Abstract
Purpose: Microwave ablation (MWA) is a recently developed thermal ablation technique that has been used for the treatment of different types of tumours. In the present study, we retrospectively evaluated the safety and efficacy of CT-guided percutaneous MWA for the treatment of colorectal cancer (CRC) pulmonary metastases.
Materials and methods: From June 2010 to June 2015, 48 unresectable lesions in 32 patients with CRC pulmonary metastases were subjected to CT-guided MWA. Imaging follow-up was with contrast-enhanced CT and 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT.
Results: Oncologic imaging showed that 42 (87.5%) of the 48 lesions in the 32 patients were completely ablated. Needle track metastatic seeding was not found, and no patient deaths occurred within 30 d after ablation. The mean hospital stay was 3 d (range, 2–7 d). Pneumothorax was the most frequent complication and occurred in 6 (12.5%) of the 48 lesions. The median survival time was 31 months (95% CI: 15.4–46.6). The 1-, 2- and 3-year survival rates were 79.5%, 63.1% and 44.4%, respectively. Univariate Cox regression analysis showed that tumour size, disease-free interval (DFI) and number of tumours were significantly related to the overall survival time (p = .007, p = .022 and p = .030, respectively). Multivariate analysis showed that tumour size was an independent prognostic factor for survival (p = .017).
Conclusion: CT-guided percutaneous MWA is a safe and effective minimally invasive method for treating CRC pulmonary metastases.
Introduction
Colorectal cancer (CRC) is a common malignancy of the human gastrointestinal tract, with approximately 1.2 million newly diagnosed cases per year worldwide [Citation1]. Pulmonary metastasis is common in patients with advanced CRC (approximately 5–15%) [Citation2]. Surgical resection is considered as a standard treatment for isolated CRC pulmonary metastases, but only a limited proportion of patients are eligible for this procedure [Citation3,Citation4]. Most patients with inoperable CRC pulmonary metastases are treated with systemic chemotherapy, which is also associated with various limitations due to side effects and drug resistance. The median survival time of patients with untreated CRC pulmonary metastases is less than 10 months, and the 5-year survival rate is less than 5% [Citation2,Citation5]. Therefore, the development of new treatment strategies for this disease is essential.
In recent years, the use of MWA to treat tumours in the lung has increased [Citation6,Citation7]. MWA offers all the benefits of radiofrequency ablation (RFA) plus some additional advantages, including reduced procedure time, enlarged ablation zone and decreased heat-sink effect [Citation6,Citation8]. In the present study, we retrospectively evaluated the clinical efficacy and safety of CT-guided percutaneous MWA for the treatment of CRC pulmonary metastases.
Materials and methods
Clinical data
This trial has been registered with Cancer Research Online (https://www.clinicaltrials.gov/), with the ClinicalTrials.gov identifier NCT02502630. All presented 32 patients had undergone surgical resection of their primary CRC with subsequent histopathological assessment. Of these patients, 21 were males and 11 were females. The median age of the patients was 63 years, with a range from 44 to 72 years. Primary tumours were cleared via R0 resections; however, metastases were found in their lungs after surgery. The tumours were either proven to be lung neoplasms that had metastasised from CRC by pathology or they were assumed to be CRC metastases based on their imaging characteristics. Among the 48 pulmonary lesions in the 32 patients, 40 and eight of the lesions were metachronous pulmonary and synchronous metastases, respectively, and 34 and 14 of the pulmonary metastases were located in the lateral and medial zones of the lung, respectively. By the end of the follow-up period, 16 patients had died due to the progression of advanced carcinoma. The details of the patients’ characteristics are shown in . The study was approved by the Ethics Committee of the hospital, and all patients gave their written informed consent prior to enrolment.
Table 1. Patients characteristics.
To receive MWA of CRC pulmonary metastases, the following inclusion criteria were used: (1) primary tumour site under control; (2) MWA technically feasible; (3) patients who had three or fewer lesions; and (4) patients with lesions that were 6 cm or smaller in maximal axial diameter.
The following exclusion criteria were used: (1) uncontrolled primary malignancy; (2) radiologic evidence of lymph node metastases or liver metastases; (3) uncorrectable coagulopathy (as indicated by an international normalised ratio >1.8 or a platelet count <75 000); (4) septicaemia; or (5) patient refusal of microwave ablation therapy.
