Abstract
Purpose: To compare local recurrence (LR) rate in patients with colorectal cancer liver metastasis (CRCLM) after surgical wedge resection (WR) or radiofrequency ablation (RFA) and to investigate predictive factors of LR.
Materials and methods: This single-centre, retrospective, institutional review board-approved study including 43 consecutive patients with 121 metastases treated by WR and 60 patients with 110 metastases treated by RFA between 2007 and 2014 with 23 and 18.5 months of follow-up, respectively. Demographics and tumour characteristics were compared using the unpaired t-test and chi-square test. Predictive factors for LR (lesion size, depth, relation to hepatic vessels, intervention, margin status) were investigated in uni- and multivariate analyses.
Results: Patient and CRCLM characteristics were similar in both groups. Mean lesion size and depth in the WR and RFA groups were 18 mm and 15 mm (p = 0.03), and 19 mm and 26 mm (p < 0.001), respectively. LR showed a trend towards difference in favour of RFA (19% and 10% in the WR and RFA groups, respectively, p = 0.06). Positive margins and lesion depth were predictive factors of LR in the WR group (p = 0.03 and p = 0.02, respectively, on uni- and multivariable analyses). Lesion depth and proximity to a vein increased the risk of positive margins on pathology after WR (p = 0.04 and p < 0.001, respectively). Our analysis did not identify any predictive factors of LR following RFA.
Conclusion: Our study showed a trend towards a lower LR rate with RFA compared to WR. Lesions located deep in the liver and/or close to large vessels are at high risk of LR following WR, while curative treatment can be obtained with RFA.
Introduction
Surgery with anatomical resection has long been considered the only curative treatment option for patients with colorectal cancer liver metastases (CRCLM) with a reported 5-year overall survival of 50–56% [Citation1,Citation2]. However, only about 30% of the patients with CRCLM are eligible for anatomic resection due to disease extension or an insufficient future liver remnant volume [Citation3]. Thus, other potentially curative therapeutic options have been developed, such as non-anatomical resections (wedge resections, WRs) or percutaneous ablation procedures (radiofrequency ablation, RFA). The advantage of WR and/or RFA for patients with CRCLM is the possibility of sparing the non-tumoural liver parenchyma allowing repeated surgical and/or ablative treatments with results similar to anatomical resection [Citation3]. Another advantage for ablation or WR is to obtain local tumour control while giving time for the expression of tumour biology (“test-of-time approach”) [Citation4]. Because the rate of recurrence of liver metastases after (anatomical and non-anatomical) surgery or RFA is between 9 and 60% [Citation5,Citation6], sparing the liver parenchyma is essential in the treatment of patients with CRCLM. The results of overall survival or the local recurrence (LR) rate following surgery and RFA are controversial [Citation7]. Nevertheless, RFA and surgical resection (both anatomical and non-anatomical) have been compared in retrospective studies showing a trend towards better progression-free survival and overall survival for surgery [Citation8,Citation9]. Moreover, the goal of the studies comparing surgery and RFA has been to evaluate the performance of each technique but not to define their respective indications on a lesion-by-lesion basis and to help select the best procedure with the best chance of cure for each patient. Furthermore, to the best of our knowledge, there is only one study in the literature comparing RFA to WR (excluding anatomical resection) [Citation10].
The purpose of the present study was to compare the LR rate in patients with CRCLM who underwent WR or RFA to identify the predictive factors of LR in each group as well as to define the prognostic factors for selection of the best therapeutic technique based on tumour characteristics.
