Abstract
Background: Given that high-intensity focussed ultrasound (HIFU) of benign thyroid nodules often causes a massive release of thyroglobulin (Tg) into the circulation, we hypothesised a greater initial Tg rise may result in a greater nodule shrinkage 6 months after ablation.
Methods: One hundred and five patients who underwent HIFU for symptomatic benign thyroid nodule from 2015 to 2016 were analysed. Serum Tg and anti-Tg autoantibody were checked on treatment day (baseline) and 4 d after treatment. The % of Tg rise = [serum Tg on day-4 – baseline serum Tg]/[baseline serum Tg] * 100 while the nodule shrinkage as measured by volume reduction ratio (VRR) = [baseline volume – volume at 6-month]/[baseline volume] * 100. Treatment success was defined as VRR >50%.
Results: At 6-month, the mean VRR was 62.2 ± 25.0% and 59 (76.6%) patients had treatment success. The mean baseline Tg level increased from 292.8 ± 672.7 ng/mL to 2022.7 ± 1759.8 ng/mL in the first-week. The % of Tg rise did not significantly correlate with either 3-month or 6-month VRR (p = 0.920 and p = 0.699, respectively). The mean % of Tg rise in the first week was not different between those with and without 6-month treatment success (368.2% vs. 1068.7%, p = 0.381). No clinical factors significantly correlated with treatment success.
Conclusions: There was an almost seven-fold increase in the mean Tg level 4 d after HIFU ablation. The % of Tg rise in the first week did not appear to correlate with the 6-month nodule shrinkage or treatment success.
Introduction
Thyroid nodules are common and although most are benign and remain relatively static, some do become large and can cause local symptoms [Citation1–3]. In this situation, thyroidectomy is usually indicated [Citation1,Citation2]. However, surgery may be associated with complications, high cost and requires a general anaesthesia. As a result, there has been a growing interest in developing less invasive, non-surgical technique in the treatment of benign thyroid nodules [Citation4–6]. For predominantly solid or solid nodules, thermal ablation techniques are highly effective [Citation4–6]. High-intensity focussed ultrasound (HIFU) is one of the ablation techniques. It utilises focussed ultrasound energy to induce ablation. Although overall it is effective in inducing significant nodule shrinkage and alleviating nodule-related symptoms and is often perceived as being less invasive because it does not require skin penetration with a needle, up to 20% of nodules may not shrink adequately [Citation7–9].
To date, no clinical markers are available to predict which nodules would shrink adequately after HIFU treatment. Thyroglobulin (Tg) is a 660 kDa dimeric protein that is produced by the follicular cells in the thyroid gland and can be readily measured in the serum before and after HIFU treatment. After nodule ablation, serum Tg level rises sharply and remains elevated and peaked before slowly returning to its pre-ablation level [Citation7,Citation10–13]. This happens because thermal ablation induces necrosis of follicular thyroid cells and that causes a massive release of Tg into the circulation. Given that nodule shrinkage depends on the extent of tissue necrosis induced by the ablation, we hypothesise that a greater rise in serum Tg may reflect a greater area of necrosis leading to a greater extent of nodule shrinkage over time. If this concept is correct, serum Tg could potentially be used as a clinical predictor of treatment success. To our knowledge, this issue has never been investigated in studies involving percutaneous laser ablation (PLA) or radiofrequency ablation. To date, only one study has examined the relationship between the rise in serum Tg in the early post-ablation period and the subsequent nodule shrinkage after microwave ablation but found no significant association [Citation12]. Given these issues, we aimed to examine the relationship between the rise in serum Tg in the first-week and the extent of nodule shrinkage 6 months after single-session HIFU ablation.
Methods
This retrospective analysis was approved by local institutional review board. All consecutive patients who underwent HIFU ablation for a symptomatic, solid or predominantly solid (<30% cystic areas), benign thyroid nodule from August 2015 to December 2016 were analysed. Over this period, HIFU ablation was only indicated for patients who did not wish to undergo surgery. Details on the eligibility for ablation were previously described [Citation9,Citation14]. The inclusions for the present study were (1) patients who only received one single ablation treatment. (2) Patients who had serum TSH, FT4, Tg and anti-thyroid autoantibodies checked on the day of treatment (baseline) and 4 d after treatment (i.e. at the first-week visit). (3) Patients who had a follow-up of at least 6 months following treatment. Patients who received two treatments to the same nodule or had two nodules treated in the same session or those who had their Tg checked on days other than day of treatment and 4 d after treatment were excluded. Also given that the presence of anti-Tg auto-antibody tends to interfere with Tg measurements and may result in falsely low Tg readings [Citation15,Citation16], patients with a titre >99 IU/ml were not included from the analysis. The extent of serum Tg rise (%) in the first week was calculated based on the formula: [serum Tg on day 4 – serum Tg at baseline]/[serum Tg at baseline] * 100. Serum Tg were rechecked at 3-month and 6-month. To see whether there was a significant association between initial change in serum Tg and subsequent treatment efficacy or success, the extent of serum Tg rise (%) in the first week were correlated with the extent of nodule shrinkage at 3- and 6-months.
