Abstract
Purpose: To assess the efficacy of percutaneous thermal ablation in treating colorectal cancer liver metastases (CRCLM), and to propose a prognostic nomogram for overall survival (OS).
Materials and methods: Seventy-one patients with CRCLM undergoing thermal ablation at our institute from 2009 to 2013 were identified and analysed to formulate a prognostic nomogram. The concordance index (C-index) and calibration curve were calculated to evaluate the predictive accuracy of the nomogram. The nomogram was compared with two current prognostic nomograms for patients with CRCLM who had undergone hepatectomy (Kattan) and selective internal radiation therapy (Fendler). Predictive validity was assessed in the validation cohort of 25 patients who had undergone thermal ablation from 2014 to 2016.
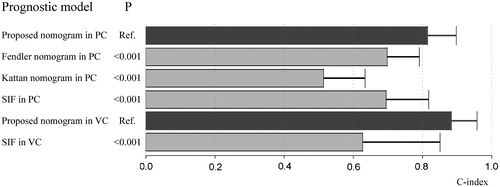
Results: The median OS in the primary cohort was 26.4 months, whereas the 1-, 3- and 5-year OS rates were 72.2%, 37.2% and 17%, respectively. The median progression-free survival was 4.2 months. After univariate and multivariate analysis, a prognostic nomogram was formulated based on four predictors, including the number of tumours, maximum diameter of the tumour, CA19–9 level and ablation margin. The C-index of the nomogram was 0.815. Based on the patients of this study, the C-index was significantly higher than that of the Fendler nomogram (C-index, 0.698) and Kattan nomogram (C-index, 0.514, p < 0.001). Predictive accuracy of the proposed nomogram was also satisfactory in the validation cohort, with a C-index of 0.884.
Conclusions: Thermal ablation was an effective therapy for CRCLM. Moreover, the nomogram was effective and simple for CRCLM patients undergoing thermal ablation.
Introduction
Colorectal cancer (CRC) accounted for 9.7% of all cancers in 2015, and a total of 1 478 000 estimated new cases were recorded worldwide [Citation1–3]. The liver is a common site for cancer metastases, and metastatic tumours are detected in up to 50% of individuals with CRC, either within a period of 3 months of initial diagnosis (synchronous) or after (metachronous); this contributes to the poor prognosis of CRC [Citation4–6]. Untreated colorectal cancer liver metastases (CRCLM) is associated with a 5-year survival rate of <1% and a median survival of 7.5 months [Citation7]. For R0-resectable CRCLM, surgical resection is the standard technique. However, the outcome of surgery is not satisfactory, with 5-year survival rates ranging from 20% to 45% [Citation8]. Neoadjuvant chemotherapy is usually used as a pre-operative therapy before surgical resection, and is potentially associated with the downstaging of unresectable CRCLM to resectable, along with metastases control [Citation8–10]. For those with potentially resectable metastases, downstaging via neoadjuvant chemotherapy followed by secondary surgery is recommended, and long-term survival or cure might be achieved. However, data indicating that neoadjuvant chemotherapy may have a significant curative effect upon overall survival (OS) are scarce [Citation11–13]. The tumours in most patients are unresectable at presentation. With improvements due to surgery and downstaging via chemotherapy, approximately 36% of all CRCLM patients become eligible for resection [Citation14,Citation15]. However, few effective treatments are available for technically “never/unlikely” resectable CRCLM. In fact, only intermediate-intensive and non-intensive palliative therapies are recommended for controlling progression [Citation8]. Thus, the adequate management of CRCLM is vital for achieving better outcome.
Thermal ablation, including radiofrequency ablation (RFA) and microwave ablation (MWA), has become more widely accepted as a curative therapy for hepatocellular carcinoma, given its comparable efficacy with surgical resection, along with its cost-effectiveness, safety and repeatability [Citation16–20]. MWA is more capable of yielding larger ablation volumes with shorter duration, and is more resistant to the “heat-sink effect” [Citation21]. However, RFA has a lower probability of injuring the surrounding tissues, as the heating process is slower and the ablation area is easier to control [Citation22]. Transcatheter arterial (chemo)embolisation (TACE/TAE) is also a minimally invasive method used for the treatment of hepatocellular carcinoma; it can be used before thermal ablation to induce tumour-feeding artery obstruction, and the combination therapy of TAE and thermal ablation could help enhance the curative efficacy [Citation23]. Thermal ablation is also a safe and effective alternative for patients with both resectable and unresectable CRCLM, with a 5-year survival rate of 17–55% and a median survival of 24–52 months after ablation [Citation24,Citation25]. The morbidity and mortality rates are reported to be 1.3–2.2% and 0.2%, respectively [Citation25,Citation26]. Moreover, ablation can maximise metastases regression and can induce a progression-free interval without the need for systemic therapy; hence, it is generally recommended in palliative settings for technically unresectable CRCLM [Citation8]. However, to our knowledge, there is no well-validated prognostic system specially designed for patients with CRCLM who are undergoing thermal ablation, and the post-operative prediction of the survival duration remains a major concern. Thus, an individualised and accurate prognostic system is required.
