Abstract
Objective: To evaluate the clinical efficacy/safety of CT-guided percutaneous microwave ablation for HCC in challenging locations using high-power microwave platforms.
Materials and methods: A retrospective review was conducted in 26 patients with 36 HCC tumours in challenging locations (hepatic dome, subcapsular, close to the heart/diaphragm/hepatic hilum, exophytic) undergoing CT-guided percutaneous microwave ablation in a single centre since January 2011. Two different microwave platforms were used both operating at 2.45 GHz: AMICA and Acculis MWA System. Patient demographics including age, sex, tumour size and location, as well as technical details were recorded. Technical success, treatment response, patients survival and complication rate were evaluated.
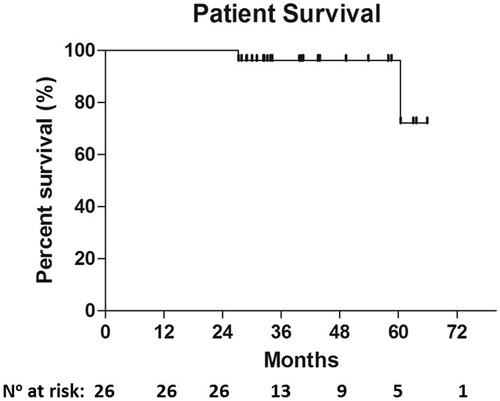
Results: Treated tumours were located in the hepatic dome (n = 14), subcapsularly (n = 16), in proximity to the heart (n = 2) or liver hilum (n = 2), while two were exophytic tumours at segment VI (n = 2). Mean tumour diameter was 3.30 cm (range 1.4–5 cm). In 3/26 patients (diameter >4 cm), an additional session of DEB-TACE was performed due to tumour size. Technical success rate was 100%; complete response rate was recorded in 33/36 tumours (91.6%). According to Kaplan–Meier analysis, survival rate was 92.3% and 72.11% at 24- and 60-month follow-up, respectively. There were no major complications; two cases of minor pneumothorax and two cases of small subcapsular haematoma were resolved only with observation requiring no further treatment.
Conclusion: CT-guided percutaneous microwave ablation for hepatocellular carcinoma tumours in challenging locations and up to 5 cm in diameter can be performed with high efficacy and safety rates.
Introduction
Hepatocellular carcinoma (HCC) represents the sixth most common neoplasm while ranks third among deaths related to cancer [Citation1]. It is not long ago since the European Association for the Study of Liver and the European Organization for Research and Treatment of Cancer (EASL-EORTC) clinical practice guidelines adopted by the Barcelona Clinic Liver Cancer (BCLC) staging and treatment strategy for the management of HCC [Citation2,Citation3]. According to the BCLC strategy, ablation is included in the curative treatments for HCC and can be proposed in very early and early disease stage [Citation3]. During thermal ablation, irreversible thermal damage is achieved with long time (1 h) exposures at 50 °C and instantaneously at 60 °C [Citation4]. In the recent years, there is a clear trend throughout the literature towards the use of personalised or innovative treatments (under reality check) for the expansion of BCLC indications [Citation5].
Different ablation techniques available in the market can be classified according to the type of energy utilised. Microwave ablation (MWA) seems to present several advantages over radiofrequency ablation (RFA) including the ability to achieve temperatures over 100 °C and the ability to produce larger ablation volumes in shorter time less affected by “heat-sink” effect and any kind of impedance-driven performance [Citation6]. In everyday clinical practice of HCC treatment, MWA has been reported to achieve lower rates of local tumour progression [Citation7]. High-power microwave platforms that use a generator frequency of 2.45 GHz, and an internal fluid cooling system for the antenna, result in larger and more spherical ablation zone within shortest ablation times [Citation8].
