Abstract
Purpose: Radiotherapy before or after resection is one of the pillars of treatment for localised high risk soft tissue sarcomas. Treatment intensification has been described with concurrent chemotherapy and hyperthermia. The aim of this study is to assess local control after multimodal treatment, focussing on the treatment of local recurrences after surgery only.
Patients and methods: Of 42 patients treated in a prospective protocol with radiotherapy and hyperthermia, nine were treated for isolated local recurrences without metastatic spread. Most patients were treated with trimodal therapy including chemotherapy with ifosfamide and underwent resection whenever possible. Median follow-up was 1.4 years.
Results: The treatment was well tolerated. Estimated disease free survival, distant metastases free survival and local control for the whole cohort after 1.5 years were 66, 73 and 88%, respectively. Neoadjuvant vs. adjuvant treatment influenced local control with a trend to statistical significance. Resection status did not influence local control. The cohort of patients treated for local recurrence after surgery alone had a significantly impaired local control compared to multimodal treatment at primary diagnosis (100 vs. 52%, p < 0.001).
Conclusions: With multimodal therapy including radiotherapy and hyperthermia local tumour control is achievable even in locally recurrent tumours. The clear-cut difference of the treatment of local recurrence in contrast to primary diagnosis might either reflect difficulties in diagnosis and treatment of local recurrences or biological aggressiveness of recurrent tumours. However, we recommend to consider multimodal treatment at primary diagnosis of high risk soft tissue sarcomas.
Introduction
The treatment of localised high-risk adult-type soft tissue sarcoma poses major challenges and requires close cooperation of a multidisciplinary team. High risk situations are defined by stage (grading, size, location) and in some studies also by age at diagnosis [Citation1]. The main pillars of treatment are complete en-block resection and pre- or postoperative radiotherapy [Citation2]. Yet, treatment results still give room for improvement. Local control is a major issue whenever complete resection with negative microscopic margins is not achievable with limb sparing surgery.
Whereas surgical therapy has become less aggressive over the last three decades, developing from amputation to limb-sparing approaches [Citation3,Citation4], efforts have been undertaken to intensify pre- and postoperative therapy.
As distant metastases are still the main type of recurrence, the addition of chemotherapy seemed promising to improve disease-free and overall survival. The agents most commonly used are doxorubicin and ifosfamide. Yet, the results of large multicentric randomised studies are conflicting. A pooled analysis of two STBSG-EORTC studies showed a significant benefit for disease-free survival [Citation5], whereas a large randomised trial published in 2012 did not point toward an effect [Citation6]. Thus, current recommendations state that neoadjuvant and/or adjuvant chemotherapy is a treatment option in high risk soft tissue sarcomas. Yet, the conflicting data warrant stringent benefit-risk analyses and open discussions with the patients. Chemotherapy is also applied in hyperthermic isolated limb perfusion at specialised centres [Citation7,Citation8]. A randomised study of neoadjuvant or adjuvant chemotherapy with or without regional hyperthermia showed a significant increase in disease free survival for combination treatment [Citation9].
Pre- or postoperative radiotherapy has been established in multimodal treatment of high risk soft tissue sarcomas of the extremities [Citation10]. Postoperative radiation with >60.0 Gy improves local control in high risk soft tissue sarcoma [Citation11,Citation12]. Preoperative irradiation with 45.0 –50.0 Gy leads to more acute wound healing complications after surgery, yet seems to have a benefit for late complications like fibrosis and joint stiffness in comparison to adjuvant treatment [Citation13–15]. Advanced radiation techniques such as intensity-modulated radiotherapy (IMRT) and image guided treatment (IGRT) seem to further reduce the risk of late complications [Citation16–18]. The role of radiotherapy is less clear for retroperitoneal sarcomas as there are no large randomised studies reported up to now. Single centre and database analyses seem to indicate a benefit for preoperative radiotherapy [Citation19–21].