Materials and methods
Study design and patient population
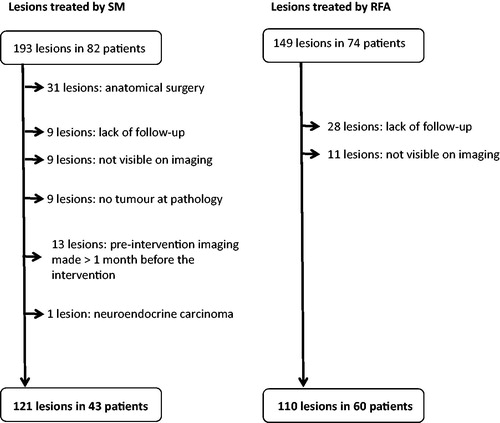
This is a retrospective, single-institution, institutional review board-approved study. Patient informed consent was waived. A database of prospective patients with CRCLM who underwent surgery or RFA at our institution from 2007 to 2014 was reviewed. One hundred and thirty-seven consecutive patients with 342 CRCLM were identified. An intention-to-treat analysis was performed. Patients were included if they had undergone WR/RFA and had available 1-year radiological follow-up since the intervention. Fifty-three patients with 111 CRCLM were excluded for the following reasons (): anatomical surgical procedures (hemi-hepatectomy, lobectomy, segmentectomy) (n = 31); lack of follow-up (n = 37); lesions that were not visible on pre-procedure imaging (e.g. lesion identified during the treatment) (n = 20); and lesions with pre-procedural imaging more than 1 month before the intervention (n = 13). Finally, if the pathological analysis revealed no tumour, or when histology revealed a different tumour type than CRCLM, these lesions were also excluded (n = 10). Patients with a combined treatment with anatomical resection (for instance right hepatectomy for five lesions and WR/RFA for one left liver metastasis) were included. None of the included patients had extrahepatic metastasis.
The final study population included 84 patients with 231 CRCLM. Forty patients with 121 CRCLM were treated by WR [mean age at CRC diagnosis: 64 years old (31–78), 11 women (26%)]. Sixty patients with 110 CRCLM were treated by RFA [mean age at CRC diagnosis: 68 years old (31–78), 15 women (25%)]. Twenty-four patients were treated by WR only (28%), 41 patients by RFA only (49%) and 19 patients (23%) were treated by combined WR and RFA for different lesions (each lesion was treated by either WR or by RFA).
Patient data
Patient demographics and CRC-related information (patient’s age at diagnosis, CRC location, synchronous/metachronous liver metastases, number of metastases, treatment date and type) were reported. Pre- and post-intervention chemotherapy was recorded. Follow-up was defined from the procedure to the final medical/radiological evaluation. Patient death was recorded.
Pathological data
An expert liver pathologist (CS, with 15 years of experience) reviewed the primary tumour and the WR samples on a lesion-by-lesion basis. Analysis of the primary tumour included pathological tumour-node-metastasis staging, grade and mucinous differentiation, the presence of a KRAS mutation and margin resection status. Analysis of the CRCLM in the WR group included the size of the metastases as well as measurement and assessment of margin resection status (positive or negative).
Radiofrequency ablation and WR
All patients were considered for RFA or WR on a case-by-case basis during a discussion at our multidisciplinary liver tumour board. Because there is no consensus in the literature on criteria to choose between RFA and WR, our policy is to contraindicate RFA in patients with lesions close to major biliary structures (left or right hepatic canal) and over 3 cm in diameter and more than five lesions to be ablated [Citation11]. Surgery is contraindicated in patients with progressive lesions after three courses of chemotherapy. The decision is then based on a discussion of each individual case among the surgeons, interventional radiologists and oncologists with specific considerations for the size and number of metastases, the vicinity of the hepatic vessels and major bile duct, primary colic tumour status, the patient’s general condition and specific contraindications to each technique. Because of the retrospective design of the study, it was not possible to obtain a detailed report on the reasons for the treatment choice in each patient. Three experienced interventional radiologists (AD, PB and RD with 20, 15 and 8 years of experience in interventional oncology, respectively) performed the RFA procedure using a standardised approach. RFA was performed under general anaesthesia, either percutaneously (n = 98 lesions) with ultrasound guidance (whenever the lesion was visible on ultrasound) or by multidetector computed tomography (MDCT) guidance or during surgery with intraoperative ultrasound (n = 12 lesions). A 200-W generator in the impedance control mode and a clustered internally cooled electrode (Covidien E-Series, Covidien, Boulder, CO) were used. A 12-min application using an automatic mode of ablation at 200-W power was performed. Contrast-enhanced MDCT was performed at the end of the procedure to evaluate whether satisfactory lesion coverage had been obtained (except for CRCLM treated by intraoperative ultrasound guidance). Two experienced hepato-pancreato-biliary surgeons (ND and NH with 20 and 15 years of experience, respectively) performed the surgical procedures. Either a laparotomy or a laparoscopy was performed depending on the number and location of metastases. The surgical technique included intraoperative ultrasound, bipolar electrocautery and an ultrasonic dissector.