The anatomical location of a hepatic tumour has been previously reported as a significant contributing factor for local recurrence following percutaneous ablation [Citation9]. Challenging tumour locations in the liver include the hepatic dome, areas close to liver hilum or to the heart and subcapsular locations or exophytic tumours and tumours abutting the diaphragm. In most of the cases, the reason for tumour remnant or local tumour progression in these challenging locations is the creation of a limited ablation volume when a more aggressive approach is required. A conservative ablation strategy resulting in undertreatment is mainly attributed to the attempt to avoid complications from either direct penetrating or more commonly thermal trauma [Citation10]. Nonetheless, careful preprocedural planning, appropriate access site selection and various intraprocedural techniques are key factors of complication-free clinical success.
The purpose of this study was to evaluate the clinical efficacy and safety of CT-guided percutaneous microwave ablation for HCC tumours in challenging locations using high-power microwave platforms with a generator frequency of 2.45 GHz and internal fluid cooling of the antenna.
Materials and methods
All patients were informed about the technique itself as well as possible benefits and complications and signed a relevant written informed consent form prior to the procedure. The authors have no conflict of interest to declare. No industry support was received for this study. The study protocol conforms to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a priori approval by the institution's human research committee.
Patient characteristics
The authors retrospectively reviewed all liver ablation cases performed in a single centre since January 2011 focussing upon microwave ablation of hepatic tumours in challenging locations using high-power microwave platforms. Inclusion criteria were high-power microwave ablation procedures performed for tumours located in the hepatic dome (periphery of segments IVa, VII, VIII) or at subcapsular location, tumours close (<1 cm) to liver hilum or to the heart, or exophytic tumours and tumours abutting the diaphragm. All other ablation techniques (e.g. radiofrequency and normal power MW) and tumours not situated in the abovementioned anatomical locations located were excluded from the study. Two different microwave devices were used, both operating at 2.45 GHz: AMICA (HS Medical, Rome, Italy) and Acculis MWA System (AngioDynamics, Amsterdam, The Netherlands). A 16 G antenna was used with the AMICA system while a 15 G antenna was used with the Acculis MWA System.
Each patient underwent laboratory tests (including liver function and coagulation tests) at least 24 h prior to the percutaneous ablation session. Preoperational imaging included contrast-enhanced CT and/or multiplane MRI.
Percutaneous ablation session
Percutaneous microwave ablation was performed using local anaesthesia and conscious sedation, extended local sterility (along with IV antibiotic prophylaxis) and computed tomography guidance. Following the initial CT scan, access site was selected. The microwave antenna was inserted within the tumour and its route was evaluated with sequential CT scans. A nontranspleural access was performed in all cases. Once the needle was correctly positioned, the ablation session was performed using specific energy amount (watt), duration (minutes) and resultant ablation volume (centimetres), according to the guidelines provided by the manufacturer. The goal was to create an ablation zone that would include the tumour and a circumferential zone of normal liver parenchyma ranging from 0.5 to 1 cm. The desired position of the antenna was the centre of the tumour, with the tip few millimetres distal to the tumour, in order to include the imaginary surgical edges, within the ablation zone. Access site and angulation were chosen in order to achieve an optimal tumour positioning within the target and to create an appropriate tract as to avoid visible blood vessels during the advancement of the antenna. Whenever necessary, additional ablation foci circumferential to the initial focus were performed and parameters were adjusted as to ablate the remaining tumour by accurate reposition of the antenna. The preferred technique used for liver dome tumours, in which the access was limited by pulmonary parenchyma, was the anterior epipericardial fat pad access. The epipericardial fat pad is a structure of variable size located in the anterior mediastinum; at cross-sectional imaging with CT, it is seen as an anlage located outside the pericardium. When present and with a diameter >1 cm, epipericardial fat pad is an attractive access route for performing ablation in the hepatic dome. However, in cases in which epipericardial fat pad access was technically not viable, a CT gantry angulated technique was used. This technique relays on the caudocranial angulation of the CT gantry, as to allow electrode access along the same angle, without traversing the lung parenchyma. Another approach used for liver dome tumours included the use of ultrasound guidance for insertion of the antenna from a caudal level heading towards the dome changing then to CT guidance (for the final reposition) once the antenna was close enough to the target. In the present study, all ablation sessions were performed with a microwave probe with internal water cooling to avoid shaft overheating and a built-in thermocouple for probe temperature monitoring. The generator’s output was up to 140 W at continuous wave of 2450 MHz. An experimental study in ex vivo bovine liver comparing four different microwave ablation devices concluded that high-power systems produce large zones of coagulation in a shorter time; additionally according to this experiment, the microwave system used in the present study achieved the largest ablation volume [Citation8].