Radiotherapy combined with concurrent chemotherapy and/or hyperthermia was first described in 1999 by Sauer et al. [Citation22]. Since then, several groups reported small retrospective series of concurrent radiochemotherapy either with interdigitated MAID chemotherapy and radiation [Citation23,Citation24] or with ifosfamide and radiotherapy [Citation25–27], partly in combination with hyperthermia. The regimens seem feasible, yet conclusions about the efficacy of the treatment are limited by patient numbers and retrospective data collection.
We focussed our analysis on nine of 42 patients followed prospectively after treatment for localised high risk soft tissue sarcomas who were treated for local recurrences without metastatic spread after surgery alone. The results were compared with the whole cohort and patients treated multimodally at primary diagnosis.
Patients and methods
Patients with high risk soft tissue sarcoma treated with a combination of radiotherapy and hyperthermia were followed prospectively from 2012 to 2016. All patients were treated in a multidisciplinary setting in the Center for Soft Tissue Sarcoma, GIST and Bone Tumors of the Comprehensive Cancer Center Tübingen. Patients treated with combined radiotherapy and hyperthermia for better local control of single disease sites in a metastatic setting were excluded from the analysis as were patients who did not finish the planned treatment at least to a radiation dose of 30.0 Gy. All patients had pre-treatment staging consisting of at least history and physical examination, biopsy, cross-sectional imaging of the tumour region and laboratory data. Pulmonary metastases were excluded by chest computed tomography (CT).
42 patients with a median age of 56 years (range 19–76 years) were treated in the prospective protocol. Nine patients were treated multimodally for recurrences after surgery only without radiotherapy, whereas 33 received multimodal treatment at initial diagnosis. Six presented with tumours smaller than 5 cm and 36 with larger tumours. Median tumour size at diagnosis was 8.7 ± 0.7 cm. Seven patients were treated for sarcomas of the upper extremities, 16 for lower extremity tumoursand 19 for tumours of the trunk. Histopathology showed one well differentiated sarcoma, 11 intermediate differentiated tumours and 30 high grade sarcomas (according to FNCLCC). AJCC stage for all patients was IIa, IIb or III. Histopathological subtypes covered the whole spectrum of adult type soft tissue sarcomas. Patient and tumour characteristics are summarised in .
Table 1. Patient characteristics of the whole cohort of 42 patients, nine were treated for isolated local recurrences after surgery alone.
Our institutional treatment guidelines follow the published IAWS protocol [Citation28] for radiotherapy and chemotherapy of localised high risk soft tissue sarcomas. Patients with large, poorly differentiated tumours (mostly in the neoadjuvant setting) under the age of 65 were offered pre-irradiation chemotherapy with 4–6 cycles of doxorubicin (60 mg/m2 day 1) and ifosfamide (3,000 mg/m2 days 1–3), repeated on day 22. Most patients were treated with two cycles of a reduced dose of ifosfamide without doxorubicin concomitantly during radiotherapy. The same ifosfamide regimen (3,000 mg/m2 days 1–2) was applied in patients treated with concurrent radiochemotherapy without sequential chemotherapy.
Hyperthermia was offered to all sarcoma patients scheduled for radiotherapy presenting without exclusion criteria such as severe cardiovascular diseases, metallic implants or claustrophobia and was performed as MR-controlled partial body hyperthermia (PBH, 20 patients), regional deep hyperthermia (RHT, 12 patients) or superficial hyperthermia (10 patients). Quality assurance guidelines according to the recommendations of the European Society for Hyperthermic Oncology were considered [Citation29]. For PBH the MR-guided hyperthermia unit BSD 2000/3 D MRI (Pyrexar Medical, formerly BSD medical corporation, Salt Lake City, UT) with Sigma-Eye or Sigma-30 applicator was used. For 14 of 20 PBH patients hyperthermia treatment planning was done using the software SigmaHyperPlan® V2.01 (Dr. Sennewald Medizintechnik GmbH, Munich, Germany) to optimise steering of phases and/or amplitudes of the applicator. MR-temperature control every 10 min with SigmaVision® V1.01 (part of SigmaHyperPlan®) was used for online adaptation of steering parameters. Patients with RHT were treated with the hyperthermia system BSD 2000/3 D (Pyrexar Medical, formerly BSD medical corporation, Salt Lake City, UT) with Sigma-30 or Sigma-60 applicator. For superficial hyperthermia the same system with the spiral applicator SA-115 was used. Hyperthermia was performed twice weekly. Depending on the tumour localisation temperature probes were located on the skin, intraluminally in bladder, rectum and vagina or in rare cases interstitially in the tumour. Target temperature was between 40 and 43 °C for at least 60 min and the maximum treatment duration per hyperthermia session was 90 min.