Nomograms have been applied as a prognostic system in many types of cancer [Citation27–29], and the performance of nomograms can be favourably compared to that of the staging or scoring systems. In 2008, Kattan et al. [Citation30] proposed a nomogram based on clinicopathological factors in patients who had undergone hepatic resection. Recently, Fendler et al. [Citation31] developed a nomogram based on four categorical variables, including prior liver surgery, carcinoembryonic antigen level, transaminase toxicity and the computed tomography (CT) size of the two largest lesions, in patients with CRCLM who had undergone selective internal radiation therapy. For patients treated with thermal ablation, such a prognostic nomogram is essential for better predicting OS.
In the present study, we aimed to evaluate the efficacy of thermal ablation for both resectable and unresectable CRCLM patients, and to establish a prognostic nomogram for the prediction of survival time. We also verified the predictive accuracy of the new nomogram and compared it with the current two prognostic nomograms.
Materials and methods
Patients
According to the inclusion criteria and exclusion criteria, a total of 96 patients with resectable/unresectable CRCLM undergoing percutaneous thermal ablation from 2009 to 2016 were included continuously at our institute. The patients’ cohort before 2013 formed the primary cohort which was analysed to formulate a prognostic nomogram, and the patients’ cohort thereafter formed the validation cohort. The study was censored on 2 January 2017. Data analysis of all patients was covered. Written informed consent was obtained from each individual for their information to be stored and used for research. Human experimentation guidelines of China were followed. This study was approved by the institutional review board of Beijing You’an Hospital Ethics Committee.
Inclusion criteria: (1) Patients with resectable CRCLM but refused surgical resection (n = 15). The reasons patients choose ablation instead of surgery mainly included the anaesthetic risk, surgical complications such as bleeding, higher surgical mortality rate and high cost of surgery. (2) Patients with tumour recurrence after surgical resection (n = 21). (3) Patients failed from previous treatments such as tumour recurrence and progression after chemotherapy (n = 12). (4) Patients with unresectable CRCLM requiring for ablation treatment (n = 48). Unresectable was defined as follows: patients medically unfit; extensive metastatic disease (number of metastases more than 4, size of metastases larger than 5 cm, non-satellite hepatic metastases, lymph node metastases beyond portal vein, hepatic artery, distant metastases in other organ/sites); critical local involvement (bilateral and/or contralateral vascular and/or biliary involvement); inadequate future liver remnant (less than 30% future liver remnant of normal non-atrophied hepatic parenchyma or less than two contiguous segments with adequate vascular inflow and drainage, and adequate biliary drainage). Exclusion criteria: (1) Patients with Child-Pugh class C. (2) Severe coagulation disorder (platelet count less than 50 × 103/μl, prothrombin activity <60%). (3) Patients with huge nodules (maximum diameter >15 cm) or large number of metastases (>10), or untreatable extrahepatic metastases. (4) Poor performance status (ECOG PS >2). (5) Patient refusal of ablation.
Pre-operative transarterial embolisation
All patients were treated following multidisciplinary tumour board consultation. A CT imaging performed before TAE was used as the reference image. For metastases with obscure margin on CT scanning, pre-operative transarterial embolisation was performed. For patients with heavy tumour burden (large tumour size and multiple lesions), pre-operative TAE was also applied. After local anaesthesia, a 5-F pigtail catheter was advanced to the supplying vessel of metastases through hepatic artery angiography. All the tumour-feeding arteries were explored and identified. Depending on the artery supply size of the metastases, 4–10 ml lipiodol (Huaihai Pharmaceutical Factory, Shanghai, China) was injected and deposited in the tumour feeding artery. A CT scan was regularly performed within 7 days after intervention to evaluate lipiodol deposition.