Treatment efficacy
Each nodule was measured by USG on the day of treatment (baseline), 3-month and 6-month. Nodule dimensions were measured using the LOGIQ e (GE Healthcare, Milwaukee, WI) scanner equipped with a 10–14 MHz linear matrix transducer. Three orthogonal diameters of the index nodule (its longest diameter and two other perpendicular diameters) were measured. In general, the longest diameter was the cranio-caudal dimension (length) of the nodule while the other two perpendicular diameters were the medio-lateral (width) and antero-posterior (depth) dimensions of the nodule. All measurements were made to the nearest 0.1 mm. To estimate nodule volume, we used the formula: volume (mL) = (width (in cm) × length (in cm) × depth (in cm)) × (π/6), where π was taken as 3.14159. The volume reduction ratio (VRR) was calculated based on the formula: [baseline volume – volume at visit]/[baseline volume] * 100. Treatment success was defined as ≥50% volume reduction at 6-month from baseline.
HIFU treatment
All treatments were performed by one person (B. H. L.) with >2 years of experience using the same USG-guided HIFU device. This device comprised an energy generator, a treatment head, a skin-cooling device, and a touch-screen interface for planning. The treatment head incorporated an image transducer (7.5 MHz, 128 elements, linear array) and HIFU transducer (3 MHz, single element, 60 mm in diameter). After positioning, patients were sedated with diazepam (10–15 mg) and pethidine (50–100 mg). Under USG guidance, the treatment head was positioned until the entire index nodule was within the treatable depth. The device computer (Beamotion version no. TUS 3.2.2, Theraclion, Paris, France) automatically divided the nodule into multiple ablation subunits (voxels). Each voxel measured approximately 7.3 mm in thickness and 5 mm in width and received a continuous 8-s pulse of HIFU energy followed by 30–40 s of cooling time before the beam was moved to the adjacent voxel. This cycle continued until all planned voxels were ablated. To ensure safety, nearby structures like the carotid artery, trachea, and skin were marked out on the treatment screen before the start of treatment by the operator. To avoid inadvertent heat injury to important surrounding structures, the device automatically selected the following safety margins: (a) 0.5 cm from the skin, (b) at least 0.5 cm from the trachea and RLN and (c) 0.2 cm from the ipsilateral carotid artery and cancelled any voxels which were within these distances. A laser-based movement detector enabled immediate power interruption when the patient moved or swallowed during ablation. To avoid skin burn, the skin was cooled by a balloon (filled with 10 °C liquids) at the tip of the treatment head. All ablations started at 204 J/pulse and increased up to 280 J/pulse until hyperechoic marks appeared at the focal point. Both the total amount of energy delivered to the nodule (in KJ) and the “on-beam” (sonification) time taken (in minutes) were automatically recorded by the device’s computer. Treatment time was taken as the time required for both planning and sonification. Patients were also asked to rate their pain during, immediately after treatment and before hospital discharge on a visual analogue scale (VAS) (0 = no pain and 10 = worse possible pain). Oral diet was resumed immediately afterwards and patients were allowed to go home two hours after treatment.
Laboratory methods
All measurement of TSH, FT4, Tg and anti-thyroid autoantibodies were carried out at our institution’s laboratory. Plasma TSH was determined by a specific two-site immunometric assay (Eleosys 2010, Boehringer Mannheim, Germany). Tg was determined by the same immunometric assay (the Immulite 2000, Diagnostic Products Corp. Roche, Los Angeles, CA). It was calibrated against the CRM- 457 standard. Serum anti-Tg and anti-TPO auto-antibodies were determined by radioimmunoassay (Bio Code, Izasa, LieÁge, Belgium) and any values >99 IU/ml were considered positive.