There are major obstacles for direct thermometry in sarcoma patients. Placement of direct thermometry probes bears the risk of implant metastases [Citation30,Citation31]. In addition, with the trimodal therapy as applied in most of our patients, chemotherapy might lead to leukopenia and thus increase the risk of infectious complications endangering the completion of radiotherapy. Thus, for quality assurance, indirect MR-thermometry [Citation32] was analysed retrospectively for four patients treated with MR-guided PBH for at least three treatments per patient with SigmaVision® V1.01. Automatic temperature evaluation in SigmaVision® V1.01 reports T90 for the contoured volume, however the given values include the 30 min heating time until the treatment temperature was reached (defined as T90*). As heatmaps were inhomogenous throughout the tumour volume due to MRI artefacts, small intratumoral volumes with homogenous MR signals were contoured manually for each treatment. Consequently, given values might correspond best to T10 to T20 of the whole tumour. Mean T90* values over the whole treatment time were calculated as well as CEM43° [Citation33,Citation34] per treatment.
All patients eligible for surgery underwent wide resection. In the case of extremity sarcomas, limb sparing surgery was performed in all patients. Most patients referred to our centre for diagnosis and treatment planning were scheduled for neoadjuvant (chemo) irradiation with hyperthermia. Details about the treatment are given in and .
Figure 1. Schematic overview of the treatment in the cohorts of patients treated for local recurrences after surgery alone and patients treated multimodally at primary diagnosis, as well as patients screened but not eligible for the analysis.
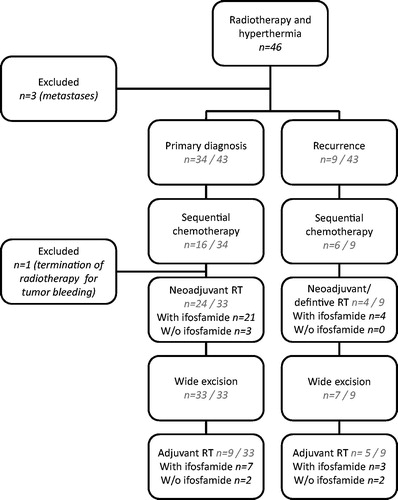
Table 2. Treatment characteristics of the whole cohort.
Evaluated parameters included estimated disease-free survival, distant metastases-free survival and local control. Factors possibly influencing local tumour control were tested for their effect on recurrences. Patients receiving multimodal treatment for local recurrence without metastatic spread after surgery only were compared to patients treated multimodally at primary diagnosis. Statistical analysis consisted of Kaplan-Meier estimation for survival curves, which were compared with the log-rank test as well as in multivariate analysis. Means are given ± standard errors and were compared with two-sided Student’s t-test for independent samples. The statistical analysis was performed with the software package SPSS 24 (SPSS Inc., Chicago, IL). Statistical significance was defined for a p values <0.05, a trend to statistical significance for p values between 0.05 and 0.10.