Ablation procedure
Ablation was performed within 14 days after TAE. Percutaneous thermal ablations were performed using RFA (Covidien, Shanghai, P.R. China or RITA Medical Systems, Mountain View, CA) or MWA (Qinghai Ltd., Nanjing, P.R. China) probes aiming at inducing a favourable necrosis zone at the tumour site. For the tumours with large size or near big vessels, MWA is preferred; while RFA is usually used to treat the tumours adjacent to critical tissues such as gallbladder or colon. With the help of radiopaque marker on the skin and instant CT scan (Toshiba, Tokyo, Japan), the final path of antenna/electrode insertion was determined. A 22-gauge needle was used as a guide and lead the antenna/electrode to the target. Intraoperative CT scans were used to confirm the angle and position of antenna/electrode. After the antenna/electrode was advanced up to the target, the ablative power, electrode ablative zone size and effective ablative duration time were designed based on tumour volume and desired ablation zone. To prevent haemorrhage and needle tract seeding, the tract was ablated from the tumour to subcutaneous tissue while withdrawing the electrodes. To avoid skin burn, ablation was stopped at about 5 cm distance to the skin. A contrast-enhanced CT was regularly performed immediately after ablation for assessment of minimal ablation margin (classified as three groups: less than 1 mm, 1–5 mm and more than 5 mm) and possible complications such as bleeding. All ablation procedures were performed under local anaesthesia with 1% lidocaine at the needle entry point and continuous electrocardiogram monitoring.
Complications
According to the Society of Interventional Radiology Clinical Practice Guidelines [Citation32,Citation33], complications of all TAE and ablations procedures were recorded and classified as major (requirement of additional therapy, increased level of care, substantial lengthened hospital stay, morbidity and disability) and minor (other complications with no consequence, requiring no therapy or nominal therapy).
Follow-up
As per our standard in-house protocol, follow-up imaging was performed at a 4-week interval for the first 3 months after ablation, and every 3 months thenceforth regularly. CT is generally recommended for follow-up, but non-radiative MRI is also recommended for younger patient. Image findings were retrospectively interpreted to evaluate technique effectiveness and relapse. End point was the event (death of disease) or last follow-up available. Progression-free survival (PFS) was defined as the time interval from date of ablation to the detection of disease progression.
Statistical analysis
Demographic and clinical characteristics of the primary cohort and validation cohort were compared using SPSS 17.0 for Windows (SPSS Inc., Chicago, IL). At univariate analysis for OS, Kaplan–Meier method was used to compare categorical variables, and COX regression was used to evaluate the continuous variables. Significant factors identified by the univariate analysis (p < 0.05) were included in the multivariate analysis. At multivariate analysis, a Cox proportional hazards regression model was calculated and final model selection was performed by a bidirectional elimination process with the Akaike information criterion [Citation34].
A prognostic nomogram to predict OS was formulated based on the Cox proportional hazards regression model, using the package of rms [Citation35] in R version 3.3.1 (http://www.r-project.org/). The concordance index (C-index) and calibration curve were calculated to evaluate the performance of the nomogram. Larger C-index value indicated better prognostic predictive accuracy. The proposed nomogram was also compared with the current two prognostic nomograms (by Kattan [Citation30] and Fendler [Citation31]) by using the rcorrp.cens package in Hmisc [Citation36] in R. The C-index and calibration curve were also calculated in the validation cohort to further access the accuracy of the nomogram. p < 0.05 was considered to be statistically significant.
Results
Patients and tumour characteristics
A total of 96 patients underwent 163 thermal ablation sessions from 2009 to 2016. Twenty-five patients presented with treatable extrahepatic/lymphatic metastases. These extrahepatic/lymphatic metastases were treated by thermal ablation (n = 5), chemotherapy (n = 9), radiotherapy (n = 8), surgical resection (n = 1) and immunotherapy (n = 1); the extrahepatic metastases failed to be treated in one case due to hepatic tumour progression. In patients with vascular/biliary tumour thrombosis, the thrombosis was treated via thermal ablation. Specific ablation procedures for tumour thrombosis have been reported in previous papers [Citation18,Citation37].
Seventy-one patients with CRCLM from 2009 to 2013 were included in the primary cohort. The patients in the primary cohort underwent an average of 1.8 (range, 1–7) sessions of ablation. Another 25 patients who underwent an average of 1.5 (range, 1–4) ablation sessions since 2014 were assigned to the validation cohort. Patients undergoing pre-operative TAE in the primary cohort (n = 59) and the validation cohort (n = 20) exhibited clear images on non-contrast enhanced CT (low-density tumour lesion and high-density lipiodol deposition). The other patients without pre-operative TAE presented with distinct low-density on CT. The patients and tumour characteristics of the primary cohort and validation cohort are listed in . Additional details of resectable and unresectable CRCLM patient subgroups in the primary cohort and validation cohort are available in Table S1 in the supplemental material.
Table 1. Demographics and characteristics of patients with CRCLM in the primary cohort and validation cohort.