Statistical analysis
Continuous variables were expressed as mean ± SD. Median and interquartile range were also presented when appropriate. Continuous variables between groups were compared using the Mann–Whitney U test. Chi-square tests were used to compare categorical variables. For correlation between two continuous variables, the Spearman’s correlation test was performed. Binary logistic regression model was performed to evaluate factors leading to treatment success. All statistical analyses were performed using SPSS version 18.0 (SPSS, Inc., Chicago, IL). All significance tests were two-tailed and those with a p value less than 0.05 were considered statistically significant.
Results
Altogether 109 patients completed single-session ablation of a symptomatic benign thyroid nodule. Four patients (3.7%) received two sequential HIFU treatments in the same session because they all had two large nodules that required treatment but there were no patients who received bilateral treatment in the same session. Since there were 29 patients who were tested positive (>99 IU/mL) for anti-Tg auto-antibody at baseline, only 76 (69.7%) were included for analysis.
shows the baseline characteristics, treatment parameters, efficacy and complications of the cohort. At 3-month, the mean nodule volume was 6.8 ± 8.4 ml and the mean VRR was 48.4 ± 22.8% (median = 47.2%; interquartile range (IQR) = 37.0%). Forty-two (55.3%) patients achieved treatment success at 3-month. At 6-month, the mean VRR increased to 63.9 ± 26.6% (median = 71.0%; IQR = 36.1%) and 59 (77.6%) patients achieved treatment success. Three (3.9%) patients suffered from a vocal cord paresis shortly after treatment but they all had a full recovery within the first 2 months. There was no local infection or skin burn in any of the patients. One patient had subclinical hypothyroidism (i.e. raised TSH and normal FT4) in the first 3 months but did not need thyroxine supplementation. Pain was most severe during ablation (VAS: 3.5 ± 4.0) but improved immediately after ablation (VAS: 1.0 ± 1.0).
Table 1. Baseline characteristics, treatment parameters, efficacy and complications after single-session high intensity focussed ultrasound.
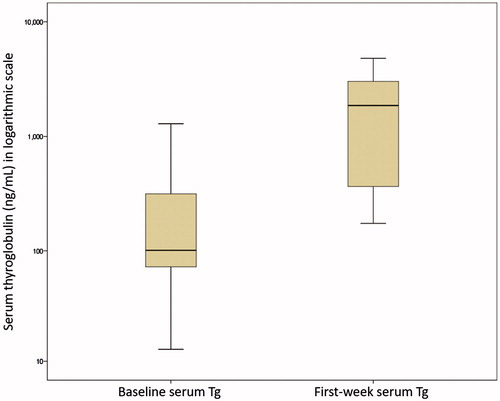
shows the box plots for serum Tg at baseline and in the first week. The mean serum Tg level increased from 292.8 ± 672.7 ng/mL at baseline to 2022.7 ± 1759.8 ng/mL in the first week. The mean extent of serum Tg rise during the first week was 2812.9 ± 8321.9% (median = 510.4%, IQR = 1045.0%). However, by 3-month and 6-month, the mean serum Tg came down to 146.2 ± 216.4 ng/mL and 132.7 ± 168.2 ng/mL, respectively. There was nobody (0.0%) who had a reduced serum Tg (i.e. equal or less than baseline) in the first week. The lowest rise in serum Tg in the first week was 72.28%.
Figure 1. Two box plots with one showing the serum thyroglobulin (Tg) on the day of treatment (baseline) and the other showing Tg 4 d after single-session high-intensity focussed ultrasound treatment (first-week).
shows the correlations between serum Tg and patient characteristics, treatment parameters and efficacy. On one hand, a higher baseline serum Tg was significantly associated with larger diameter nodule (ρ = 0.372, p = 0.007), larger volume nodule (ρ = 0.323, p = 0.020) and longer “on-beam” time (ρ = 0.273, p = 0.035). On the other hand, the serum Tg in the first week did not significantly correlated with any of the treatment parameters including the 3-month or 6-month VRR (p = 0.973 and p = 0.758, respectively). Similarly, the extent of serum Tg rise (%) in the first week did not significantly correlated with any of the treatment parameters including the 3-month or 6-month VRR (p = 0.920 and p = 0.699, respectively). The mean % rise in serum Tg in the first week was not significantly different between those with treatment success (n = 59) and those without treatment success (n = 17) (368.2% vs. 1068.7%, p = 0.381).
Table 2. Bivariate spearman’s correlations between percentage change in serum thyroglobulin (Tg) during the first week and baseline characteristics, treatment parameters and efficacy after single-session high intensity focussed ultrasound (HIFU) ablation.
