Abstract
Background: The post-operative morbidity and mortality after CRS-HIPEC has been widely evaluated. However, there is a major discrepancy between rates reported due to different metrics and time of analysis used.
Objective: To evaluate the legitimacy of 90-day morbidity and mortality based on the National Cancer Institute’s Common Terminology Criteria for Adverse Events (NCI-CTCAE) v4.0 classification as criteria of quality for cytoreductive surgery combined with hyperthermic intraperitoneal chemotherapy (CRS-HIPEC).
Methods: A prospective database of all patients undergoing CRS-HIPEC for peritoneal carcinomatosis between 2004 and 2015 was queried for 90-day morbidity and mortality and survival.
Results: Among 881 patients, the 90-day major complication rate based on NCI-CTCAE classification and Clavien-Dindo’s classification were 51% (n = 447 patients) and 25% (n = 222 patients), respectively. Among patients who presented with a 90-day complication based on the NCI-CTCAE classification, 50% (n = 225 patients) presented a medical complication not reported by Clavien-Dindo’s classification. After surgery, 24 patients (2.7%) died of post-operative complications, for only 10 (42%) of them the death occurred within 30-day after surgery. Occurrence of major complication based on either NCI-CTCAE classification, Clavien-Dindo’s classification or the medical complication not reported by Clavien-Dindo’s classification all negatively impacts the overall survival.
Conclusion: Among commonly reported morbidity’s classification, 90-day morbidity based on NCI-CTCAE classification represents a legitimate metric of CRS-HIPEC quality. Post-operative morbidity after CRS-HIPEC should be reported using 90-day NCI-CTCAE classification.
Introduction
Peritoneal carcinomatosis (PC) is a common evolution of abdominal cancers. It may be primary arising from the peritoneum itself, or secondary to another type of cancer, especially those of gastrointestinal or gynaecological origin. Without aggressive multimodal therapeutic approaches, it is associated with a poor prognosis [Citation1]. Since its origin in the 1990s, CRS-HIPEC has been increasingly used as the curative treatment for several aetiologies of peritoneal carcinomatosis [Citation2,Citation3].
Over the following 20 years, interest in CRS-HIPEC has increased, but the concept was not universally embraced by the medical community due to concerns regarding morbidity and mortality which are reported to be between 18.7–42% and 0–10%, respectively [Citation4–6]. The post-operative morbidity and mortality after CRS-HIPEC has been extensively evaluated. However, there is a major discrepancy between rates reported due to different metrics and time period used for analysis [Citation7]. Verwaal et al., described the 30 days toxicity of CRS-HIPEC of 65% (grade 3–5 NCI-CTCAE), with a surgical complication rate of 35% [Citation8]. In addition, Smeenk et al., also described the treatment related (grade 3–5) 30 days toxicity of 54%, with surgical complications of 38% [Citation9]. At our institution, we recently evaluated a 25-year experience with 1125 procedures [Citation10] and reported major complications within a 90-day time period according to NCI-CTCAE [Citation11], whereas most previous studies evaluated 30-day morbidity according to the surgical classification by Clavien-Dindo’s classification [Citation12]. In hepatopancreatobiliary (HPB) operations, Mise et al. reported a 90-day overall mortality rate that was significantly higher than the 30-day mortality and better represented mortality [Citation13]. Furthermore, recent studies suggested that 90-day surveillance should be mandatory after major surgical procedures in order to better define post-operative complications [Citation14].
Using a large prospectively maintained institutional database, this study compared the accuracy of the reporting criteria of post-CRS-HIPEC morbidity and mortality using 90-day definitions assessed based on either surgical classification by Clavien-Dindo’s classification [Citation12] or by NCI-CTCAE v 4.0 (Bethesda, MD) [Citation11].
Methods
Study population
Our prospectively maintained institutional peritoneal carcinomatosis database was queried to identify all patients who underwent HIPEC for PC at Lyon Sud Hospital, France, between January 2004 and September 2015. At our institution, 2004 was the year when a sufficient experience was achieved for our centre to be considered as a referral centre for PC treatment [Citation10]. The study was performed in accordance with the precepts established by the Declaration of Helsinki. For each patient who underwent a complete CRS-HIPEC, the following data were extracted: gender, age at time of surgery, origin of PC, survival after CRS-HIPEC and post-operative morbidity and mortality.