Results
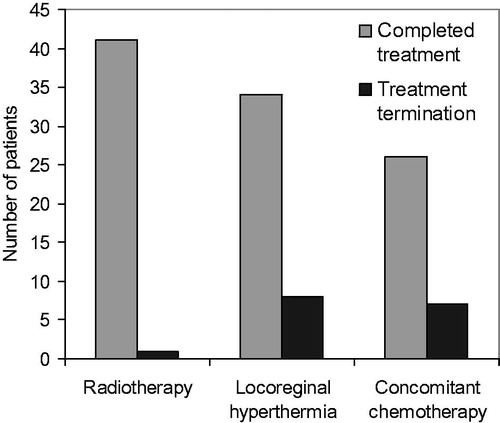
Overall the treatment was well tolerated. Radiotherapy was completed in all but one patient. The treatment termination in this case was not indicated for toxicity, but for massive progression of the tumour (trunk, undifferentiated sarcoma, NOS, grade 3) with new metastatic manifestations. The patient was started on palliative systemic therapy with trabectedin, which has stabilised the disease for 1.5 years. The patient is still on treatment. Full dose concomitant chemotherapy with ifosfamide was administered in 26 of 33 patients for whom the treatment had been planned. 33 patients completed at least one cycle of chemotherapy (50% of the planned dose). Hyperthermia was terminated for severe skin toxicity due to the combination of radiotherapy and hyperthermia, oedema of extremity or lack of tolerability of the bolus pressure or circulatory problems during treatment. 28 patients completed all planned treatments (ten or more) and 34 patients completed at least eight treatments (.
Figure 2. Multimodal treatment with radiotherapy and hyperthermia complemented with concomitant chemotherapy in 33 patients. Radiotherapy was completed in all but one patient. 34 of 42 patients underwent eight or more hyperthermia treatments twice weekly. 26 of 33 patients completed the full two cycles of concomitant chemotherapy with ifosfamide.
For four patients undergoing neoadjuvant treatment with PBH, indirect MR-thermometry indicated that the treatment goal of 40–42 °C was reached, CEM43° was high overall, yet with rather large standard errors ().
Table 3. Indirect MR-thermometry data were evaluated for four patients in the neoadjuvant setting.
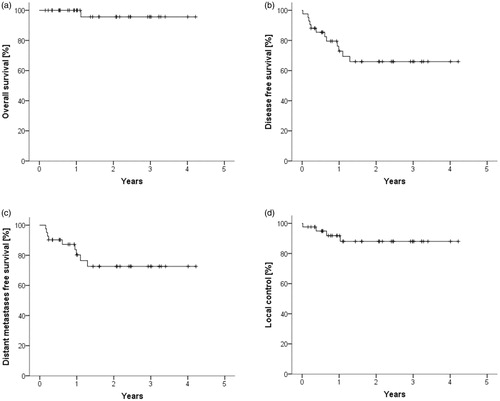
The median follow-up of the patients was 1.4 years. The estimated mean overall survival was 4.1 ± 0.13 years, the 1.5 year estimated overall survival was 96%. Mean disease free survival, distant metastases free survival and local control were 3.0 ± 0.29 , 3.3 ± 0.28 and 3.8 ± 0.21 years, respectively. The 1.5 year rates were 66%, 73% and 88% for disease free survival, distant metastases free survival and local control, respectively (. All local failures were located inside of the irradiated volume.
Figure 3. Estimated overall survival (a), disease free survival (b), distant metastases free survival (c) and local control (d) are shown after a median follow up of 1.4 years.