Complete/incomplete treatment was defined according to margin resection status in the WR group and the first imaging follow-up performed 4 weeks after ablation in the RFA group based on current recommendations [Citation12]. The presence of recurrence (date and type of recurrence) was recorded based on the results of the imaging follow-up. LR rate was evaluated by a lesion-by-lesion analysis.
Image analysis
Image analysis was performed by two experienced radiologists (AD and NVV, with 20 and 5 years of experience in liver imaging, respectively) who reached a consensus. MDCT or magnetic resonance imaging (MRI) results performed 4 weeks before and after treatment, then at intervals according to institutional guidelines (MDCT or MRI at 3-month intervals for the first year, then every 6 months for the next 2 years without recurrence and then every year) were reviewed. All imaging follow-up exams were analysed. Abdominal MDCT included unenhanced and contrast-enhanced phases (arterial and venous phases – 35 and 70 s following contrast medium injection, respectively). A standard liver MRI protocol was used [T2-weighted half-Fourier single-shot turbo spin-echo imaging, cross-sectional fast multiplanar gradient-echo pulse, DW imaging (b factors of 50, 400, 800 s/mm2), fat-suppressed T2-weighted imaging, gadoxetic acid-enhanced dynamic T1-weighted images using a fat-suppressed three-dimensional spoiled gradient-echo sequence, volumetric interpolated breath-hold examination (VIBE; Siemens, Erlangen, Germany) after a bolus injection of gadoxetic acid (Primovist; Bayer Schering Pharma, Berlin, Germany) obtained before contrast injection and in the arterial, venous, delayed and hepatobiliary phases (30, 70 s, 3 and 20 min after contrast material injection, respectively)]. The type of imaging performed was recorded (MDCT or MRI).
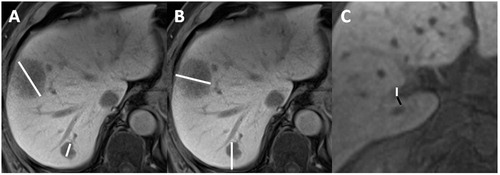
The size of the metastases (defined as the largest diameter of the lesion), depth (defined as the distance from the tumour centre to the nearest hepatic capsule) and distance from the veins (all portal or hepatic veins >3 mm, veins located <10 mm of the metastasis; when the lesion was in direct contact with the vein, the distance was reported as 0 mm) were recorded on the pre-procedure imaging ().
Figure 2. Example of measurements made on the pre-intervention exam. (A,B) 70-year-old male with CRCLM in segments I, VI and VII. (C) 68-year-old man with CRCLM in segments I, IV, V, VI and VIII. (A) Contrast-enhanced (hepatobiliary phase, 20 min after primovist injection) T1-weighted MRI axial acquisition showing two CRCLM with measurement of the lesion size (white lines) defined as the largest lesion diameter. (B) Contrast-enhanced (hepatobiliary phase) T1-weighted MRI axial acquisition showing two CRCLM with measurement of lesion depth (white lines) defined as the distance from the deepest part of the lesion to the nearest hepatic capsule. (C) Contrast-enhanced (hepatobiliary phase) T1-weighted MRI axial acquisition showing a segment I CRCLM with vessel proximity analysis: distance between the lesion and the vessel (black line), diameter of the vein (white line) was measured and type of vessel (right portal vein) was noticed. CRCLM: colorectal cancer liver metastasis; MRI: magnetic resonance imaging.
Complete/incomplete treatment in patients treated by RFA was defined as complete/incomplete lesion ablation on follow-up imaging (4 weeks after ablation): incomplete treatment was defined as the presence of viable remnant tumour in the treated area [Citation12].
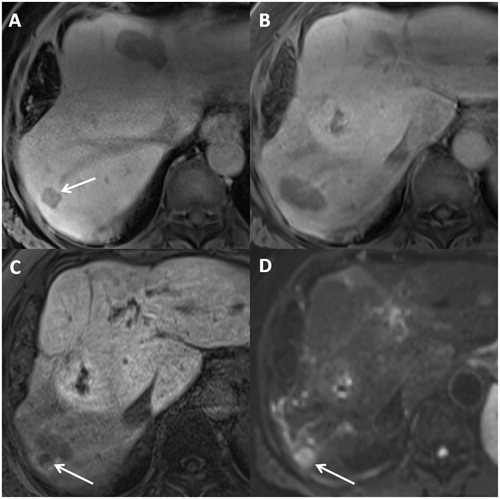
All available radiological exams were reviewed to identify LR, which was considered on a lesion-by-lesion basis and was defined as the appearance of a new lesion with tumoural characteristics (MDCT: a nodule with peripheral enhancement on the portal venous phase; MRI: high signal on B800 DWI and diffusion restriction on restriction MAP, arterial and portal enhancement, moderate to high signal on T2-weighted images) at the location of the initial lesion or in direct contact with the RFA scar or WR margin [Citation12] (). Hepatic recurrence was defined as newly identified lesions in the hepatic parenchyma (except in direct contact to the resected/ablated area). Based on the imaging follow-up and patient data, extrahepatic recurrence was defined as newly identified extrahepatic metastases.