Outcome measures
Postprocedural contrast-enhanced CT was performed before discharge in order to assess the ablation zone, defined as the area of low attenuation surrounding the target and an ablative margin, and the potential immediate postprocedural complications. All patients were monitored overnight and were then discharged. Technical success, treatment response, patient survival and complication rates were evaluated. Follow-up protocol was consisted of clinical visits and MRI at 1, 3, 6, 12 and 24 months. MR imaging included conventional T1W and T2W sequences, dynamic contrast-enhanced imaging during arterial, portal venous and 3-min delayed phases as well as functional imaging [diffusion MR imaging and apparent diffusion coefficient mapping]. Treatment response was assessed according to the mRECIST criteria [Citation11,Citation12]. Patient survival was estimated using Kaplan–Meier analysis. Complications were classified as major or minor according to international reporting standards [Citation13]. Pain scores were recorded using a 0 (no pain) to 10 (maximum pain) visual analogue score 2 h after the procedure and at days 1, 2 and 3 postablation.
Results
A total of 36 HCC tumours in 26 patients (male = 18, female = 8, mean age 68.8 years) were treated with CT-guided percutaneous microwave ablation. Treated tumours were mainly located in the hepatic dome (n = 14; 38.8%; all located less than 1 cm from the diaphragm) and subcapsularly (n = 16; 44.4%), while the remaining tumours were located in proximity to the heart (n = 2; 5.5%) and liver hilum (n = 2; 5.5%) (). Finally, two exophytic tumours at segment VI were treated (n = 2; 5.5%). Tumour location and size are analytically reported in . Underlying cirrhosis was present in all patients (26/26); cirrhosis was associated with hepatitis B (16/26), hepatitis C (7/23), alcoholic liver disease (1/26) and nonalcoholic steatohepatitis (2/26). The vast majority (23/26) of the patients included in the study were class A according the Child–Pugh score; only 3/26 patients were class B. Alpha-fetoprotein was increased in 24/26 patients.
Figure 1. (A) Computed tomography axial scan post-IV contrast medium injection in arterial phase – HCC located in left hepatic lobe close to the liver hilum. (B) Computed tomography axial scan without IV contrast medium injection – tip of the microwave antenna is located at the distal end of the tumour. (C) Computed tomography axial scan post-IV contrast medium injection in portal venous phase immediately postablation – the ablation zone covers the whole tumour plus a safety margin. (D) MRI post-IV contrast medium injection in arterial phase one year postablation illustrates no viable tumour.
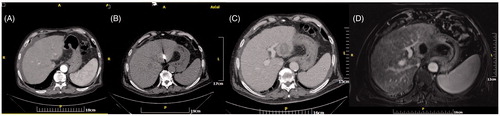
Figure 2. (A) Computed tomography axial scan without IV contrast medium injection – microwave antenna is placed in the centre of an HCC tumour located subcapsularly at the hepatic dome. (B) Computed tomography axial scan post-IV contrast medium injection in arterial phase immediately postablation – the ablation zone covers the whole tumour plus a safety margin. (C) Computed tomography axial scan post-IV contrast medium injection in portal venous phase immediately postablation – the ablation zone covers the whole tumour plus a safety margin. (D) Computed tomography coronal reconstruction post-IV contrast medium injection in portal venous phase immediately postablation – the round ablation zone covers the whole tumour plus a safety margin.
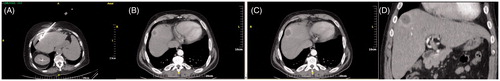
Figure 3. (A,B) Computed tomography axial scan post-IV contrast medium injection in arterial (A) and portal venous (B) phase immediately postablation – the ablation zone extends subcapsularly with gas at the centre. (C,D) MRI post-IV contrast medium injection in arterial phase, one (C) and two (D) years postablation illustrates capsular retraction, no contrast medium uptake and no viable tumour.