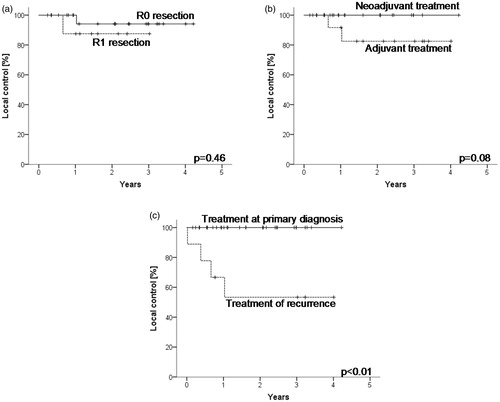
Possible prognostic factors tested for their influence on local tumour control after multimodal treatment of the whole cohort were age at diagnosis, recurrent vs. initial diagnosis, localisation, grading, resection status, additional sequential chemotherapy and concurrent radiosensitizing chemotherapy. Tumour grading as well as localisation in the upper extremity vs. lower extremity vs. trunk were not prognostic. Adjuvant treatment yielded inferior results to neoadjuvant treatment, with a trend to statistical significance. Neither sequential nor concurrent chemotherapy influenced local tumour control. Local control was slightly worse for positive resection status compared to microscopically complete resection, yet without statistical significance. Local control was highly significantly worse after multimodal treatment of recurrent disease compared to patients treated with multimodal treatment at initial diagnosis (. Disease free survival and distant metastases free survival were not significantly influenced by any of the tested factors (data not shown). Multivariate analysis of local control (recurrent vs. primary disease, neoadjuvant vs. adjuvant treatment and resection status) did not reveal independent prognostic factors.
Figure 4. Resection status did not influence local control (a). Adjuvant treatment in comparison to preoperative irradiation led to worse estimated local control with a trend to statistical significance (b). The most significant factor influencing local control was the treatment of local recurrences after surgery only in comparison to multimodal treatment at primary diagnosis (c). Multivariate analysis did not reveal independent prognostic factors among resection status, adjuvant vs. neoadjuvant treatment or treatment or recurrences.
The clear-cut difference between patients treated at primary diagnosis and with recurrent disease (although not significant in multivariate analysis) raised the question as how these cohorts were compared concerning known prognostic factors. Tumour size was significantly smaller for recurrent sarcomas (p = 0.04). Tumour grading was not different between the groups. On the other hand, more recurrent tumours were located at the trunk. Treatment characteristics were unfavourable in the recurrent tumours with more patients treated in the adjuvant setting or with macroscopic disease ().
Table 4. Comparison of known prognostic factors in the cohort of patients treated for local recurrences after surgery alone vs. patients treated multimodally at primary diagnosis.
Discussion
Our results demonstrate a significantly worse local control in patients treated multimodally for isolated local recurrences after surgery only. However, interpretation of our results is limited by small patient numbers and retrospective definition of the compared cohorts of recurrent tumours vs. therapy at primary diagnosis. The interpretation of local tumour control might also be influenced by salvage systemic therapy for distant metastases.
Distant metastases and disease free survival are in line with Stubbe et al. describing neoadjuvant radiochemotherapy with ifosfamide and hyperthermia [Citation27]. Local control of the mixed population in this cohort is between local control described for neoadjuvant treatment [Citation25] and definitive treatment [Citation26] with the same regimen. As expected, it is lower than for patient populations restricted to neoadjuvant treatment [Citation27,Citation35].
Whereas resection status is one of the strongest predictors of local control in soft tissue sarcomas undergoing resection only [Citation36,Citation37], results are conflicting when surgery is combined with irradiation [Citation38,Citation39]. Dagan et al. reported a 69% five-year local control rate for contaminated margins vs. 95% for marginal or wide excision [Citation39]. Ahmad et al. analysed a large retrospective cohort of 384 patients and did not find a significant difference in local control for negative and positive pathological resection margins [Citation38]. Our data suggest similar results like those reported by Ahmad et al., yet are limited by low numbers of local recurrences and comparatively short follow up.
The most striking predictor for local recurrence was multimodal treatment of local recurrences after surgery alone in comparison to multimodal treatment at primary diagnosis. Despite the fact that recurrence was not a significant prognosticator for local control in multivariate analysis, the comparison of known prognostic factors in the cohorts of recurrent tumours and sarcomas treated after primary diagnosis revealed conflicting trends. Whereas tumour size favoured the group of recurrent tumours, grading did not differ between cohorts and recurrences had unfavourable characteristics concerning localisation and treatment modality.