Treatment methods
Preoperative preparation
Preoperative routine examinations, including routine blood, hepatorenal function, coagulation function, carcino-embryonic antigen, ECG, and the thoracoabdominal CT or 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT examinations, were performed to rule out ablation contraindications. The patients were fasted for 12 h prior to ablation and received intramuscular injections of diazepam (10 mg) and morphine (10 mg) 30 min prior to ablation.
Instruments and equipment
A KY-2000 microwave multifunctional therapeutic apparatus (Kangyou Medical Instruments, Nanjing, China) was used for the ablation. This system consisted of a microwave generator with a frequency of 2450 MHz, a power output of 10–100 W (continuously adjustable) and a 15-cm high-strength water-cooled antenna with an external diameter of 1.9 mm. CT-guided imaging (Somatom Emotion 16, Siemens, Erlangen, Germany) was used.
Ablation process
The percutaneous needle entry point and the needle path toward the lesion were determined based on tumour size, location, and morphology in addition to adjacent structures and access route. The antenna position was confirmed by CT fluoroscopy. After marking the skin and sterile prepping and draping, 2% lidocaine was used for topical infiltration anaesthesia at the puncture site and insertion route. During the procedure, an appropriate amount of conscious sedation (morphine/diazepam) was used according to the reaction of the patient. Tumours with a maximal diameter ≤3 cm were treated by placing an applicator in the centre of the tumour. Nodules >3 cm were treated at two to three different sites based on tumour size and shape. The selected power range was 50–70 W with ablation times ranging from 4 to 16 min. Overlapping ablations were performed, aiming for the ablation of a circumferential safety margin of 0.5–1.0 cm beyond the borders of the target lesion to ensure the complete ablation of all tumour tissue. To prevent the seeding of malignant cells in the puncture track during the removal of the antenna, puncture-track coagulation was routinely performed at the end of the procedure. During the procedure, patients were monitored by continuous pulse oximetry and ECG and blood pressure was measured every 5 min. After the procedure, CT scanning was performed to check for complications, such as pneumothorax or haemorrhage.
Follow-up, complications and response
Routine follow-up imaging was contrast-enhanced CT or 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET)/CT. CT scans were acquired 24 h after MWA as the baseline images. The follow up visits occur at 1, 3 and 6 months and yearly. Additional CT scans were also performed in patients who developed symptoms suggesting disease progression, including cough, haemoptysis or dyspnoea. Complications were considered to be related to treatment if they occurred within 30 d of ablation. The response to MWA was classified as either complete or incomplete ablation [Citation9].
Statistical analyses
Overall survival (OS) was defined as the time from the date of MWA to the date of death or the date of the final follow-up. DFI was the period of time from the resection of the primary lesion of intestinal cancer to the first sign of pulmonary metastases. All data were analysed with the SPSS software package 13.0 (SPSS, Chicago, IL). OS rates were evaluated with a Kaplan–Meier analysis. A Cox regression analysis was performed to evaluate the prognosis factors for pulmonary metastases from CRC using univariate and multivariate analyses. Differences with p values less than 0.05 were considered to be significant.
Results
Evaluation of short-term effects
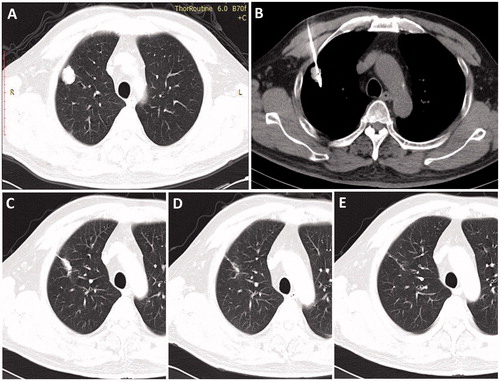
The mean hospital stay was 3 d (range, 2–7 d). The evaluation of oncologic imaging 3 months after ablation indicated that 42 (87.5%) of the 48 lesions in the 32 patients were completely ablated. Typical CT images obtained during the follow-up are shown in .