Factors leading to treatment success
By binary logistic regression, none of the clinical factors examined including baseline nodule diameter (OR = 0.705, 95%CI = 0.416–1.809, p = 0.705), baseline nodule volume (OR = 0.975, 95%CI= 0.895–1.062, p = 0.559), total energy given (OR = 0.959, 95%CI = 0.857–1.138, p = 0.866), total “on-beam” time (OR = 0.988, 95%CI = 0.944–1.042, p = 0.992), power per voxel (OR = 0.645, 95%CI = 0.973–1.017, p = 0.645), baseline Tg (OR = 1.001, 95%CI = 0.999–1.004, p = 0.492), first-week Tg (OR = 1.000, 95%CI = 0.999–1.001, p = 0.621), extent of Tg rise in the first week (OR = 0.998, 95%CI = 0.994–1.001, p = 0.247) turned out to be significantly associated with treatment success at 6 months.
Discussion
Similar to other studies, our data showed that there was a significant rise in serum Tg level in the first week after HIFU ablation [Citation7,Citation13]. On an average, there was an almost seven-fold increase in serum Tg (292.8 ± 672.7 ng/mL to 2022.7 ± 1759.8 ng/mL) in the first week. This appeared to be similar in magnitude to another study reporting a baseline Tg level of 118 ± 390 ng/mL rising to 963 ± 3376 ng/mL in the first week following PLA of benign thyroid nodules [Citation11]. It is worth noting that in that study, the serum Tg level actually peaked in the first 24 h of ablation and gradually fell afterwards and by 3 months onward, the mean serum level became less than the baseline [Citation11]. Although serum Tg on the first ablation day was not available in the present study, our data did show that the mean serum Tg level fell below the baseline level from 3 months onwards. Perhaps, this is a reflection of the lower nodule volume following the ablation.
However, in contrast to our study hypothesis, our data did not find a significant correlation between the extent of serum Tg rise in the first week and the 3-month or 6-month VRR (p = 0.920 and p = 0.699, respectively). Although baseline Tg significantly correlated with the pre-ablation nodule size, nodule volume and treatment time, the extent of serum Tg rise in the first week did not correlate with either 6-month VRR or treatment success. Also those with treatment success (n = 59) had comparable extent of Tg rise as those without treatment success (n = 17) (368.2% vs. 1068.7%, p = 0.381).
Consistent with our previous analyses [Citation17,Citation18], there were no identifiable clinical predictors including baseline nodule diameter (p = 0.558), nodule volume (p = 0.964), total energy given (p = 0.959), total “on-beam” time (p = 0.594), power per voxel (p = 0.554), baseline Tg (p = 0.903), first-week Tg (p = 0.871), and extent of Tg rise in the first week (p = 0.998) for 6-month treatment success. Perhaps, future studies could focus more on this issue as this has an important bearing on patient selection for HIFU ablation as well as thermal ablation of benign thyroid nodules in general.
Given that HIFU remains a relatively new procedure with few centres around the world adopting it as a treatment for benign thyroid nodules, clinicians should be critical in analysing its risk to benefit ratio relative to other more established thermal ablation techniques. For example, a higher risk of temporary vocal cord paresis following HIFU treatment was noted relatively to other ablation techniques. Nevertheless, this represented our earlier experience and we are confident this injury is preventable and its rate could be lowered over time [Citation19].
Despite these findings, we would like to acknowledge several shortcomings. First, our study was a moderately sized study and so, our results were prone to type II errors (i.e. some of the non-significant findings including the lack of significant correlation between extent of serum Tg rise and subsequent nodule shrinkage might have been due to inadequate power of the study). Second, since there was an upper limit for the Tg assay (>4800 ng/mL), it was difficult to obtain an accurate measurement when the serum level was extremely high.
Conclusion
On an average, there was a seven-fold increase in the mean serum Tg level in the first week after single-session HIFU ablation of benign thyroid nodule. However, unlike our hypothesis, the extent of serum Tg rise in the first week did not correspond to the subsequent extent of nodule shrinkage.
Disclosure statement
All authors had nothing to disclose. No competing financial interests exist.
References
- Gharib H, Papini E, Garber JR, et al. (2016). AACE/ACE/AME Task Force on Thyroid Nodules. American Association of Clinical Endocrinologists, American College of Endocrinology, and Associazione Medici Endocrinologi Medical Guidelines for Clinical Practice for the diagnosis and management of thyroid nodules – 2016 Update. Endocr Pract 22:622–39.