OS and PFS in the primary cohort
The Kaplan–Meier estimates of the survival rates of the primary cohort at 1-, 3- and 5-year were 72.2%, 37.2% and 17%, respectively; the median OS was 26.4 months. Patients with resectable metastases presented a significantly better survival (100%, 53.5% and 35.7% at 1-, 3- and 5-year, respectively; median OS, 55.4 months) as compared to those with unresectable CRCLM (57.6%, 17.3% and 0% at 1-, 3- and 5-year, respectively; median OS, 13.9 months; p < 0.001). The 1-, 3- and 5-year PFS rates of the primary cohort were 29.6%, 9.4% and NR (not reached), respectively; the median PFS was 4.2 months.
Univariate and multivariate analysis for OS of the primary cohort
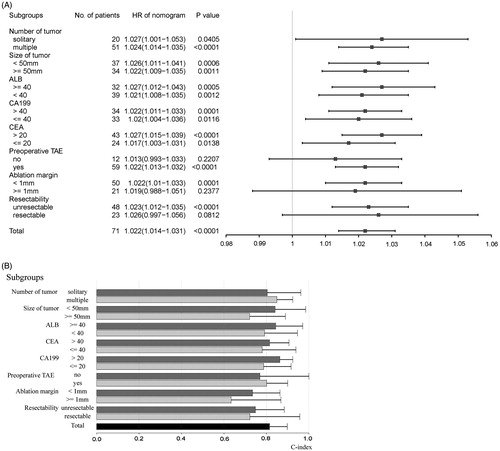
Most of the factors such as HCV co-infection and alcohol diseases showed no relevance with OS on univariate analysis. The following eight factors were found to be significantly associated with the OS of the primary cohort (p < 0.05): pre-operative TAE, ablation margin, resectability, albumin, CEA level, CA19–9 level, number of tumours and maximum diameter of the tumour (). Using the bidirectional elimination process with the Akaike information criterion, four variables were identified to construct the Cox proportional hazards regression model: number of tumours (HR, 1.211), maximum diameter of the tumour (HR, 1.014), CA19–9 level (HR, 1.002) and ablation margin (HR, 0.41) (). Among these variables, the number of tumours was a single independent risk factor (p = 0.007).
Table 2. Univariate and multivariate analysis of prognostic factors for primary cohort.
Prognostic nomogram for OS of the primary cohort
Based on the results of multivariate analysis and the Cox proportional hazards regression model, the nomogram was formulated using all four prognostic factors for OS in the primary cohort (). The C-index of the nomogram was 0.815 (95% CI, 0.732–0.898), indicating a relatively satisfactory predictive accuracy. The calibration curves were plotted (), and showed good agreement in the predictive survival assessed via nomogram and direct observation at 1 or 3 years after ablation.
Figure 1. Prognostic nomogram for overall survival of patients with colorectal cancer liver metastases undergoing ablation. To use the nomogram, for each variable, draw a line straight upward to the Points axis at the same vertical position. The total points were calculated. Draw a line straight down to survival axis to find the patient’s probability of 1-, 3- and 5-year survival at the same vertical position. For patients with more than 10 tumours, number of tumour was regarded as 10. CA19–9: carbohydrate antigen 19–9.
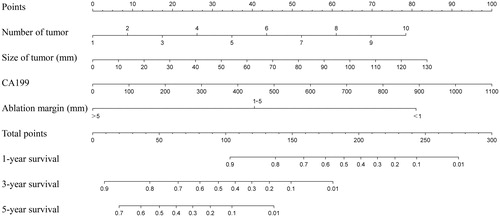
Figure 2. The calibration curve of nomogram for predicting survival at (A) 1 year and (B) 3 years in the primary cohort and at (C) 1 year in the validation cohort. Nomogram-predicted survival probability is plotted on the x-axis while actual OS is plotted on the y-axis. Thin grey line represents the reference line. OS: overall survival.
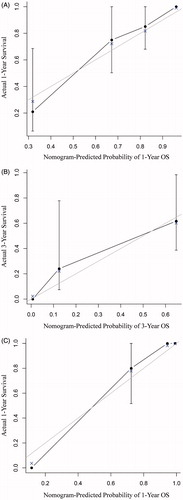
Predictive accuracy of the proposed nomogram in different subgroups in the primary cohort
The prognostic score for each individual was calculated using the nomogram and was analysed in different subgroups. The proposed nomogram showed significant relevance with survival (p < 0.05) in most of the subgroups (13 of the 16 subgroups), with the HR ranging from 1.017 to 1.027 (). In the three subgroups (12 patients without pre-operative TAE, 21 patients with ablation margin ≥1 mm, and 23 patients with resectable CRCLM), the nomogram did not show any significant relevance with the OS (p > 0.05). In most of the subgroups (15 of the 16 subgroups), the C-indices were above 0.7, ranging from 0.721 to 0.863 (), which was relatively high. Only in one subgroup (21 patients with ablation margin ≥1 mm), C-index was not that high (0.634; ).