There is only scarce data about local control and survival after curative treatment of local recurrences of soft tissue sarcoma. Liu et al. analysed the significance of resection status at primary diagnosis and recurrence for extremity tumours and found a local control rate of appr. 80% at 1 year [Citation40]. For retroperitoneal sarcoma a local control rate of appr. 50% at 1 year was reported for recurrent tumours [Citation41]. Daigeler et al. specifically reported on 135 patients with recurrent soft tissue sarcoma. Yet, they focus on overall survival and do not report on local control after diagnosis of recurrence. In addition, the analysed population consisted of local recurrences with and without simultaneous metastatic spread [Citation42]. In our cohort 1.5 year local control rate for patients treated with local recurrences of soft tissue sarcomas was 53%. The question arises, whether the striking difference in the treatment of primary and recurrent tumours is due to difficulties in the diagnosis and treatment of recurrent tumours or rather reflecting a more aggressive biology of recurrent tumours. On the one hand radiological diagnosis and treatment, e.g. planning of surgery and radiotherapy, are more challenging in pre-treated tissue. On the other hand, tumours recurring after aggressive resection might differ biologically from tumours that can be cured after primary diagnosis by surgery alone [Citation43].
In conclusion, multimodal treatment with radiotherapy and hyperthermia, in most cases also in combination with surgery and concurrent ifosfamide, for high risk soft tissue sarcomas might be able to alleviate the negative prognostic value of microscopically positive resection margins. In addition, local control rate in recurrent disease after surgery is significantly lower than in primary disease. Despite the insecurities in the interpretation of the data concerning the reason for this difference, we recommend to consider multimodal treatment at primary diagnosis generously.
Acknowledgements
This study was partly funded by the “EKFS-Forschungskolleg Tübingen” (2015_Kolleg.14) of the Else-Kröner-Fresenius foundation.
Disclosure statement
The authors report no conflicts of interest.
References
- Gilbert NF, Cannon CP, Lin PP, Lewis VO. (2009). Soft-tissue sarcoma. J Am Acad Orthop Surg 17:40–7.
- Abraham JA, Baldini EH, Butrynski JE. (2010). Management of adult soft-tissue sarcoma of the extremities and trunk. Expert Rev Anticancer Ther 10:233–48.
- Rosenberg SA, Tepper J, Glatstein E, et al. (1982). The treatment of soft-tissue sarcomas of the extremities: prospective randomized evaluations of (1) limb-sparing surgery plus radiation therapy compared with amputation and (2) the role of adjuvant chemotherapy. Ann Surg 196:305–15.
- Williard WC, Hajdu SI, Casper ES, Brennan MF. (1992). Comparison of amputation with limb-sparing operations for adult soft tissue sarcoma of the extremity. Ann Surg 215:269–75.
- Le Cesne A, Ouali M, Leahy MG, et al. (2014). Doxorubicin-based adjuvant chemotherapy in soft tissue sarcoma: pooled analysis of two STBSG-EORTC phase III clinical trials. Ann Oncol 25:2425–32.
- Woll PJ, Reichardt P, Le Cesne A, et al. (2012). Adjuvant chemotherapy with doxorubicin, ifosfamide, and lenograstim for resected soft-tissue sarcoma (EORTC 62931): a multicentre randomised controlled trial. Lancet Oncol 13:1045–54.
- Andreou D, Werner M, Pink D, et al. (2016). Histological response assessment following neoadjuvant isolated limb perfusion in patients with primary, localised, high-grade soft tissue sarcoma. Int J Hyperthermia 32:159–64.
- Rastrelli M, Campana LG, Valpione S, et al. (2016). Hyperthermic isolated limb perfusion in locally advanced limb soft tissue sarcoma: a 24-year single-centre experience. Int J Hyperthermia 32:165–72.