Figure 1. Axial CT images of a 71-year-old man with a 2.2-cm metastatic tumour in right lung from primary colorectal cancer. (A) Image obtained immediately before MWA, (B) image obtained during MWA, (C) image obtained 3 months after ablation, (D) image obtained 1 year after MWA and (E) image obtained 3 years after MWA.
Complications
The adverse events were classified according to the Common Terminology Criteria for Adverse Events version 4.03 (CTCAE) [Citation10]. Pneumothorax was the most common complication and occurred in 6 patients (12.5%). One patient with severe pneumothorax received thoracic cavity drainage. The 5 patients who experienced mild pneumothorax did not require treatment. Other complications included three patients (6.3%) with severe chest pain, one patient (2.1%) with a small amount of pleural effusion and four patients (8.3%) with low fevers of less than 38.5 °C, which lasted for 1–2 d. In our series, intrapulmonary bleeding has appeared in four cases did not require treatment. Procedure-related complications are encountered in . No needle track seeding occurred. No procedure-related deaths occurred within the 30-d period after ablation.
Table 2. Complications of percutaneous microwave ablations (48 sessions).
Follow-up and survival
Follow-up was performed for all 32 patients, and the median survival time was 31 months (95% CI: 15.4–46.6). Of 48 tumours, eight demonstrated local recurrence. Four tumours were treated with a second MWA, and none of them presented residual disease during follow-up. The median DFI was 15 months from the time of the primary procedure. The 1-, 2- and 3-year survival rates were 79.5%, 63.1% and 44.4%, respectively (). Univariate Cox regression analysis showed that tumour size, DFI and the number of tumours significantly correlated with OS (p = .007, p = .022 and p = .030, respectively) (). Multivariate analysis showed that tumour size was an independent prognostic factor for survival (p = .017) ().
Figure 2. Kaplan–Meier estimates of the overall survival (OS) for 32 patients. The median survival time was 31 months (95% CI: 15.4–46.6).
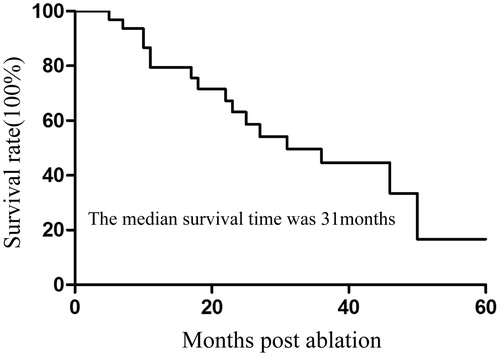
Figure 3. Kaplan–Meier estimates of the overall survival (OS) for 32 patients divided into two subgroups according to tumour size (p = .007). Continuous line: patients with tumour ≤3 cm; dotted line, patients with tumour >3 cm.
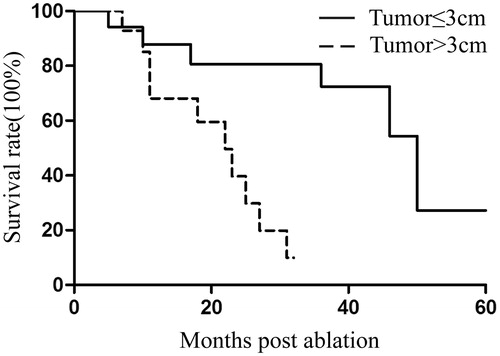
Figure 4. Kaplan–Meier estimates of the overall survival (OS) for 32 patients divided into two subgroups according to DFI (p = .022). Continuous line: patients with DFI >12 months; dotted line, patients with DFI ≤12 months.
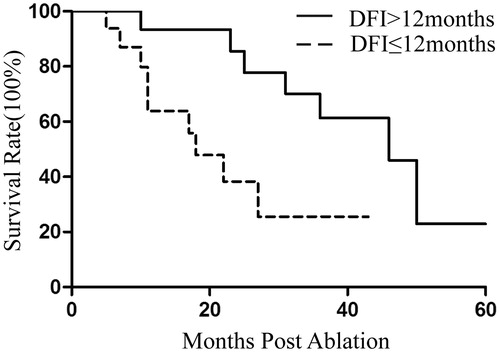
Figure 5. Kaplan–Meier estimates of the overall survival (OS) for 32 patients divided into two subgroups according to tumour number (p = .030). Continuous line: patients with one lesion; dotted line, patients with >1 lesions.