Figure 3. Plots to show (A) the hazard ratios of the total points calculated by the proposed nomogram in different subgroups and (B) the C-indices of the total points calculated by the proposed nomogram in different subgroups in the primary cohort. HR: hazard ratio; ALB: albumin; CA19–9: carbohydrate antigen 19–9; CEA: carcinoembryonic antigen; TAE: transarterial embolisation.
Comparison between the nomogram and single independent factor in the primary cohort
A prognostic model was constructed using the single independent factor (number of tumours), and the C-index of this model for OS prediction was found to be 0.695. The single factor model was significantly less accurate for predicting survival as compared to the proposed nomogram (p < 0.001) ().
Comparison between the nomogram and the current two prognostic systems in the primary cohort
The C-indices of the Fendler and Kattan nomograms in the primary cohort were 0.698 and 0.514, respectively. The predictions of the proposed nomogram were significantly more concordant with the outcome, as compared to both the Fendler and Kattan nomograms (p < 0.001 for both) ().
Validation of the nomogram
In the validation cohort, the 1-, 3- and 5-year OS rates were 71.9%, NR and NR, respectively; moreover, the median OS was 15.7 months, which was similar to the survival in the primary cohort (p > 0.05). The median PFS of the validation cohort was 7 months, and the 1-, 3- and 5-year PFS rates were 40.1%, NR and NR, respectively.
The C-index of the proposed nomogram in the validation cohort was 0.884 (95% CI, 0.81–0.958). The calibration plot showed good agreement between the predictions by nomogram and actual survival at 1 year after ablation (). In contrast, a prognostic model constructed using the number of tumours presented a C-index of 0.627. Moreover, the single factor model in the validation cohort was significantly less accurate than the proposed nomogram in predicting survival ().
Complications
The ablation and TAE procedures were well tolerated by most individuals. A total of 79 TAE sessions were performed, and 5 (6.3%) minor complications and 1 (1.3%) major complications were noted. Minor complications included two cases of hypertension and three cases of slight fever. The major complication included one case of intrahepatic haematoma that required percutaneous transhepatic drainage. Among all the 163 thermal ablation sessions, the minor and major complication rates were 16.6% (27 of 163 ablation sessions) and 2.5% (4 of 163 ablation sessions), respectively. Minor complications included fever (n = 7), nausea (n = 5), abdominal pain (n = 10) and transient hepatic dysfunction (n = 5). The major complications included biloma (n = 2) and intrahepatic haematoma (n = 2), which were well managed by percutaneous transhepatic drainage and transcatheter arterial embolisation, respectively. There were no deaths related to ablation.
Discussion
For patients who cannot undergo surgical resection, thermal ablation can help maximise the necrosis of metastases and induce a progression-free interval without the need for systemic therapy [Citation38,Citation39]. In the present study, those with unresectable CRCLM in the primary cohort had a median OS of 13.9 months. Given that the median OS of untreated CRCLM patients was approximately 7.5 months [Citation7], thermal ablation offers potential benefit in prolonging survival in unresectable CRCLM patients. Thus, our results suggest that thermal ablation can be used for technically unresectable CRCLM.
In CRCLM patients, the presence of extrahepatic/lymphatic metastases usually indicates more advanced tumour stage. In the present study, these extrahepatic/lymphatic metastases were treated by various modalities, including thermal ablation, chemotherapy, radiotherapy, surgical resection and immunotherapy. Survival analysis of the primary cohort indicated that both extrahepatic and lymphatic metastases were not significantly related to survival (). This result suggests that with appropriate and effective management, extrahepatic/lymphatic metastases may not significantly influence the treatment outcome.
We proposed using a nomogram to predict the prognosis of patients with CRCLM who were undergoing thermal ablation. Four prognostic factors were included in the nomogram, and the number of tumours was found to be a single independent predictor. For patient subgroups with 1 to 10 tumours in the primary cohort, the median survival were 32.7, NA (not available), 29.0, 34.6, 9.5, 16.3, NA, 13.9, 4.8, 6.7, respectively, thus indicating a potential decreasing trend in the median OS. The number of tumours was assessed as a continuous variable with a linear effect rather than as a categorical variable. The HR of the number of tumours in the Cox regression model was 1.211 (95% CI, 1.054–1.392), which implies that one additional metastasis leads to an increase in the disease-related death risk by approximately 21%.