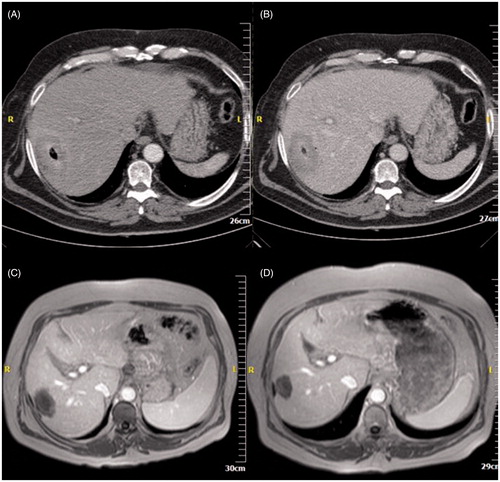
Table 1. Tumours’ location with corresponding diameter.
Mean tumour diameter was 3.30 cm (range 1.4–5 cm). Technical success rate was 100%; complete response rate was recorded in 33/36 tumours (91.6%). In all three tumours (3/36; 8.3%) with remnant (partial response), the diameter ranged between 4.5 and 5 cm. In these 3/26 patients due to the tumour size (diameter >4 cm), a 2nd insertion/ablation circle and an additional session of DEB-TACE were performed in the same session. Kaplan–Meier estimated survival rate was 96.15% at 48-month and 72.11% at 60-month follow-up ().
The artificial ascites or pneumothorax technique was not performed in this study. An angulated approach was performed in 11/14 tumours located at hepatic dome, while an anterior epipericardial fat pad approach was performed in the remaining 3/14 tumours. All subcapsular tumours were treated using an indirect approach through normal liver parenchyma.
There were no major complications; two cases of minor pneumothorax and two cases of small subcapsular haematoma were noted, which required only observation and auto-resolved without any further treatment. No complication occurred following an anterior approach. There were no reports of right hemidiaphragmatic hernias, paralyses, increase of diaphragm thickness or right pleural effusions. Mean pain score according to VAS was 1:1.97 ± 1.838 2 h following the procedure, 0.62 ± 0.817 at day 1 postablation, 0.03 ± 0.172 at day 2 postablation and 0 at day 3 postablation
Discussion
Microwave ablation is an established treatment option for inoperable HCC as it produces large ablation volume in short time and is less affected by “heat-sink” effect compared to other ablative technologies [Citation6]. According to the results of this study, microwave ablation can be safely performed in challenging hepatic locations including the hepatic dome, subcapsular location or locations in proximity to the heart and diaphragm. Additionally, microwave ablation in these locations was reported to achieve very satisfactory efficacy rates concerning total tumour necrosis (33/36 tumours; 91.6%). However, it must be noted that each hepatic location warrants a different approach and individualised strategy in order to achieve total tumour necrosis without significant complications.
Artificial ascites and hydrodissection using 5% dextrose are quite commonly used during ablation of exophytic or other tumours adjacent to intestine [Citation14–18]. In the present study, artificial ascites was not provoked as none of the tumours was at a distance of less than 2 cm from adjacent organs.
Pain during the procedure and postablation of subcapsular hepatic tumours is very common. A frequent approach to reduce pain in the early and sub-early postablation period includes the formation of artificial ascites by injection of 5% dextrose [Citation14,Citation15]. However, in a recent study by Hakimé et al., it was clarified that artificial ascites prevents only immediate postprocedural pain, which re-appears intensively after 4 days [Citation16]. All subcapsular tumours in these studies were approached through an indirect route via normal hepatic parenchyma; tumour seeding was not noted in our study. Although it has been reported in the literature that the direct puncture of subcapsular hepatic tumours is a safe and effective technique, in a study by Francica et al, a single case of tumour seeding has been reported; however, the authors highlighted the fact that this particular tumour had previously undergone biopsy [Citation19].