Surgical management and HIPEC
The technique used at our institution was previously described [Citation10]. In brief, the diagnosis of PC was suspected or established pre-operatively, and all CRS-HIPEC procedures were approved in a multidisciplinary conference. Under general anaesthesia and complete hemodynamic monitoring, an exploratory laparotomy was performed through a midline incision for thorough abdominal exploration, which included collection of samples for the review of cytology and pathology. Utilising a combination of organ resection and peritonectomy, CRS was performed according to the location of tumour deposits with the goal of complete cytoreduction of macroscopic disease [Citation15]. At the end of the operation, HIPEC was administered under general anaesthesia using the closed abdomen technique. The cytotoxic agents used, alone or in combination, were cisplatin, mitomycin C, oxaliplatin, doxorubicin and/or irinotecan.
Post-operative morbidity and mortality
Post-operative complications were evaluated within 90 days after surgery and assessed based on both Clavien-Dindo’s classification [Citation12] or by the NCI-CTCAE v4.0 [Citation11]. Only major complications (grade 3, 4 and 5) were recorded according to each classification. For didactic purposes, “medical complications” were defined as complications reported by NCI-CTCAE classification and not reported by Clavien-Dindo’s classification, and “surgical complications” were defined as complications reported by both NCI-CTCAE and Clavien-Dindo’s classification. The date of complications requiring treatment with interventional radiology, gastro-intestinal endoscopy, and surgery were recorded to define the time after surgery.
Statistical analysis
Continuous data without a normal distribution were reported in terms of medians with ranges and compared with the Mann–Whitney U-test. Categorical data were compared with chi-square tests. Overall survival (OS) was calculated from the date of CRS-HIPEC to the last known date of follow-up. Estimates of survival were calculated using the Kaplan–Meier method and compared using the log-rank test. A p values of less than 0.05 was considered statistically significant. Missing data were not included in the analysis. The statistical analyses were performed with SPSS version 19.0 (SPSS Inc., IBM, Chicago, IL).
Results
Population
Between January 2004 and September 2015, 881 patients, 298 males (34%) and 583 females (66%) underwent CRS-HIPEC. The median age at time of surgery was 59 years (range 17–81), with a median Peritoneal Cancer Index of 9 (0–36). The patients had PC from colorectal cancer (n = 281), epithelial ovarian carcinoma (n = 215), pseudomyxoma peritonei (PMP; n = 162), gastric cancer (n = 70), malignant mesothelioma peritoneal (MMP; n = 73), peritoneal serous carcinoma (n = 24), appendiceal (n = 13), small bowel (n = 11), sarcomatosis (n = 6) and other rare origins (n = 26).
Post-operative morbidity and mortality
Based on NCI-CTCAE and Clavien-Dindo’s classifications, the 90-day major complication rate was 51% (n = 447 patients) and 25% (n = 222 patients), respectively. Among patients who suffered from a major complication based on the NCI-CTCAE classification, 50% (n = 225 patients) of patients who suffered from a medical complication were not accounted for when using the Clavien-Dindo’s classification ().
Figure 1. Comparative rate of 30-day morbidity and mortality compared to 90-day morbidity and mortality, and major complication based on Clavien-Dindo’s classification to NCI-CTCAE classification.
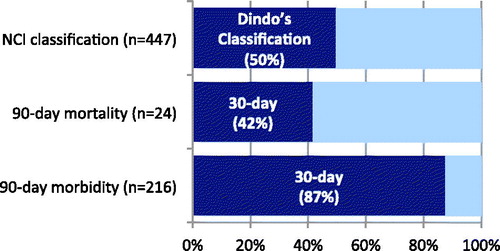
After surgery, 24 patients (2.7%) died of post-operative complications; 10 (42%) of these deaths occurred within 30 days after surgery ().
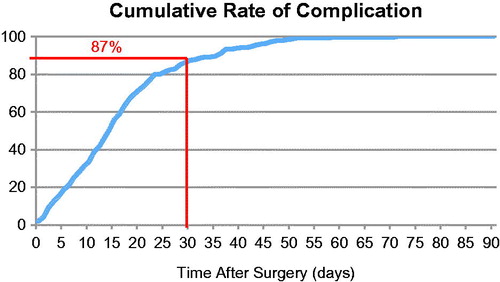
Among all patients who had a major complication that required a new intervention (surgical, radiological or endoscopic), the date of re-intervention was determined for 216 patients; 189 (87.5%) of these complications occurred within 30 days after surgery (). represents the rate of major complication requiring an invasive treatment over time after surgery.