Although the other three factors in the nomogram, including the maximum diameter of the tumour, CA19–9 level and ablation margin (), were not single independent factors (p > 0.05), the nomogram showed a significantly more accurate predictive performance as compared to the single factor model (number of tumours) in the primary cohort (). With these three factors, the model was quite stable in predicting survival in the validation cohort; moreover, the C-index was 0.884 (95% CI, 0.81–0.958), which was significantly higher than that of the single factor model (number of tumours; 0.627). The maximum diameter of the tumour and CA19–9 level were used as continuous variables, which could yield additional prognostic information than if they were used as categorical variables. Moreover, the ablation margin was a protective factor, and a larger margin indicated a better prognosis. We believe that diameter of tumour, ablation margin and CA19–9 level could be important markers for patient survival; however, due to the limited sample size, these factors did not yield a significant independent effect on survival. Hence, the prognostic value of the maximum diameter of the tumour, CA19–9 level, and ablation margin need to be validated in future research.
The proposed nomogram also had good predictive accuracy in most of the subgroups in the primary cohort (); however, for the subgroup of patients with an ablation margin of ≥1 mm, the nomogram did not show a significant effect on survival (p > 0.05) and the C-index was low (0.634). We further assessed this subgroup and found that the number of events (only four cases of death) was too small to support efficient survival analysis; hence, the HR value and C-index in this subgroup may not be meaningful. Further analysis with larger samples may help to comprehensively evaluate the predictive ability of patients with ablation margin ≥1 mm. Overall, the proposed nomogram had quite a favourable prognostic performance in both the subgroups and the entire primary cohort, as well as in the validation cohort.
Two nomograms have been proposed by Kattan et al. [Citation30] and Fendler et al. [Citation31] for the prediction of survival in CRCLM patients. The Kattan nomogram included both continuous and categorical clinicopathological factors. However, for the Kattan nomogram, the C-index in his study was 0.61 [30], which was relatively low as compared to that in the present study [Citation40]. Moreover, the predictive performance of the Kattan nomogram was unfavourable in our patient cohort, with a C-index of merely 0.514. Given that the Kattan nomogram was formulated based on patients with CRCLM undergoing surgical resection, it might not be suitable for individuals treated with other modalities such as thermal ablation. Although the area under the receiver-operating characteristic curve was 0.83 for the Fendler nomogram [Citation31], that nomogram failed to show a similar predictability in the present population (C-index, 0.698). Both these nomograms were significantly less accurate than the proposed nomogram in predicting survival in the primary cohort (p < 0.001) (). However, the predictive performance of the proposed nomogram needs to be further validated in future studies with a larger population of patients who are undergoing thermal ablation.
The present study has certain limitations. The limited sample size remains a problem for achieving more efficient statistical analysis. The number of tumours was used as a linear variable, but not all the differences in the survival rates between neighbouring numbers were statistically significant. Second, transarterial (chemo)embolisation could have an positive effect on disease control [Citation41], as previous studies showed that transarterial (chemo)embolisation could induce disease partial response [Citation42]. And neoadjuvant TACE was found to be effective in downsizing lesions, thus the group of patients eligible for ablation could be enlarged and survival could be improved [Citation43]. Although the effect of TAE might not be as significant as curative therapies, it may lead to additional subjective bias in our data. However, due to the limited number of patients without pre-operative transarterial embolisation, and the baseline heterogeneity between patients with/without TAE, this bias was difficult to evaluate in the present study. Third, the follow-up was not sufficiently long to yield a more precise outcome, especially in patients with a better ablation margin; hence, the nomogram could not be effectively assessed in subgroups with a limited number of deceased cases.
Conclusions
In conclusion, our results indicate the efficacy and safety of CT-guided percutaneous thermal ablation for patients with CRCLM. Furthermore, the proposed nomogram was specifically designed for patients with CRCLM undergoing thermal ablation, and could help precisely estimate the prognosis after ablation. Further studies are needed to validate the predictability of this nomogram.
| Abbreviations | ||
| CRC | = | colorectal cancer |
| CRCLM | = | colorectal cancer liver metastases |
| OS | = | overall survival |
| RFA | = | radiofrequency ablation |
| MWA | = | microwave ablation |
| CT | = | computed tomography |
| ECOG PS | = | Eastern Cooperative Oncology Group Performance Status |
| TACE | = | transarterial chemoembolisation |
| TAE | = | transarterial embolisation |
| PFS | = | progression-free survival |
| C-index | = | concordance index |
| NR | = | not reached |
| HR | = | hazard ratio |
| CI | = | confidence interval |
| NA | = | not available |
| HCV | = | hepatitis C virus |
| ALT | = | alanine aminotransferase |
| TBIL | = | total bilirubin |
| PLT | = | platelet |
| PT | = | prothrombin time |
| PTA | = | prothrombin activity |
| AFP | = | alpha-fetoprotein |
| CEA | = | carcinoembryonic antigen |
| CA19–9 | = | carbohydrate antigen 19–9 |
| DFI | = | disease-free interval |
| ALB | = | albumin |
| PC | = | primary cohort |
| Ref.: reference; SIF | = | single independent factor |
| VC | = | validation cohort |
Supplemental File
Download PDF (173.1 KB)Acknowledgements
The institutional review board of You’an Hospital Ethics Committee has approved this study. Informed consent was obtained from all individual participants included in the study. Human experimentation guidelines of China were followed in the conduct of this clinical research. The work described has not been published or accepted elsewhere, in whole or in part. All the authors listed have seen and approved the manuscript that is enclosed, contributed significantly to the work.