The preferred technique used for liver dome tumours, in which the access was limited by pulmonary parenchyma, was the anterior epipericardial fat pad access. The epipericardial fat pad is a structure of variable size located in the anterior mediastinum; at cross-sectional imaging with CT, it is seen as an anlage located outside the pericardium. When present and with a diameter >1 cm, epipericardial fat pad is an attractive access route for performing ablation in the hepatic dome. However, in cases in which epipericardial fat pad access was technically not viable, a CT gantry angulated technique was used. This technique relays on the caudocranial angulation of the CT gantry, as to allow electrode access along the same angle, without traversing the lung parenchyma. Another approach used for liver dome tumours included the use of ultrasound guidance for insertion of the antenna from a caudal level heading towards the dome changing then to CT guidance (for the final reposition) once the antenna was close enough to the target. Tumours located high in the hepatic dome can be treated using an angulated approach, thus inserting the antenna from a lower level and approaching the tumour from a transhepatic route. With this approach, longer microwave antennas (19–25 cm length) are necessary in the vast majority of the cases. Alternatively, artificial pneumothorax can be induced for a transthoracic approach, the artificial pleural effusion or ascites technique can be used for tumours of the hepatic dome abutting the diaphragm, or even a direct transpulmonary approach can be utilised [Citation20–23]. Additionally, tumours at the hepatic dome have been treated under combined imaging guidance using fluoroscopy, ultrasound and computed tomography [Citation24]. All cases of hepatic dome ablation in our study were approached either with angulation technique (11/14 tumours) or were approached anteriorly through the epicardial fat pad (3/14). During this latter approach, advantage is taken of the epicardial fat pad which, in certain patients with favourable anatomy, provides a potential access window in order to avoid transthoracic or transpleural approaches [Citation25]. It was the authors’ preference to proceed using the anterior epipericardial or angulated gantry approaches, due to local expertise following numerous CT-guided biopsies using the particular techniques. Notably, studies comparing these approaches are not available.
According to experimental porcine models, microwave ablation of pulmonary parenchyma close to the heart can mitigate cardiac complications including cardiac tissue injury and arrhythmias; the rate of these complications decreased with increasing antenna distance in both parallel and perpendicular orientation [Citation26]. One might expect that the same principle could be valid for microwave ablation of hepatic tumours in proximity to the heart. In this study, both hepatic tumours located in proximity to the heart were 5 cm in diameter; during ablation of each tumour, the antenna was located at least 5–10 mm away from the cardiac tissue. Although no cardiac complications were noted, it should be highlighted that the herein presented study did not include sufficient patients to exclude the possibility of such a serious complication. In both cases, residual disease was expected and was treated with a DEB-TACE session performed at the same day; repeat ablation was deemed technically challenging and dangerous due to the proximity to the heart.
Mean pain scores were minimal both immediately after the procedure and during the 48-h follow-up, while the vast majority of the patients did not experience any pain 3 days after the ablation. Although pain comparison between various ablation techniques (hydrodissection) and other covariates that could influence pain outcomes (e.g. tumour location, tumour size, number of tumours and number of punctures) was beyond the scope of this study, none of the patients experienced moderate to severe pain (e.g. >4 according to VAS).
Combining TACE and ablation has been reported to improve overall survival rate and provide better prognosis in patients with large-sized HCC [Citation27]. Combinations of RFA or MWA to TACE are governed by similar efficacy and outcomes [Citation28–31]. According to systematic reviews and meta-analysis, percutaneous radiofrequency and microwave ablation demonstrated similar efficacy for the treatment of HCC, but with an apparent superiority of MWA in larger neoplasms [Citation32]. The superiority of microwave ablation in large diameter HCC is reported in most literature studies [Citation33–35]. Moreover, various studies comparing the specific microwave system used in the present study to low-frequency microwave generators reported significantly higher ablation volumes, longer time to progression and lower progression rates for high-power/high-frequency systems [Citation36].
Limitations of our study include the retrospective nature and the small patient sample especially in certain challenging locations such as close to the heart or to the liver hilum. Moreover, the number of cases sizing between 4.5 and 5 cm was insufficiently small to draw safe conclusions on the efficacy of the method. Additionally, there was no comparison between RFA and MWA or between low- and high-power microwave platforms in terms of efficacy and safety in these challenging locations.