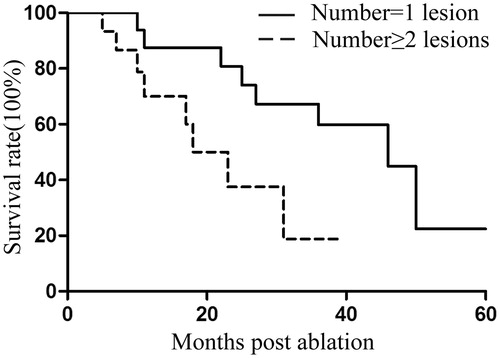
Table 3. Univariate and multivariate analyses of overall survival in patients.
Discussion
Thermal ablation has emerged as a safe, highly effective and minimally invasive technology to treat solid tumours [Citation11]. Thermal ablation therapy mainly includes RFA, MWA and cryotherapy [Citation12]. Compared with RFA, MWA exhibits better thermal conductivity, achieves higher intratumoural temperatures and is associated with a shorter ablation time, larger tumour ablation volume, lower susceptibility to heat-sink effects and greater ablation scope. Furthermore, grounding pads are not required because multiple MWA antennae may be used simultaneously [Citation13–15]. MWA is a relatively new technology and has been applied for the treatment of benign tumours, malignant primary and metastatic hepatomas, and neoplasms of the lungs, kidney, pancreas, papillary thyroid and adrenal glands [Citation16–26].
In the literature, different degrees of benefit have been reported for patients treated with MWA [Citation13,Citation19]. Anna et al. [Citation27] showed that the complete ablation rate was 90.3%. In the present study, the complete ablation rate was 87.5%. The clinical efficacy was clear, and the objectives of cytoreduction were achieved. Yan et al. [Citation28,Citation29] showed that complete ablation for CRC pulmonary metastases greater than 3 cm in diameter was difficult using percutaneous lung RFA. Our results showed that MWA for tumours less than 3 cm in diameter produced a good clinical outcome. However, the local control rate for larger tumours was poor. This finding may be due to the fact that some tumours were so large and irregularly shaped that the ablation-induced heat could not effectively kill all of the tumour cells. Our findings corroborate their results [Citation28,Citation29].
Pneumothorax is the most common complication associated with MWA therapy, with a reported incidence rate of 15–45% [Citation30]. In our study, the most common complication was pneumothorax and the incidence was 12.5%. One patient with moderate pneumothorax received thoracic cavity drainage immediately after the procedure. Postoperative pleural effusion is an exudate reacting to the adjacent thermal damage [Citation31]. In the current cohort, one patient presented with a small amount of pleural effusion, which resolved within 1 month without drainage. Needle tract metastatic seeding is also a serious complication after thermal ablation [Citation32,Citation33]. No needle track seeding occurred in our study. Intrapulmonary bleeding is a common occurrence after pulmonary ablation, and it is generally self-limited. In the current cohort, four patients had intrapulmonary bleeding after MWA. The incidence was 8.3% (4/48), similar with the other studies [Citation28,Citation34]. The complication rate was low. Therefore, MWA is relatively safe for treating CRC pulmonary metastases.
Previous studies have shown that MWA therapy can improve the survival rate of patients with pulmonary metastases [Citation30,Citation35]. Aimin et al. [Citation36] have reported a 38.3-month median OS in the solitary metastasis group after lung MWA. We followed up all 32 patients in our study. The median DFI was 15 months from the time of the primary operation, and the median survival time was 31 months, similar to the reported studies [Citation36]. Belforie et al. [Citation19] reported cancer-specific mortalities of 69%, 54% and 49% at 1, 2 and 3 years in 56 patients treated with MWA. In the present study, the 1-, 2- and 3-year survival rates were 79.5%, 63.1% and 44.4%, respectively. Reported independent predictors of OS include location of primary disease for lung metastasis [Citation37], tumour stage [Citation36], number of metastases [Citation37], tumour size [Citation20,Citation36,Citation37] and extrapulmonary metastasis [Citation38]. In the present study, multivariate analysis showed that tumour size was an independent prognostic factor for survival. Our results were concordant with those from previous studies [Citation20,Citation36,Citation37]. Our study showed that patients with tumour diameters ≤3 cm, a DFI >12 months and solitary CRC pulmonary metastases survived longer. A cut-off tumour diameter of 3 cm was effective for identifying patients who might have a good prognosis and an increased chance of survival. Our results also imply that MWA can prolong the survival time of patients who have small tumours, which is similar to previously reported results [Citation39].