Figure 3. Example of LR after RFA: 63-year-old male with CRCLM diagnosed 2 years after primary cancer diagnostic. Metastasis in segment VI was treated by RFA with LR at 13 months after treatment with apparition of multiple new metastasis. (A) Contrast-enhanced (hepatobiliary phase, 20 min after primovist injection) T1-weighted axial MR image, acquired before RFA, evidencing a hepatic metastasis in segment VII (arrow). (B) Contrast-enhanced (hepatobiliary phase) T1-weighted axial MR image, acquired 1 month after RFA, showing the physiologic appearance of resection zone after RFA. (C,D) Contrast-enhanced (hepatobiliary phase) T1-weighted axial MR image and DWI image, acquired 13 months after RFA, evidencing a LR (arrow) with apparition of a new lesion beside RFA scar. WR: wedge resection; RFA: radiofrequency ablation; CRCLM: colorectal cancer liver metastasis; MR: magnetic resonance; DWI: diffusion weighted imaging.
Statistical analysis
Statistical analysis was performed with Anaconda 2.7 (Python programming language) and R3.1.3 with the python module Rpy2 (Python Software Foundation, Wilmington, DE). Categorical data were summarised as the percentage of the total group. Differences in quantitative data distributions between the two groups were assessed using an unpaired Student’s t-test. Differences in frequencies for categorical data were assessed using the chi-square test. The odds ratio was reported as the relative risk for LR (changing from non-LR to LR state).
Univariate analysis and multivariate logistic regression were performed using LR as a dependent variable. Predictive factors were clinically selected differentially for WR and RFA groups. Predictive factors were defined as lesion size, lesion depth in the liver, distance from vascular structures [distance was defined as close to the metastasis (0 or 1 mm) and far (>1 mm between the lesion and the vessel)], and resection margins in case of WR. Additional logistic regression was performed in the WR group using positive margins as a dependent variable and the same predictive factors as above. A p value <0.05 was considered to be statistically significant.
Results
Patient data
Demographics data were similar between the two groups (). The number of total CRCLM per patient was not statistically different in the WR and RFA groups [3.77 (1–10) and 2.83 (1–10), respectively, p = 0.06]. The number of CRCLM treated by RFA by patient was lower than by WR (1.5 and 2.5 mean lesions by patients treated by RFA and WR, respectively, p < 0.001). Ninety percent of the patients treated by RFA had one or two lesions. Survival was not different between the two groups (93% of survival in both groups, p = 0.73; after 33.5 and 34 months of mean follow-up in WR and RFA group, respectively, p = 0.89). The number of patients followed by MDCT (and not by MRI) was not different in the WR and RFA groups (11.6 and 10%, respectively, p = 0.79).
Table 1. Baseline characteristics of the study patients.
Pathological data
No pathological differences were observed in the primary tumour between the two groups ().
Radiofrequency ablation and WR
Because the definition of complete treatment was different for the two study groups, the rate of complete treatment could not be compared (). Complete treatment was obtained in 62% of resections (75 lesions) in the WR group (negative resection margins on pathology). Complete tumour treatment was obtained in 91% of the procedures (100 lesions) in the RFA group, as assessed on the first follow-up imaging examination. Patients treated by WR received post-procedure chemotherapy significantly more often than those treated by RFA (58.7% vs. 37.3%, respectively, p < 0.001) while a similar percentage of patients received pre-procedure chemotherapy (79.3% vs. 82.7%, respectively, p = 0.82). The mean follow-up period after the procedure was longer in the WR group [23 months (3–71), standard deviation (SD): 0.14] than in the RFA group [18 months (3–61), SD: 0.12] (p = 0.03).
Table 2. Metastasis characteristics.