Conclusion
CT-guided percutaneous microwave ablation for the treatment of challenging HCC tumours up to 5 cm including subcapsular or exophytic targets and those located in the hepatic dome or close to heart/diaphragm/hepatic hilum resulted in satisfactory efficacy and safety rates. Choosing the proper approach is a prerequisite for technical and clinical success.
Disclosure statement
The authors report no declarations of interest.
References
- Lencioni R, Crocetti L. (2012). Local-regional treatment of hepatocellular carcinoma. Radiology 262:43–58.
- European Association For The Study Of The Liver; European Organisation For Research And Treatment Of Cancer. (2012). EASL-EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol 56:908–43.
- de Lope CR, Tremosini S, Forner A, et al. (2012). Management of HCC. J Hepatol 56(Suppl 1):S75–S87.
- Rhim H, Goldberg SN, Dodd GD, et al. (2001). Essential techniques for successful radio-frequency thermal ablation of malignant hepatic tumors. Radiographics 21:S17–S35.
- Mazzaferro V, Lencioni R, Majno P. (2014). Early hepatocellular carcinoma on the procrustean bed of ablation resection and transplantation. Semin Liv Dis 34:415–26.
- Amabile C, Ahmed M, Solbiati L, et al. (2017). Microwave ablation of primary and secondary liver tumours: ex vivo, in vivo, and clinical characterisation. Int J Hyperthermia 33:34–42.
- Potretzke TA, Ziemlewicz TJ, Hinshaw JL, et al. (2016). Microwave versus radiofrequency ablation treatment for hepatocellular carcinoma: a comparison of efficacy at a single center. J Vasc Interv Radiol 27:631–8.
- Hoffmann R, Rempp H, Erhard L, et al. (2013). Comparison of four microwave ablation devices: an experimental study in ex vivo bovine liver. Radiology 268:89–97.
- Mulier S, Ni Y, Jamart J, et al. (2005). Local recurrence after hepatic radiofrequency coagulation: multivariate meta-analysis and review of contributing factors. Ann Surg 242:158–71.
- Howenstein MJ, Sato KT. (2010). Complications of radiofrequency ablation of hepatic, pulmonary, and renal neoplasms. Semin Intervent Radiol 27:285–95.
- Lencioni R, Llovet JM. (2010). Modified RECIST (mRECIST) assessment for hepatocellular carcinoma. Semin Liver Dis 30:52–60.
- Lencioni R. (2013). New data supporting modified RECIST (mRECIST) for hepatocellular carcinoma. Clin Cancer Res 19:1312–14.
- Leoni CJ, Potter JE, Rosen MP, et al. (2001). Classifying complications of interventional procedures: a survey of practicing radiologists. J Vasc Interv Radiol 12:55–9.
- Nishimura M, Nouso K, Kariyama K, et al. (2012). Safety and efficacy of radiofrequency ablation with artificial ascites for hepatocellular carcinoma. Acta Med Okayama 66:279–84.
- Song I, Rhim H, Lim HK, et al. (2009). Percutaneous radiofrequency ablation of hepatocellular carcinoma abutting the diaphragm and gastrointestinal tracts with the use of artificial ascites: safety and technical efficacy in 143 patients. Eur Radiol 19:2630–40.
- Hakimé A, Tselikas L, Otmezguine Y, et al. (2015). Artificial ascites for pain relief during microwave ablation of subcapsular liver tumors. Cardiovasc Intervent Radiol 38:1557–62.
- Kondo Y, Yoshida H, Shiina S, et al. (2006). Artificial ascites technique for percutaneous radiofrequency ablation of liver cancer adjacent to the gastrointestinal tract. Br J Surg 93:1277–82.
- Kitchin D, Lubner M, Ziemlewicz T, et al. (2014). Microwave ablation of malignant hepatic tumours: intraperitoneal fluid instillation prevents collateral damage and allows more aggressive case selection. Int J Hyperthermia 30:299–305.