In conclusion, CT-guided, cool-circulation MWA therapy achieves local tumour control for CRC pulmonary metastases. Specifically, it can effectively control the progression of tumours and prolong the survival of patients and is associated with several advantages: it is a simple procedure that is minimally invasive and relatively safe. Thus, it is expected to become one of the preferred methods for patients with CRC pulmonary metastases who cannot tolerate surgical treatment. However, multicenter studies should be conducted to obtain further evidence regarding the benefits of microwave therapy for patients with CRC pulmonary metastases.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Jemal A, Bray F, Center MM, et al. (2011). Global cancer statistics. CA Cancer J Clin 61:69–90.
- Kim HK, Cho JH, Lee HY, et al. (2014). Pulmonary metastasectomy for colorectal cancer: how many nodules, how many times? World J Gastroenterol 20:6133–45.
- Watanabe K, Nagai K, Kobayashi A, et al. (2009). Factors influencing survival after complete resection of pulmonary metastases from colorectal cancer. Br J Surg 96:1058–65.
- Chua TC, Thornbury K, Saxena A, et al. (2010). Radiofrequency ablation as an adjunct to systemic chemotherapy for colorectal pulmonary metastases. Cancer 116:2106–14.
- Gonzalez M, Robert JH, Halkic N, et al. (2012). Survival after lung metastasectomy in colorectal cancer patients with previously resected liver metastases. World J Surg 36:386–91.
- Ferguson CD, Luis CR, Steinke K. (2017). Safety and efficacy of microwave ablation for medically inoperable colorectal pulmonary metastases: single-centre experience. J Med Imaging Radiat Oncol 61:243–9.
- Sidoff L, Dupuy DE. (2017). Clinical experiences with microwave thermal ablation of lung malignancies. Int J Hyperthermia 33:25–33.
- Xiong L, Dupuy DE. (2016). Lung ablation: whats new? J Thorac Imaging 31:228–37.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria - a 10-year update. Radiology 273:241–60.
- National Cancer Institute, National Institutes of Health, US Department of Health and Human Services. Common Terminology Criteria for Adverse Events (CTCAE) Version 4.0. Published: May 28, 2009; Revised version 4.03 June 14, 2010. Available online: http://evsncinihgov/ftp1/CTCAE/CTCAE_403_2010-06-14_QuickReference_85x11pdf (Accessed July 31, 2016).
- Vogl TJ, Naguib NN, Lehnert T, Nour-Eldin NE. (2011). Radiofrequency, microwave and laser ablation of pulmonary neoplasms: clinical studies and technical considerations – review article. Eur J Radiol 77:346–57.
- Liang P, Wang Y, Yu X, Dong B. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation – complications among cohort of 1136 patients. Radiology 251:933–40.
- Wolf FJ, Grand DJ, Machan JT, et al. (2008). Microwave ablation of lung malignancies: effectiveness, CT findings, and safety in 50 patients. Radiology 247:871–9.
- Bhardwaj N, Strickland AD, Ahmad F, et al. (2010). Liver ablation techniques: a review. Surg Endosc 24:254–65.
- Chu KF, Dupuy DE. (2014). Thermal ablation of tumours: biological mechanisms and advances in therapy. Nat Rev Cancer 14:199–208.
- Li X, Fan WJ, Zhang L, et al. (2013). CT-guided percutaneous microwave ablation of liver metastases from nasopharyngeal carcinoma. J Vasc Interv Radiol 24:680–4.
- Liang P, Wang Y, Zhang D, et al. (2008). Ultrasound guided percutaneous microwave ablation for small renal cancer: initial experience. J Urol 180:844–8; discussion 8.
- Wang Y, Liang P, Yu X, et al. (2009). Ultrasound-guided percutaneous microwave ablation of adrenal metastasis: preliminary results. Int J Hyperthermia 25:455–61.