Impact of post-operative complications on overall survival
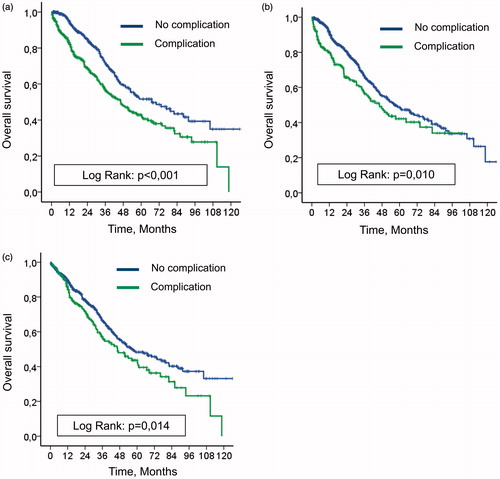
The median follow-up time was 23.6 months (range, 0.16–125.9 months), and the median OS was 53.0 months (95% CI: 44.0–62.1 months). For patients who presented with or without a 90-day major complication based on NCI-CTCAE classification, the median OS times were 46.4 months and 67.0 months, respectively (p < 0.001, ). Median OS times for patients who presented with or without a 90-day major complication based on the Clavien-Dindo’s classification were 46.3 months and 56.3 months, respectively (p = 0.010, ). Median OS times for patients who presented with or without a 90-day major medical complication (not graded by the Clavien-Dindo’s classification) were 46.4 months and 57.3 months, respectively, (p = 0.014, ).
Figure 3. (a) Impact on overall survival of 90-day complication based on NCI-CTCAE classification. (b) Impact on overall survival of 90-day complication based on Clavien-Dindo’s classification. (c) Impact on overall survival of 90-day complication based on medical complication not reported by Clavien-Dindo’s classification.
Discussion
Our study confirmed that the NCI-CTCAE definition for 90-day morbidity and mortality better describes post-operative outcomes after CRS-HIPEC. This was especially apparent for post-operative mortality, which was shown to be not adequately evaluated in a period of 30 days with the Clavien-Dindo’s classification. Using this 90-day definition to report post-operative morbidity and mortality after CRS-HIPEC would more accurately standardise the literature with regard to post-operative events. Our findings also support the practice that morbidity and mortality rates within 90 days should be routinely reported for the purposes of education, research and quality improvement.
The evaluation of post-operative complications in surgical oncology trials is pivotal to define the optimal patient management [Citation16] and is as important as the assessment of toxicities in chemotherapy trials. The Clavien-Dindo’s classification defined a classification of post-operative complications, which has been adopted widely in surgical practice [Citation12]. This classification categorises post-operative complications broadly into five grades of severity (grade I, II, IIIa, IIIb, IVa, IVb and V). The American NCI-CTCAE has been used widely to evaluate and define the toxicity of chemotherapy or radiotherapy [Citation17]. It considers both medical and surgical complications. The grading definition has a long tradition in reporting medication toxicity and adverse effects, and includes 370 specific types of complications including acute and late effects of both surgical and medical effects, while using five grades of severity [Citation18]. A recent study by Lehmann et al., reported a significantly higher rate of major complication after CRS-HIPEC in 147 patients when comparing the NCI-CTCAE to the Clavien-Dindo classification (8.1% vs. 25.1%; p = 0.001) [Citation19]. Although the NCI-CTCAE classification provided the same number and types of complications, the severity in grading the complications were different in the Lehmann et al., study. They also indicated that the interpretation of complication grade after CRS-HIPEC was inconsistent between different evaluators, which could impair the validity of data among centres. The present study confirmed that the NCI-CTCAE classification reported significantly more major complications, but also suggested that these major complications missed by Clavien-Dindo’s classification significantly impacted survival. Currently, for a procedure that includes a surgical procedure and toxicity of chemotherapy, there continues to be debate over the most appropriate complication grading system with either one that is strictly surgically based such as the Calvien-Dindo classification or one that considers both surgical and medical toxicity adverse events such as the NCI-CTCAE grading system. Therefore, to standardise reporting of complications after CRS-HIPEC, the NCI-CTCAE grading system was proposed in a consensus conference in Milan in 2006 [Citation20], which should remain the current consensus to compare surgical outcomes for CRS-HIPEC.