Disclosure statement
The authors declare that they have no competing interests.
Additional information
Funding
References
- Mahasneh A, Al-Shaheri F, Jamal E. (2017). Molecular biomarkers for an early diagnosis, effective treatment and prognosis of colorectal cancer: current updates. Exp Mol Pathol 102:475–83.
- Verma M, Kumar V. (2017). Epigenetic biomarkers in colorectal cancer. Mol Diagn Ther 21:153–65.
- Ferlay J, Soerjomataram I, Dikshit R, et al. (2015). Cancer incidence and mortality worldwide: sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 136:E359–86.
- Millikan KW, Staren ED, Doolas A. (1997). Invasive therapy of metastatic colorectal cancer to the liver. Surg Clin North Am 77:27–48.
- Wagner JS, Adson MA, Van Heerden JA, et al. (1984). The natural history of hepatic metastases from colorectal cancer. A comparison with resective treatment. Ann Surg 199:502–8.
- Bozzetti F, Doci R, Bignami P, et al. (1987). Patterns of failure following surgical resection of colorectal cancer liver metastases. Rationale for a multimodal approach. Ann Surg 205:264–70.
- Stangl R, Altendorf-Hofmann A, Charnley RM, et al. (1994). Factors influencing the natural history of colorectal liver metastases. Lancet 343:1405–10.
- Van Cutsem E, Cervantes A, Nordlinger B, et al. (2014). Metastatic colorectal cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol 25:iii1–9.
- Huang L, Li TJ, Zhang JW, et al. (2014). Neoadjuvant chemotherapy followed by surgery versus surgery alone for colorectal cancer: meta-analysis of randomized controlled trials. Medicine 93:e231.
- Ji JH, Park SH, Lee J, et al. (2013). Prospective phase II study of neoadjuvant FOLFOX6 plus cetuximab in patients with colorectal cancer and unresectable liver-only metastasis. Cancer Chemother Pharmacol 72:223–30.
- Kim JY, Kim JS, Baek MJ, et al. (2013). Prospective multicenter phase II clinical trial of FOLFIRI chemotherapy as a neoadjuvant treatment for colorectal cancer with multiple liver metastases. J Korean Surg Soc 85:154–60.
- Kataoka M, Kanda M, Ishigure K, et al. (2017). The COMET open-label phase II study of neoadjuvant FOLFOX or XELOX treatment combined with molecular targeting monoclonal antibodies in patients with resectable liver metastasis of colorectal cancer. Ann Surg Oncol 24:546–53.
- Nasti G, Piccirillo MC, Izzo F, et al. (2013). Neoadjuvant FOLFIRI + bevacizumab in patients with resectable liver metastases from colorectal cancer: a phase 2 trial. Br J Cancer 108:1566–70.
- Cirocchi R, Trastulli S, Boselli C, et al. (2012). Radiofrequency ablation in the treatment of liver metastases from colorectal cancer. Cochrane Database Syst Rev 6:CD006317.
- Adam R, Wicherts DA, de Haas RJ, et al. (2009). Patients with initially unresectable colorectal liver metastases: is there a possibility of cure? J Clin Oncol 27:1829–35.
- Kim YS, Lim HK, Rhim H, et al. (2014). Ablation of hepatocellular carcinoma. Best Pract Res Clin Gastroenterol 28:897–908.
- Li W, Wang Y, Gao W, et al. (2016). Combination of transcatheter arterial chemoembolization and CT-guided percutaneous segment ablation for hepatocellular carcinoma therapy: a retrospective study. Medicine 95:e5422.
- Zheng JS, Long J, Sun B, et al. (2014). Transcatheter arterial chemoembolization combined with radiofrequency ablation can improve survival of patients with hepatocellular carcinoma with portal vein tumour thrombosis: extending the indication for ablation? Clin Radiol 69:e253–63.
- Li JJ, Zheng JS, Cui SC, et al. (2015). C-arm Lipiodol CT in transcatheter arterial chemoembolization for small hepatocellular carcinoma. World J Gastroenterol 21:3035–40.