- Francica G, Meloni MF, de Sio I, et al. (2016). Radiofrequency and microwave ablation of subcapsular hepatocellular carcinoma accessed by direct puncture: safety and efficacy. Eur J Radiol 85:739–43.
- de Baère T, Dromain C, Lapeyre M, et al. (2005). Artificially induced pneumothorax for percutaneous transthoracic radiofrequency ablation of tumors in the hepatic dome: initial experience. Radiology 236:666–70.
- Zhang D, Liang P, Yu X, et al. (2013). The value of artificial pleural effusion for percutaneous microwave ablation of liver tumour in the hepatic dome: a retrospective case-control study. Int J Hyperthermia 29:663–70.
- Iwai S, Sakaguchi H, Fujii H, et al. (2012). Benefits of artificially induced pleural effusion and/or ascites for percutaneous radiofrequency ablation of hepatocellular carcinoma located on the liver surface and in the hepatic dome. Hepatogastroenterology 59:546–50.
- Rhim H, Lim HK. (2009). Radiofrequency ablation for hepatocellular carcinoma abutting the diaphragm: the value of artificial ascites. Abdom Imaging34:371–80.
- Basile A, Calcara G, Montineri A, et al. (2008). Application of a new combined guiding technique in RF ablation of subphrenic liver tumors. Eur J Radiol 66:321–4.
- Brennan DD, Ganguli S, Brecher CW, Goldberg SN. (2008). Thinking outside the abdominal box: safe use of the epi-pericardial fat pad window for percutaneous radiofrequency ablation of hepatic dome tumors. J Vasc Interv Radiol 19:133–6.
- Carberry GA, Nocerino E, Mason PJ, et al. (2017). Pulmonary microwave ablation near the heart: antenna positioning can mitigate cardiac complications in a porcine model. Radiology 282:892–902.
- Ni JY, Liu SS, Xu LF, et al. (2013). Transarterial chemoembolization combined with percutaneous radiofrequency ablation versus TACE and PRFA monotherapy in the treatment for hepatocellular carcinoma: a meta-analysis. J Cancer Res Clin Oncol 139:653–9.
- Ginsburg M, Zivin SP, Wroblewski K, et al. (2015). Comparison of combination therapies in the management of hepatocellular carcinoma: transarterial chemoembolization with radiofrequency ablation versus microwave ablation. J Vasc Interv Radiol 26:330–41.
- Iezzi R, Pompili M, Posa A, et al. (2014). Combined locoregional treatment of patients with hepatocellular carcinoma: State of the art. World J Gastroenterol 22:1935–42.
- Iezzi R, Pompili M, Gasbarrini A, Bonomo L. (2014). Sequential or combined treatment? That is the question. Radiology 272:612–13.
- Liu C, Liang P, Liu F, et al. (2011). MWA combined with TACE as a combined therapy for unresectable large-sized hepotocellular carcinoma. Int J Hyperthermia 27:654–62.
- Facciorusso A, Di Maso M, Muscatiello N. (2016). Microwave ablation versus radiofrequency ablation for the treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Int J Hyperthermia 32:339–44.
- Xu Y, Shen Q, Wang N, et al. (2017). Percutaneous microwave ablation of 5-6 cm unresectable hepatocellular carcinoma: local efficacy and long-term outcomes. Int J Hyperthermia 33:247–54.
- Meloni MF, Chiang J, Laeseke PF, et al. (2017). Microwave ablation in primary and secondary liver tumours: technical and clinical approaches. Int J Hyperthermia 33:15–24.
- Li X, Zhang L, Fan W, et al. (2011). Comparison of microwave ablation and multipolar radiofrequency ablation, both using a pair of internally cooled interstitial applicators: results in ex vivo porcine livers. Int J Hyperthermia 27:240–8.
- Vogl TJ, Hagar A, Nour-Eldin NA, et al. (2016). High-frequency versus low-frequency microwave ablation in malignant liver tumours: evaluation of local tumour control and survival. Int J Hyperthermia 32:868–75.