Morbidity after CRS-HIPEC significantly impact long-term outcomes. For Simkens et al., patients with complications requiring intervention had a reduced survival compared with those without complications [Citation21]. In the same study, the post-operative complications requiring intervention were significantly associated with an increased risk of early recurrence (OR 2.3) and decreased overall survival. For Baratti et al., five-year disease-specific survival was 14.3% for patients who experienced major complications and 52.3% for those who did not. Furthermore, five-year overall survival was 11.7% for patients who experienced major complications and 58.8% for those who did not. The post-operative morbidity was an independent prognostic factor for disease-free survival [Citation22]. Those two studies graded the complication based on NCI-CTCAE at 30 days. In addition, patients who were disease-free 5 years after CRS-HIPEC had experienced less post-operative complication than those with recurrent disease [Citation23]. This has been similarly observed after surgery for colorectal liver metastases where post-operative morbidity was associated with early recurrence (OR 4.7) [Citation24]. Reported recurrence rates in patients with post-operative complications after radical resection for colorectal cancer were also increased [Citation25]. Finally, anastomotic leak has been described as a risk factor for both local and systemic recurrence after curative surgery in patients with rectal cancer [Citation26]. The consequence of complications on recurrence and survival may be a result of delay or omission of adjuvant therapy [Citation26]. Our study was in accordance with current literature and underlines the importance to well define and describe post-operative morbidity and mortality after CRS-HIPEC.
After CRS-HIPEC, morbidity reported varies widely from 0% to 62% [Citation27]; this significant heterogeneity in the reported literature is due to the variety of morbidity grading tools used across inconsistent time periods for evaluation. Although Clavien-Dindo’s classification of post-operative morbidity and mortality is commonly used to describe surgical morbidity [Citation12], this morbidity classification mainly describes surgical complication. Generally, in surgical oncology and particularly for HIPEC procedures, patients receive perioperative systemic chemotherapy known to be associated with medical issues and additional morbidity. Therefore, evaluation of medical morbidity would better reflect the whole panel of complications. The NCI-CTCAE classification includes both surgical and medical morbidity [Citation11] and is routinely used in oncology trials. In the present study, the morbidity based on the Clavien-Dindo’s classification underestimates post-operative morbidity compared to the NCI-CTCAE classification, with nearly 50% of complications not reported (25% of major complications based on Clavien-Dindo’s classification vs. 51% based on NCI-CTCAE). In the present study, “medical complications” not reported by Clavien-Dindo’s classification also significantly impacts overall survival after CRS-HIPEC, highlighting the importance of evaluating all post-operative major complications. NCI-CTCAE classification appears as a better classification to describe post-CRS-HIPEC morbidity and mortality.
Historically, 30-day mortality was used to measure performance across a wide range of surgical disciplines [Citation28]. A recent survey evaluating eight surgical procedures for cancers (gastric, colon, breast, renal, lung, rectal, bladder and oesophageal) led to a recommendation for a 30-day definition as an international standard, because it was perceived that the great majority of surgery-related deaths were captured [Citation29]. The accuracy of post-operative mortality in terms of capturing surgery-related deaths and excluding deaths due to underlying disease depends on the follow-up time over which deaths are tracked [Citation29,Citation30]. Therefore, alternate definitions of post-operative mortality are frequently used, including in-hospital mortality, 90-day mortality and 100-day mortality [Citation30]. For Simkens et al., the majority of treatment-related deaths after CRS-HIPEC occurred after the 30th post-operative day; therefore, treatment-related mortality is substantially higher than what is described by the 30-day mortality rate alone [Citation31]. A similar underestimation was found in a recent, single-centre study describing a 30-day mortality rate of 2.4% and a 90-day mortality rate of 7.1% after CRS-HIPEC [Citation32]. Recent studies in HPB surgery demonstrated that 30-day mortality underestimates the number of deaths directly related to surgery by approximately 50% [Citation33,Citation34]. For Mise et al., the 90-day overall mortality rate captured most surgery-related deaths and excluded most disease-related deaths. Therefore, it represents a legitimate measure of surgical quality in HPB surgery [Citation13]. For Schwarz et al., one-third of severe adverse events and readmissions were reported more than 30 days after pancreatic surgery [Citation14]. Our study also reports a 30-day mortality that underestimates post-operative mortality by 50% in CRS-HIPEC patients. In the present study, most major complications occurred within 30 day, but more than 50% of deaths related to post-operative complications occurred after 30 days. Evaluating mortality related to complex surgical procedures such as HPB surgery or CRS-HIPEC, appears to be better described using a 90-day time period for its definition.