- Issels RD, Lindner LH, Verweij J, et al. (2010). Neo-adjuvant chemotherapy alone or with regional hyperthermia for localised high-risk soft-tissue sarcoma: a randomised phase 3 multicentre study. Lancet Oncol 11:561–70.
- Wang D, Abrams RA. (2014). Radiotherapy for soft tissue sarcoma: 50 years of change and improvement. Am Soc Clin Oncol Educ Book 2014:244–51.
- Beane JD, Yang JC, White D, et al. (2014). Efficacy of adjuvant radiation therapy in the treatment of soft tissue sarcoma of the extremity: 20-year follow-up of a randomized prospective trial. Ann Surg Oncol 21:2484–9.
- Yang JC, Chang AE, Baker AR, et al. (1998). Randomized prospective study of the benefit of adjuvant radiation therapy in the treatment of soft tissue sarcomas of the extremity. J Clin Oncol 16:197–203.
- Davis AM, O'Sullivan B, Bell RS, et al. (2002). Function and health status outcomes in a randomized trial comparing preoperative and postoperative radiotherapy in extremity soft tissue sarcoma. J Clin Oncol 20:4472–7.
- Davis AM, O’Sullivan B, Turcotte R, et al. (2005). Late radiation morbidity following randomization to preoperative versus postoperative radiotherapy in extremity soft tissue sarcoma. Radiother Oncol 75:48–53.
- O’Sullivan B, Davis AM, Turcotte R, et al. (2002). Preoperative versus postoperative radiotherapy in soft-tissue sarcoma of the limbs: a randomised trial. Lancet 359:2235–41.
- O’Sullivan B, Griffin AM, Dickie CI, et al. (2013). Phase 2 study of preoperative image-guided intensity-modulated radiation therapy to reduce wound and combined modality morbidities in lower extremity soft tissue sarcoma. Cancer 119:1878–84.
- Roper B, Heinrich C, Kehl V, et al. (2015). Study of preoperative radiotherapy for sarcomas of the extremities with intensity-modulation, image-guidance and small safety-margins (PREMISS). BMC Cancer 15:904.
- Wang D, Zhang Q, Eisenberg BL, et al. (2015). Significant reduction of late toxicities in patients with extremity sarcoma treated with image-guided radiation therapy to a reduced target volume: results of radiation therapy oncology group RTOG-0630 Trial. J Clin Oncol 33:2231–8.
- Molina G, Hull MA, Chen YL, et al. (2016). Preoperative radiation therapy combined with radical surgical resection is associated with a lower rate of local recurrence when treating unifocal, primary retroperitoneal liposarcoma. J Surg Oncol 114:814–20.
- Nussbaum DP, Rushing CN, Lane WO, et al. (2016). Preoperative or postoperative radiotherapy versus surgery alone for retroperitoneal sarcoma: a case-control, propensity score-matched analysis of a nationwide clinical oncology database. Lancet Oncol 17:966–75.
- Smith MJ, Ridgway PF, Catton CN, et al. (2014). Combined management of retroperitoneal sarcoma with dose intensification radiotherapy and resection: long-term results of a prospective trial. Radiother Oncol 110:165–71.
- Sauer R, Schuchardt U, Hohenberger W, et al. (1999). Neoadjuvant radiochemotherapy in soft tissue sarcomas. Optimization of local functional tumor control. Strahlenther Onkol 175:259–66.
- Lehane C, Ho F, Thompson SR, et al. (2016). Neoadjuvant chemoradiation (modified Eilber protocol) versus adjuvant radiotherapy in the treatment of extremity soft tissue sarcoma. J Med Imaging Radiat Oncol 60:539–44.
- Look Hong NJ, Hornicek FJ, Harmon DC, et al. (2013). Neoadjuvant chemoradiotherapy for patients with high-risk extremity and truncal sarcomas: a 10-year single institution retrospective study. Eur J Cancer 49:875–83.
- Eckert F, Gani C, Kluba T, et al. (2013). Effect of concurrent chemotherapy and hyperthermia on outcome of preoperative radiotherapy of high-risk soft tissue sarcomas. Strahlenther Onkol 189:482–5.
