interferon gamma, and melphalan. J Clin Oncol 13:264–73
- Hohenberger P, Haier J, Schlag PM. (1997). Rhabdomyolysis and renal function impairment after isolated limb perfusion–comparison between the effects of perfusion with rhTNF alpha and a 'triple-drug' regimen. Eur J Cancer 33:596–601.
- Eggimann P, Chioléro R, Chassot PG, et al. (1995). Systemic and hemodynamic effects of recombinant tumor necrosis factor alpha in isolation perfusion of the limbs. Chest 107:1074–82.
- Zwaveling JH, Maring JK, Clarke FL, et al. (1996). High plasma tumor necrosis factor (TNF)-alpha concentrations and a sepsis-like syndrome in patients undergoing hyperthermic isolated limb perfusion with recombinant TNF-alpha, interferon-gamma, and melphalan. Crit Care Med 24:765–70.
- Daryanani D, Komdeur R, Ter Veen J, et al. (2001). Continuous leakage measurement during hyperthermic isolated limb perfusion. Ann Surg Oncol 8:566–72.
- Alexander C, Omlor G, Berberich R, et al. (1993). Rapid measurement of blood leakage during regional chemotherapy. Eur J Nucl Med 20:187–91.
- Van Ginkel RJ, Limburg PC, Piers DA, et al. (2002). Value of continuous leakage monitoring with radioactive iodine-131-labeled human serum albumin during hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan. Ann Surg Oncol 9:355–63.
- Rastrelli M, Campana LG, Valpione S, et al. (2016). Hyperthermic isolated limb perfusion in locally advanced limb soft tissue sarcoma: a 24-year single-centre experience. Int J Hyperthermia 32:165–72.
- Andreou D, Werner M, Pink D, et al. (2016). Histological response assessment following neoadjuvant isolated limb perfusion in patients with primary, localised, high-grade soft tissue sarcoma. Int J Hyperthermia 32:159–64
- Belgrano V, Ben-Shabat I, Bergh P, et al. (2016). Isolated limb perfusion as a treatment option for rare types of tumours. Int J Hyperthermia 32:595–9.
- Ben-Shabat I, Belgrano V, Hansson C, et al. (2017). The effect of perfusate buffering on toxicity and response in isolated hepatic perfusion for uveal melanoma liver metastases. Int J Hyperthermia 22:1–17.
- Hendriks JM, Grootenboers MJ, Schramel FM, et al. (2004). Isolated lung perfusion with melphalan for resectable lung metastases: a phase I clinical trial. Ann Thorac Surg 78:1919–26.
- Den Hengst WA, Van Putte BP, Hendriks JM, et al. (2010). Long-term survival of a phase I clinical trial of isolated lung perfusion with melphalan for resectable lung metastases. Eur J Cardiothorac Surg 38:621–7.
- Den Hengst WA, Hendriks JM, Balduyck B, et al. (2014). Phase II multicenter clinical trial of pulmonary metastasectomy and isolated lung perfusion with melphalan in patients with resectable lung metastases. J Thorac Oncol 9:1547–53.
- Pass HI, Mew DJ, Kranda KC, et al. (1996). Isolated lung perfusion with tumor necrosis factor for pulmonary metastases. Ann Thorac Surg 61:1609–17.
- Pogrebniak HW, Witt CJ, Terrill R, et al. (1994). Isolated lung perfusion with tumor necrosis factor: a swine model in preparation of human trials. Ann Thorac Surg 57:1477–83.
- Sleijfer S, van Ginkel RJ, van der Mark TW, et al. (1997). Effects of hyperthermic isolated limb perfusion with tumor necrosis factor-alpha and melphalan on pulmonary function assessments. J Immunother 20:202–7.
- Kristoffersen US, Straalman K, Schmidt G, et al. (2009). Radiation exposure to surgical staff during hyperthermic isolated limb perfusion with 99mTechnetium labeled red blood cells. Int J Hyperthermia 25:86–9.
- Sedger LM, McDermott MF. (2014). TNF and TNF-receptors: from mediators of cell death and inflammation to therapeutic giants - past, present and future. Cytokine Growth Factor Rev 25:453–72.
- Gurrola-Díaz CM, Sánchez-Enriquez S, Oregon-Romero E, et al. (2009). Establishment of a cut-point value of serum TNF-alpha levels in the metabolic syndrome. J Clin Lab Anal 23:51–6.
- Xiao H, Chen L, Luo G, et al. (2014). Effect of the cytokine levels in serum on osteosarcoma. Tumour Biol 35:1023–8.
- Karayiannakis AJ, Syrigos KN, Polychronidis A, et al. (2001). Serum levels of tumor necrosis factor-alpha and nutritional status in pancreatic cancer patients. Anticancer Res 21:1355–8.
- McCullagh A, Rosenthal M, Wanner A, et al. (2010). The bronchial circulation–worth a closer look: a review of the relationship between the bronchial vasculature and airway inflammation. Pediatr Pulmonol 45:1–13.
- Zanzonico P, Heller S. (2000). The intraoperative gamma probe: basic principles and choices available. Semin Nucl Med 30:33–48.
- Wierts R, de Jong JR, Lazarenko SV, et al. (2011). Characteristics of a new system for monitoring the leakage factor during regional hyperthermic isolated limb perfusion. Nucl Med Commun 32:853–62.