- Belfiore G, Ronza F, Belfiore MP, et al. (2013). Patients' survival in lung malignancies treated by microwave ablation: our experience on 56 patients. Eur J Radiol 82:177–81.
- Wei Z, Ye X, Yang X, et al. (2015). Microwave ablation plus chemotherapy improved progression-free survival of advanced non-small cell lung cancer compared to chemotherapy alone. Med Oncol 32:464.
- Cheng M, Fay M, Steinke K. (2016). Percutaneous CT-guided thermal ablation as salvage therapy for recurrent non-small cell lung cancer after external beam radiotherapy: a retrospective study. Int J Hyperthermia 32:316–23.
- Facciorusso A, Di Maso M, Muscatiello N. (2016). Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 32:339–44.
- Yue W, Chen L, Wang S, Yu S. (2015). Locoregional control of recurrent papillary thyroid carcinoma by ultrasound-guided percutaneous microwave ablation: a prospective study. Int J Hyperthermia 31:403–8.
- Veltri A, Gazzera C, Rotondella C, et al. (2012). Image-guided microwave ablation of hepatic tumours: preliminary experience. Radiol Med 117:378–92.
- Carrafiello G, Ierardi AM, Piacentino F, et al. (2012). Microwave ablation with percutaneous approach for the treatment of pancreatic adenocarcinoma. Cardiovasc Intervent Radiol 35:439–42.
- Zhou W, Wang R, Liu X, et al. (2017). Ultrasound-guided microwave ablation: a promising tool in management of benign breast tumours. Int J Hyperthermia 33:263–70.
- Ierardi AM, Coppola A, Lucchina N, Carrafiello G. (2017). Treatment of lung tumours with high-energy microwave ablation: a single-centre experience. Med Oncol 34:5.
- Yan TD, King J, Sjarif A, et al. (2006). Percutaneous radiofrequency ablation of pulmonary metastases from colorectal carcinoma: prognostic determinants for survival. Ann Surg Oncol 13:1529–37.
- Yan TD, King J, Sjarif A, et al. (2007). Treatment failure after percutaneous radiofrequency ablation for nonsurgical candidates with pulmonary metastases from colorectal carcinoma. Ann Surg Oncol 4:1718–26.
- Lu Q, Cao W, Huang L, et al. (2012). CT-guided percutaneous microwave ablation of pulmonary malignancies: results in 69 cases. World J Surg Oncol 10:80.
- Li M, Yu XL, Liang P, et al. (2012). Percutaneous microwave ablation for liver cancer adjacent to the diaphragm. Int J Hyperthermia 28:218–26.
- Yu J, Liang P, Yu XL, et al. (2012). Needle track seeding after percutaneous microwave ablation of malignant liver tumors under ultrasound guidance: analysis of 14-year experience with 1462 patients at a single center. Eur J Radiol 81:2495–9.
- Yamauchi Y, Izumi Y, Hashimoto K, et al. (2011). Needle-tract seeding after percutaneous cryoablation for lung metastasis of colorectal cancer. Ann Thorac Surg 92:e69–71.
- Ko WC, Lee YF, Chen YC, et al. (2016). CT-guided percutaneous microwave ablation of pulmonary malignant tumors. J Thorac Dis 8:S659–S665.
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. (2011). Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 261:643–51.
- Zheng A, Ye X, Yang X, et al. (2016). Local efficacy and survival after microwave ablation of lung tumors: a retrospective study in 183 patients. J Vasc Interv Radiol 27:1806–14.
- de Baere T, Auperin A, Deschamps F, et al. (2015). Radiofrequency ablation is a valid treatment option for lung metastases: experience in 566 patients with 1037 metastases. Ann Oncol 26:987–91.
- Matsui Y, Hiraki T, Gobara H, et al. (2015). Long-term survival following percutaneous radiofrequency ablation of colorectal lung metastases. J Vasc Interv Radiol 26:303–10. quiz 11.
- Liu H, Steinke K. (2013). High-powered percutaneous microwave ablation of stage I medically inoperable non-small cell lung cancer: a preliminary study. J Med Imaging Radiat Oncol 57:466–74.