Image analysis
The mean size of metastases was significantly larger in the WR group [18 mm (3–90), SD: 0.11] than in the RFA group [15 mm (3–31), SD: 0.06] (p = 0.03) (). Metastases were significantly deeper in the liver parenchyma in the RFA group [mean depth 26 mm (3–51), SD: 0.08] than in the WR group [mean depth: 20 mm (6–59), SD: 0.11] (p < 0.001). The distance between the lesion to the nearest vascular structures was similar in both groups (p = 0.25). The results show a trend towards a lower LR rate with RFA, 10% (11 lesions), than with WR, 19% (23 lesions) (p = 0.06) with a time to LR of 8 and 9.5 months, respectively (p = 0.56). The same trend was found for hepatic recurrence: 78.5% (95 lesions) in the WR group and 66% (73 lesions) in the RFA group (p = 0.05) and a time to HR of 7.5 months (1–39) and 8 months (1–35), respectively (p = 0.81). Rate of extrahepatic recurrence was not different between the two groups (p = 0.15).
Predictive factors for LR
Both positive margins (p = 0.02) and the depth of metastases (p = 0.03) were predictive factors of LR in the WR group in logistic regression analysis (). For depth of metastases, the relative risk of LR increased by a factor of 1.12 by depth millimetre (odds ratio). No predictive factors were identified for LR in the RFA group. Complementary multivariate analyses in the WR group showed that lesion depth (p = 0.04) and proximity to a vascular structure (p < 0.001) increased the risk of positive margins.
Table 3. Predictive factors for LR and positive margins.
Discussion
This study shows that there is a trend towards lower LR and HR rates with RFA than with WR (LR: 10% vs. 19%, respectively, p = 0.06; and HR: 66% vs. 78%, respectively, p = 0.05). Furthermore, we showed that positive margins and metastases located deep in the liver parenchyma and close to veins were predictive of a greater risk of LR after WR, while no predictive factors of LR were found for RFA. As a more personalised approach is taken in medicine, it is important to compare these two techniques and to identify tumour characteristics that are risk factors for LR to help multidisciplinary tumour boards to propose the optimal therapeutic option. The findings in the present study are a first step in this direction by identifying predictive factors of LR for WR.
In the present study, RFA resulted in a LR rate of 10% after a mean follow-up of 18 months, which is in the low range compared to previous studies (9–40% with follow-up between 17 and 50 months) [Citation6,Citation13]. One previous study compared the LR rate between RFA and WR in solitary CRCLM and reported a rate of 55% following RFA at 37 months of follow-up [Citation10]. The authors suggested that this high rate could be due to the use of unenhanced MDCT during the procedure, resulting in less reliable evaluation of ablation margins, and that RFA should be limited to patients who are poor surgical candidates. However, in that study, the median size of RFA treated lesions was 2.4 centimetres. Our series was more selective for size and always used contrast-enhanced MDCT to validate the ablation zone at the end of the procedure.
As shown in , there are only a few studies reporting resection margin status and LR rate following WR. White et al. [Citation10] described a LR rate of 12% after non-anatomical resection, compared to 19% in our study. In our study, 38% of removed lesions had positive margins which is similar to that in the most experienced hepatobiliary centres working on extending surgical indications [Citation14]. Positive margins remain the major risk of LR and overall survival after surgery [Citation14].
Table 4. Summary of large study cohorts with WR.
The present study has a negative selection bias: patients treated by WR had significantly more post-intervention chemotherapy (59% in the WR and 37% in the RFA groups, p < 0.001). This difference might be due to the relatively high rate of positive margin in the WR group (in contrast to the low rate of incomplete treatment after RFA). Recent data from Nordlinger et al. [Citation15] suggest that the high rate of perioperative chemotherapy in our surgical group could have favoured better progression-free survival in this group compared to the RFA group. Evidence supporting post-RFA chemotherapy was also confirmed in a recent study [Citation16].
In our study, the mean size of the lesion was smaller in the RFA group than in the surgical group. Although they were statistically different (15 mm vs. 18 mm, respectively, p = 0.03), this may not be clinically relevant. Both groups had small lesions which were less than the limit of 30 mm for RFA [Citation11] and are considered to be small lesions for surgery [Citation17]. In our study, RFA lesions were pre-selected based on their size (<30 mm) and the distance from the major bile duct. This follows the recent expert consensus on quality criteria for RFA ablation of CRCLM [Citation11] and explains why tumour size did not appear to be a negative prognostic factor for LR in our study.