The data reported herein should be evaluated in the context of their limitations. First, this study represents a retrospective analysis of patients treated at a single institution. However, the databases used are prospectively maintained and managed by a trained, full time personnel using abstracted data and standardised algorithms similar to those used in the management of large, national data sets. Second, we have no information on the date of occurrence of first medical complications. However, the date of complications requiring treatment with interventional radiology, gastrointestinal endoscopy and surgery were recorded for all patients, allowing an analysis for complication requiring invasive treatment.
Conclusions
Among commonly reported morbidity’s classification, 90-day morbidity based on NCI-CTCAE classification represents a legitimate metric of CRS-HIPEC quality. Post-operative morbidity after CRS-HIPEC should be reported using the 90-day NCI-CTCAE classification, and it should be routinely reported for the purposes of education, research and quality improvement.
Acknowledgements
The authors thank Isabelle Bonnefoy and Peggy Jourdan-Enfer for data management.
Disclosure statement
The authors report no conflicts of interest relevant to this article.
References
- Sadeghi B, Arvieux C, Glehen O, et al. (2000). Peritoneal carcinomatosis from non-gynecologic malignancies: results of the EVOCAPE 1 multicentric prospective study. Cancer 88:358–63.
- Sugarbaker PH, Cunliffe WJ, Belliveau J, et al. (1989). Rationale for integrating early postoperative intraperitoneal chemotherapy into the surgical treatment of gastrointestinal cancer. Semin Oncol 16(4 Suppl 6):83–97.
- Gilly FN, Sayag AC, Carry PY, et al. (1991). Intra-peritoneal chemo-hyperthermia (CHIP): a new therapy in the treatment of the peritoneal seedings. Preliminary report. Int Surg 76:164–7.
- Ahmed S, Stewart JH, Shen P, et al. (2014). Outcomes with cytoreductive surgery and HIPEC for peritoneal metastasis. J Surg Oncol 110:575–84.
- Vaira M, Robella M, Mellano A, et al. (2014). Iterative procedures combining cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for isolated peritoneal recurrence. Int J Hyperthermia 30:565–9.
- Tan G, Chia C, Kumar M, et al. (2017). 201 consecutive cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC) procedures in a single Asian tertiary centre. Int J Hyperthermia 33:288–94.
- Chua TC, Yan TD, Saxena A, Morris DL. (2009). Should the treatment of peritoneal carcinomatosis by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy still be regarded as a highly morbid procedure?: a systematic review of morbidity and mortality. Ann Surg 249:900–7.
- Verwaal VJ, van Tinteren H, Ruth SV, Zoetmulder FA. (2004). Toxicity of cytoreductive surgery and hyperthermic intra-peritoneal chemotherapy. J Surg Oncol 85:61–7.
- Smeenk RM, Verwaal VJ, Zoetmulder FA. (2006). Toxicity and mortality of cytoreduction and intraoperative hyperthermic intraperitoneal chemotherapy in pseudomyxoma peritonei–a report of 103 procedures. Eur J Surg Oncol 32:186–90.
- Passot G, Vaudoyer D, Villeneuve L, et al. (2016). What made hyperthermic intraperitoneal chemotherapy an effective curative treatment for peritoneal surface malignancy: a 25-year experience with 1,125 procedures. J Surg Oncol 113:796–803.
- National Cancer Institute. Common Terminology Criteria for Adverse Events v.3.0and v.4.0 (CTCAE). Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm [last accessed 14 Jun 2011].
- Dindo D, Demartines N, Clavien PA. (2004). Classification of surgical complications: a new proposal with evaluation in a cohort of 6336 patients and results of a survey. Ann Surg 240:205–13.
- Mise Y, Vauthey JN, Zimmitti G, et al. (2015). Ninety-day postoperative mortality is a legitimate measure of hepatopancreatobiliary surgical quality. Ann Surg 262:1071–8.
- Schwarz L, Bruno M, Parker NH, et al. (2015). Active surveillance for adverse events within 90 days: the standard for reporting surgical outcomes after pancreatectomy. Ann Surg Oncol 22:3522–9.
- Sugarbaker PH. (1995). Peritonectomy procedures. Ann Surg 221:29–42.