- Haugen BR, Alexander EK, Bible KC, et al. (2016). 2015 American Thyroid Association Management Guidelines for Adult Patients with Thyroid Nodules and Differentiated Thyroid Cancer: The American Thyroid Association Guidelines Task Force on Thyroid Nodules and Differentiated Thyroid Cancer. Thyroid 26:1–133.
- Durante C, Costante G, Lucisano G, et al. (2015). The natural history of benign thyroid nodules. JAMA 313:926–35.
- Gharib H, Hegedüs L, Pacella CM, et al. (2013). Clinical review: nonsurgical, image-guided, minimally invasive therapy for thyroid nodules. J Clin Endocrinol Metab 98:3949–57.
- Sung JY, Baek JH, Kim KS, et al. (2013). Single-session treatment of benign cystic thyroid nodules with ethanol versus radiofrequency ablation: a prospective randomized study. Radiology 269:293–300.
- Wong KP, Lang BH. (2013). Use of radiofrequency ablation in benign thyroid nodules: a literature review and updates. Int J Endocrinol 2013:428363.
- Korkusuz H, Sennert M, Fehre N, et al. (2014). Local thyroid tissue ablation by high-intensity focused ultrasound: effects on thyroid function and first human feasibility study with hot and cold thyroid nodules. Int J Hyperthermia 30:480–5.
- Kovatcheva RD, Vlahov JD, Stoinov JI, Zaletel K. (2015). Benign solid thyroid nodules: US-guided high-intensity focused ultrasound ablation-initial clinical outcomes. Radiology 276:597–605.
- Lang BH, Woo YC, Wong CK. (2017). High intensity focused ultrasound (HIFU) treatment for symptomatic benign thyroid nodules: a prospective study. Radiology. [Epub ahead of print]. doi: 10.1148/radiol.2017161640
- Cakir B, Topaloglu O, Gul K, et al. (2006). Effects of percutaneous laser ablation treatment in benign solitary thyroid nodules on nodule volume, thyroglobulin and anti-thyroglobulin levels, and cytopathology of nodule in 1 yr follow-up. J Endocrinol Invest 29:876–84.
- Valcavi R, Riganti F, Bertani A, et al. (2010). Percutaneous laser ablation of cold benign thyroid nodules: a 3-year follow-up study in 122 patients. Thyroid 20:1253–61.
- Heck K, Happel C, Grünwald F, Korkusuz H. (2015). Percutaneous microwave ablation of thyroid nodules: effects on thyroid function and antibodies. Int J Hyperthermia 31:560–7.
- Korkusuz H, Sennert M, Fehre N, et al. (2015). Localized thyroid tissue ablation by high intensity focused ultrasound: volume reduction, effects on thyroid function and immune response. Rofo 187:1011–5.
- Lang BH, Woo YC, Chiu KW. (2017). Single-session high-intensity focused ultrasound treatment in large-sized benign thyroid nodules. Thyroid 27:714–21.
- Spencer CA, Takeuchi M, Kazarosyan M, et al. (1998). Serum thyroglobulin autoantibodies: prevalence, influence on serum thyroglobulin measurement, and prognostic significance in patients with differentiated thyroid carcinoma. J Clin Endocrinol Metab 83:1121–7.
- Spencer C, LoPresti J, Fatemi S. (2014). How sensitive (second-generation) thyroglobulin measurement is changing paradigms for monitoring patients with differentiated thyroid cancer, in the absence or presence of thyroglobulin autoantibodies. Curr Opin Endocrinol Diabetes Obes 21:394–404.
- Lang BH, Wong CKH, Ma EPM. (2017). Single-session high intensity focused ablation (HIFU) versus open cervical hemithyroidectomy for benign thyroid nodule: analysis on early efficacy, safety and voice quality. Int J Hyperthermia. [Epub ahead of print]. doi: 10.1080/02656736.2017.1305127
- Lang BH, Woo YC, Chiu KW. (2017). High-intensity focused ablation (HIFU) of single benign thyroid nodule rarely alters underlying thyroid function. Int J Hyperthermia. [Epub ahead of print]. doi: 10.1080/02656736.2017.1318456
- Lang BH, Woo YC, Chiu KW. (2017). Vocal cord paresis following single-session high intensity focused ablation (HIFU) treatment of benign thyroid nodules: incidence and risk factors. Int J Hyperthermia. [Epub ahead of print]. doi: 10.1080/02656736.2017.1328130