Although it has been used increasingly in the past decade, the selection criteria for WR have not been as extensively evaluated in the literature as those for RFA [Citation18]. Multiple metastases and tumour size >50 mm have been suggested to be poor prognostic factors for survival (for both anatomical and non-anatomical resections) [Citation19]. In the present study, we included other factors such as the depth of the lesion and proximity to vascular structures because WR of deep lesions close to vascular structures is technically more difficult despite the use of standard of care surgical method such as intraoperative ultrasound and ultrasonic scissors. In our study, the relative risk of LR after WR increased by 1.12-fold by millimetre of depth, emphasising the importance of this criterion when selecting the optimal treatment strategy during the multidisciplinary tumour board meeting. The vicinity of a vessel within 10 mm from the lesion has an odds ratio of 2.9 for the R1 status.
The present study has several limitations. First, its retrospective design is a classical limitation, although patients were included consecutively to reduce a selection bias. Second, certain patients received both treatments simultaneously or separately. This induced an overlap in the cohort limiting the relevance of survival comparison (even if we found no difference in survival between the two groups). However, we are able to evaluate a large number of lesions, which would have not been possible with a patient-by-patient analysis. Third, decision criteria for the choice between WR and RFA by the multidisciplinary tumour board were not clear. This is due to the absence of clear criteria in the literature to guide the choice of treatment based on the lesions. However, both tumour and patient characteristics were in the same range (<3 cm) and fulfilled the usual selection criteria for WR and RFA. Fourth, some patients were followed by MDCT which is less sensitive than MRI to evaluate CRCLM [Citation20], although most patients underwent MRI (11.6 and 10% of the patients were followed by MDCT in the WR and RFA groups, respectively). Finally, patients in the WR group were followed up significantly longer than those in the RFA group (23 and 18.5 months, respectively, p = 0.03) but recurrences occurred before the follow-up period was half over in both groups, so we do not feel that this represents a significant bias.
In conclusion, despite a trend towards a lower LR rate with RFA than with WR, this study shows that a personalised approach based on lesion location should play a key role in the treatment choice in patients with CRCLM. Lesions located deep in the liver and/or close to large vessels are at a high risk of LR after WR, while curative treatment is possible with RFA.
| Abbreviations | ||
| CRCLM | = | colorectal cancer liver metastasis |
| CRC | = | colorectal cancer |
| LR | = | local recurrence |
| MDCT | = | multidetector computed tomography |
| MRI | = | magnetic resonance imaging |
| RFA | = | radiofrequency ablation |
| SD | = | standard deviation |
| WR | = | wedge resection |
Disclosure statement
The authors disclose no conflict of interest.
References
- de Haas RJ, Wicherts DA, Andreani P, et al. (2011). Impact of expanding criteria for resectability of colorectal metastases on short- and long-term outcomes after hepatic resection. Ann Surg 253:1069–79.
- Saiura A, Yamamoto J, Koga R, et al. (2014). Favorable outcome after repeat resection for colorectal liver metastases. Ann Surg Oncol 21:4293–9.
- Moris D, Ronnekleiv-Kelly S, Rahnemai-Azar AA, et al. (2017). Parenchymal-sparing versus anatomic liver resection for colorectal liver metastases: a systematic review. J Gastrointest Surg 21:1076–85.
- Livraghi T, Solbiati L, Meloni F, et al. (2003). Percutaneous radiofrequency ablation of liver metastases in potential candidates for resection: the “test-of-time” approach. Cancer 97:3027–35.
- Akgul O, Cetinkaya E, Ersoz S, et al. (2014). Role of surgery in colorectal cancer liver metastases. World J Gastroenterol 20:6113–22.
- Minami Y, Kudo M. (2013). Radiofrequency ablation of liver metastases from colorectal cancer: a literature review. Gut Liver 7:1–6.
- Frankel TL, D’Angelica MI. (2014). Hepatic resection for colorectal metastases. J Surg Oncol. 109:2–7.
- Ko S, Jo H, Yun S, et al. (2014). Comparative analysis of radiofrequency ablation and resection for resectable colorectal liver metastases. World J Gastroenterol 20:525–31.