- Kim BJ, Caudle AS, Gottumukkala V, Aloia TA. (2016). The impact of postoperative complications on a timely return to intended oncologic therapy (RIOT): the role of enhanced recovery in the cancer journey. Int Anesthesiol Clin 54:e33–46.
- National Cancer Institute. Cancer Therapy Evaluation Program. Common terminology criteria for adverse events v3.0 (CTCAE). Available from: http://ctep.cancer.gov/protocolDevelopment/electronic_applications/ctc.htm. [last accessed 10 Jun 2013].
- Trotti A, Colevas AD, Setser A, et al. (2003). CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol 13:176–81.
- Lehmann K, Eshmuminov D, Slankamenac K, et al. (2016). Where oncologic and surgical complication scoring systems collide: time for a new consensus for CRS/HIPEC. World J Surg 40:1075–81.
- Younan R, Kusamura S, Baratti D, et al. (2008). Morbidity, toxicity, and mortality classification systems in the local regional treatment of peritoneal surface malignancy. J Surg Oncol 98:253–7.
- Simkens GA, van Oudheusden TR, Luyer MD, et al. (2015). Serious postoperative complications affect early recurrence after cytoreductive surgery and HIPEC for colorectal peritoneal carcinomatosis. Ann Surg Oncol 22:2656–62.
- Baratti D, Kusamura S, Iusco D, et al. (2014). Postoperative complications after cytoreductive surgery and hyperthermic intraperitoneal chemotherapy affect long-term outcome of patients with peritoneal metastases from colorectal cancer: a two-center study of 101 patients. Dis Colon Rectum 57:858–68.
- Goere D, Malka D, Tzanis D, et al. (2013). Is there a possibility of a cure in patients with colorectal peritoneal carcinomatosis amenable to complete cytoreductive surgery and intraperitoneal chemotherapy? Ann Surg 257:1065–71.
- Kaibori M, Iwamoto Y, Ishizaki M, et al. (2012). Predictors and outcome of early recurrence after resection of hepatic metastases from colorectal cancer. Langenbeck’s Arch Surg 397:373–81.
- Law WL, Choi HK, Lee YM, Ho JW. (2007). The impact of postoperative complications on long-term outcomes following curative resection for colorectal cancer. Ann Surg Oncol 14:2559–66.
- Mirnezami A, Mirnezami R, Chandrakumaran K, et al. (2011). Increased local recurrence and reduced survival from colorectal cancer following anastomotic leak: systematic review and meta-analysis. Ann Surg 253:890–9.
- Halkia E, Kopanakis N, Nikolaou G, Spiliotis J. (2015). Cytoreductive surgery and HIPEC for peritoneal carcinomatosis. A review on morbidity and mortality. J BUON 20:S80–S7.
- Khuri SF, Daley J, Henderson W, et al. (1998). The Department of Veterans Affairs’ NSQIP: the first national, validated, outcome-based, risk-adjusted, and peer-controlled program for the measurement and enhancement of the quality of surgical care. National VA Surgical Quality Improvement Program. Ann Surg 228:491–507.
- Damhuis RA, Wijnhoven BP, Plaisier PW, et al. (2012). Comparison of 30-day, 90-day and in-hospital postoperative mortality for eight different cancer types. Br J Surg 99:1149–54.
- Russell EM, Bruce J, Krukowski ZH. (2003). Systematic review of the quality of surgical mortality monitoring. Br J Surg 90:527–32.
- Simkens GA, van Oudheusden TR, Braam HJ, et al. (2016). Treatment-related mortality after cytoreductive surgery and HIPEC in patients with colorectal peritoneal carcinomatosis is underestimated by conventional parameters. Ann Surg Oncol 23:99–105.
- Tabrizian P, Shrager B, Jibara G, et al. (2014). Cytoreductive surgery and hyperthermic intraperitoneal chemotherapy for peritoneal carcinomatosis: outcomes from a single tertiary institution. J Gastrointest Surg 18:1024–31.
- Mise Y, Day RW, Vauthey JN, et al. (2016). After pancreatectomy, the “90 days from surgery” definition is superior to the “30 days from discharge” definition for capture of clinically relevant readmissions. J Gastrointest Surg 20:77–84. discussion
- Swanson RS, Pezzi CM, Mallin K, et al. (2014). The 90-day mortality after pancreatectomy for cancer is double the 30-day mortality: more than 20,000 resections from the national cancer data base. Ann Surg Oncol 21:4059–67.