- Eckert F, Matuschek C, Mueller AC, et al. (2010). Definitive radiotherapy and single-agent radiosensitizing ifosfamide in patients with localized, irresectable soft tissue sarcoma: a retrospective analysis. Radiat Oncol 5:55.
- Stubbe F, Agaimy A, Ott O, et al. (2016). Effective local control of advanced soft tissue sarcoma with neoadjuvant chemoradiotherapy and surgery: a single institutional experience. Cancer Radiother 20:6–13.
- Hartmann J, Schütte J. (2009). Neoadjuvant chemotherapy of localised soft tissue sarcomas( Neoadjuvante Chemotherapie des lokalisierten Weichteilsarkoms). Onkologe 15:389–97. German
- Bruggmoser G, Bauchowitz S, Canters R, et al. (2011). Quality assurance for clinical studies in regional deep hyperthermia. Strahlenther Onkol 187:605–10.
- Hughes TM, Thomas JM. (2000). Sarcoma metastases due to iatrogenic implantation. Eur J Surg Oncol 26:50–2.
- Pai VD, Puri A, Epari S, Gulia A. (2015). Iatrogenic implantation of soft tissue sarcoma at skin graft donor site: delayed manifestation of an avoidable complication. South Asian J Cancer 4:100–1.
- Hartmann J, Gellermann J, Brandt T, et al. (2017). Optimization of single voxel MR spectroscopy sequence parameters and data analysis methods for thermometry in deep hyperthermia treatments. Technol Cancer Res Treat 16:470–81.
- Dewey WC. (2009). Arrhenius relationships from the molecule and cell to the clinic. Int J Hyperthermia 25:3–20.
- van Rhoon GC, Samaras T, Yarmolenko PS, et al. (2013). CEM43 degrees C thermal dose thresholds: a potential guide for magnetic resonance radiofrequency exposure levels? Eur Radiol 23:2215–27.
- Curtis KK, Ashman JB, Beauchamp CP, et al. (2011). Neoadjuvant chemoradiation compared to neoadjuvant radiation alone and surgery alone for Stage II and III soft tissue sarcoma of the extremities. Radiat Oncol 6:91.
- Kainhofer V, Smolle MA, Szkandera J, et al. (2016). The width of resection margins influences local recurrence in soft tissue sarcoma patients. Eur J Surg Oncol 42:899–906.
- Tang YW, Lai CS. (2012). The significance of close but negative excision margin for treatment of soft-tissue sarcoma. Ann Plast Surg 69:633–6.
- Ahmad R, Jacobson A, Hornicek F, et al. (2016). The width of the surgical margin does not influence outcomes in extremity and truncal soft tissue sarcoma treated with radiotherapy. Oncologist 21:1269–76.
- Dagan R, Indelicato DJ, McGee L, et al. (2012). The significance of a marginal excision after preoperative radiation therapy for soft tissue sarcoma of the extremity. Cancer 118:3199–207.
- Liu CY, Yen CC, Chen WM, et al. (2010). Soft tissue sarcoma of extremities: the prognostic significance of adequate surgical margins in primary operation and reoperation after recurrence. Ann Surg Oncol 17:2102–11.
- Lehnert T, Cardona S, Hinz U, et al. (2009). Primary and locally recurrent retroperitoneal soft-tissue sarcoma: local control and survival. Eur J Surg Oncol 35:986–93.
- Daigeler A, Zmarsly I, Hirsch T, et al. (2014). Long-term outcome after local recurrence of soft tissue sarcoma: a retrospective analysis of factors predictive of survival in 135 patients with locally recurrent soft tissue sarcoma. Br J Cancer 110:1456–64
- Brennan MF. (2007). Local recurrence in soft tissue sarcoma: more about the tumor, less about the surgeon. Ann Surg Oncol 14:1528–9.