- Bai H, Huangz X, Jing L, et al. (2015). The effect of radiofrequency ablation vs. liver resection on survival outcome of colorectal liver metastases (CRLM): a meta-analysis. Hepatogastroenterology 62:373–7.
- White RR, Avital I, Sofocleous CT, et al. (2007). Rates and patterns of recurrence for percutaneous radiofrequency ablation and open wedge resection for solitary colorectal liver metastasis. J Gastrointest Surg 11:256–63.
- Gillams A, Goldberg N, Ahmed M, et al. (2015). Thermal ablation of colorectal liver metastases: a position paper by an international panel of ablation experts, the Interventional Oncology Sans Frontières Meeting 2013. Eur Radiol 25:3438–54.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria - a 10-year update. Radiology 273:241–60.
- Liu M, Huang G-L, Xu M, et al. (2017). Percutaneous thermal ablation for the treatment of colorectal liver metastases and hepatocellular carcinoma: a comparison of local therapeutic efficacy. Int J Hyperthermia 33:446–53.
- van Dam RM, Lodewick TM, van den Broek MAJ, et al. (2014). Outcomes of extended versus limited indications for patients undergoing a liver resection for colorectal cancer liver metastases. HPB 16:550–9.
- Nordlinger B, Sorbye H, Glimelius B, et al. (2013). Perioperative FOLFOX4 chemotherapy and surgery versus surgery alone for resectable liver metastases from colorectal cancer (EORTC 40983): long-term results of a randomised, controlled, phase 3 trial. Lancet Oncol 14:1208–15.
- Zhang K, Yu J, Zhou F, et al. (2016). Impact of timing and cycles of systemic chemotherapy on survival outcome of colorectal liver metastases patients treated by percutaneous microwave ablation. Int J Hyperthermia 32:531–8.
- Nordlinger B, Guiguet M, Vaillant JC, et al. (1996). Surgical resection of colorectal carcinoma metastases to the liver. A prognostic scoring system to improve case selection, based on 1568 patients. Association Française de Chirurgie. Cancer 77:1254–62.
- Bartlett EK, Simmons KD, Wachtel H, et al. (2015). The rise in metastasectomy across cancer types over the past decade. Cancer 121:747–57.
- Wei AC, Greig PD, Grant D, et al. (2006). Survival after hepatic resection for colorectal metastases: a 10-year experience. Ann Surg Oncol 13:668–76.
- Bajpai S, Sahani DV. (2009). Recent progress in imaging of colorectal cancer liver metastases. Curr Colorectal Cancer Rep 5:99–107.
- Viganò L, Capussotti L, Lapointe R, et al. (2013). Early recurrence after liver resection for colorectal metastases: risk factors, prognosis, and treatment. A LiverMetSurvey-based study of 6,025 patients. Ann Surg Oncol 21:1276–86.
- Pawlik TM, Scoggins CR, Zorzi D, et al. (2005). Effect of surgical margin status on survival and site of recurrence after hepatic resection for colorectal metastases. Ann Surg 241:715–22 (discussion 722–724).
- Choti MA, Sitzmann JV, Tiburi MF, et al. (2002). Trends in long-term survival following liver resection for hepatic colorectal metastases. Ann Surg 235:759–66.
- de Jong MC, Pulitano C, Ribero D, et al. (2009). Rates and patterns of recurrence following curative intent surgery for colorectal liver metastasis: an international multi-institutional analysis of 1669 patients. Ann Surg 250:440–8.
- Fong Y, Fortner J, Sun RL, et al. (1999). Clinical score for predicting recurrence after hepatic resection for metastatic colorectal cancer: analysis of 1001 consecutive cases. Ann Surg 230:309–18. discussion 318–21.
- House MG, Ito H, Gönen M, et al. (2010). Survival after hepatic resection for metastatic colorectal cancer: trends in outcomes for 1,600 patients during two decades at a single institution. J Am Coll Surg 210:744–52. 752–5.
- Gold JS, Are C, Kornprat P, et al. (2008). Increased use of parenchymal-sparing surgery for bilateral liver metastases from colorectal cancer is associated with improved mortality without change in oncologic outcome: trends in treatment over time in 440 patients. Ann Surg 247:109–17.
- DeMatteo RP, Palese C, Jarnagin WR, et al. (2000). Anatomic segmental hepatic resection is superior to wedge resection as an oncologic operation for colorectal liver metastases. J Gastrointest Surg 4:178–84.