- Nishikawa H, Kimura T, Kita R, et al. (2013). Radiofrequency ablation for hepatocellular carcinoma. Int J Hyperthermia 29:558–68.
- Poch FG, Rieder C, Ballhausen H, et al. (2016). The vascular cooling effect in hepatic multipolar radiofrequency ablation leads to incomplete ablation ex vivo. Int J Hyperthermia 32:749–56.
- Poulou LS, Botsa E, Thanou I, et al. (2015). Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 7:1054–63.
- Guo W, He X, Li Z, et al. (2015). Combination of transarterial chemoembolization (TACE) and radiofrequency ablation (RFA) vs. surgical resection (SR) on survival outcome of early hepatocellular carcinoma: a meta-analysis. Hepato-gastroenterology 62:710–14.
- Hamada A, Yamakado K, Nakatsuka A, et al. (2012). Radiofrequency ablation for colorectal liver metastases: prognostic factors in non-surgical candidates. Jpn J Radiol 30:567–74.
- Solbiati L, Ahmed M, Cova L, et al. (2012). Small liver colorectal metastases treated with percutaneous radiofrequency ablation: local response rate and long-term survival with up to 10-year follow-up. Radiology 265:958–68.
- Livraghi T, Solbiati L, Meloni MF, et al. (2003). Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–51.
- Lang BH, Wong CK. (2015). Validation and comparison of nomograms in predicting disease-specific survival for papillary thyroid carcinoma. World J Surg 39:1951–8.
- Al-Daghmin A, English S, Kauffman EC, et al. (2014). External validation of preoperative and postoperative nomograms for prediction of cancer-specific survival, overall survival and recurrence after robot-assisted radical cystectomy for urothelial carcinoma of the bladder. BJU Int 114:253–60.
- Wang Y, Li J, Xia Y, et al. (2013). Prognostic nomogram for intrahepatic cholangiocarcinoma after partial hepatectomy. J Clin Oncol 31:1188–95.
- Kattan MW, Gonen M, Jarnagin WR, et al. (2008). A nomogram for predicting disease-specific survival after hepatic resection for metastatic colorectal cancer. Ann Surg 247:282–7.
- Fendler WP, Ilhan H, Paprottka PM, et al. (2015). Nomogram including pretherapeutic parameters for prediction of survival after SIRT of hepatic metastases from colorectal cancer. Eur Radiol 25:2693–700.
- Gaba RC, Lewandowski RJ, Hickey R, et al. (2016). Transcatheter therapy for hepatic malignancy: standardization of terminology and reporting criteria. J Vasc Interv Radiol 27:457–73.
- Cardella JF, Kundu S, Miller DL, et al. (2009). Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol 20:S189–S91.
- Harrell FE Jr, Lee KL, Mark DB. (1996). Multivariable prognostic models: issues in developing models, evaluating assumptions and adequacy, and measuring and reducing errors. Stat Med 15:361–87.
- Harrell FE Jr. (2013). rms: regression modeling strategies. R package version 4.0-0. Computer software. Available from: http://CRAN.R-project.org/package=rms [last accessed 10 Mar 2017].
- Harrell FE Jr. (2013). Hmisc: Harrell miscellaneous. R package version 3.12-2. Computer software. Available from: http://CRAN.R-project.org/package=Hmisc [last accessed 10 Mar 2017].
- Long J, Zheng JS, Sun B, et al. (2016). Microwave ablation of hepatocellular carcinoma with portal vein tumor thrombosis after transarterial chemoembolization: a prospective study. Hepatol Int 10:175–84.
- Liu M, Huang GL, Xu M, et al. (2017). Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia 33:446–53.
- Amabile C, Ahmed M, Solbiati L, et al. (2017). Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia 33:34–42.
- Brentnall AR, Cuzick J. (2016). Use of the concordance index for predictors of censored survival data. Stat Methods in Med Res 25:962280216680245.
- De Groote K, Prenen H. (2015). Intrahepatic therapy for liver-dominant metastatic colorectal cancer. World J Gastrointest Oncol 7:148–52.
- Gruber-Rouh T, Naguib NN, Eichler K, et al. (2014). Transarterial chemoembolization of unresectable systemic chemotherapy-refractory liver metastases from colorectal cancer: long-term results over a 10-year period. Int J Cancer 134:1225–31.
- Vogl TJ, Dommermuth A, Heinle B, et al. (2014). Colorectal cancer liver metastases: long-term survival and progression-free survival after thermal ablation using magnetic resonance-guided laser-induced interstitial thermotherapy in 594 patients: analysis of prognostic factors. Invest Radiol 49:48–56.