Abstract
Objective: Computed tomography (CT)-guided radiofrequency ablation (RFA) results in a high radiation dose. This study aimed to assess low-dose CT protocols for guiding RFA and oncologic outcomes for the treatment of small renal cell carcinoma (RCC).
Materials and methods: Between December 2011 and December 2014, CT-guided RFA was performed in 31 patients with 31 biopsy-proven RCCs (median, 2.1 cm). RFA included planning, targeting, monitoring and survey phases. The dose length product (DLP), CT dose index volume (CTDIvol), effective dose, number of scans, scan range, tube current and exposure time of RFA phases were compared. The 3-year recurrence-free survival rate was recorded. Nonparametric or parametric repeated-measures ANOVA with Dunn’s or Tukey–Kramer multiple comparisons and Kaplan–Meier analysis were used for statistical analysis.
Results: The median total DLP, CTDIvol and effective dose of CT-guided RFA procedures per session were 1238.8 mGy (range 517.4–3391.7 mGy), 259.7 mGy (10.7–67.9 mGy) and 18.6 mSv (7.8–50.9 mSv), respectively. The median DLP, CTDIvol, effective dose, number of scans, tube current and exposure time during the targeting phase were higher than those during the other phases (p < 0.001). The scan range in the targeting phase was the same as that in the monitoring phase (p > 0.05) but smaller than those in the planning and survey phases (p < 0.001). The 3-year recurrence-free survival rate was 96.7%.
Conclusions: Low-dose CT protocols for guiding RFA may reduce radiation dose without compromising oncologic outcomes. Reducing the number of scans during the targeting phase contributes to dose reduction.
Introduction
Image-guided radiofrequency ablation (RFA) is a minimally invasive therapy for the treatment of small renal cell carcinoma (RCC) in patients who are poor surgical candidates [Citation1–3]. Percutaneous RFA can be guided by various imaging modalities, including ultrasonography (US) [Citation4,Citation5], computed tomography (CT) [Citation1–3] and magnetic resonance imaging (MRI) [Citation6,Citation7]. Among these, CT is the most often used imaging modality to guide RFA because it provides favourable image resolution and because radiologists are familiar with the use of CT for various interventional procedures. However, repeated CT scans are required for lesion localisation, targeting, monitoring and survey phases [Citation8]. Accordingly, a high radiation dose is delivered to patients during RFA procedures [Citation9].
Several studies have reported on the radiation dose of CT-guided RFA when treating renal tumours [Citation10–12]. However, to our knowledge, few studies have focussed on what CT protocols should be used to reduce the high radiation dose during CT-guided RFA procedures. We hypothesised that low-dose CT protocols contribute to dose reduction while preserving good oncologic outcomes. The purpose of this study was to assess low-dose CT protocols guiding RFA and oncologic outcomes for the treatment of small RCC.
Materials and methods
This retrospective study was approved by our institutional review board, and the need for informed consent was waived.
Patients
Patients included in the study had a single renal mass, histologically proven RCC and underwent percutaneous RFA. Forty-one patients with 41 RCCs who met these inclusion criteria and who had undergone CT-guided RFA between December 2011 and December 2014 were found in our database. Of these 41 patients, 10 were excluded because their CT scans did not contain detailed information on radiation dose, that is only the overall dose information was available and that for individual scans was missing. In total, 31 patients (23 men and 8 women; age range, 34–80 years; median, 57 years) were included for analysis. The median body mass index was 24.6 kg/m2 (range 18.3–31.8 kg/m2).
Histologic diagnosis was obtained via percutaneous biopsy. US- and CT-guided biopsies were performed in 29 and two patients on the same day as RFA, respectively. The 31 RCC subtypes consisted of 22 clear cell, 2 papillary, 1 chromophobe and 6 unclassified. The size of the tumour was determined according to the maximum length measured on axial, sagittal and coronal images of pre-RFA CT or MRI examination. The median RCC size was 2.1 cm (range 1.2–3.9 cm). These tumours consisted of 15 exophytic and 16 endophytic RCCs. These patients were all used as a part of study population in Kim et al. [Citation13]. CT scans were performed 1 month following RFA and every 6 months thereafter for the second year. Between the third and fifth year, CT scans were performed yearly.
CT-guided RFA procedures
Imaging of CT-guided RFA procedures was performed with a 64-channel multidetector CT scanner (Aquilion, Toshiba Medical Systems, Otawara, Japan). The tube current was modulated automatically (Sure Exposure 3 D, Toshiba Medical Systems, Otawara, Japan) during all scans. CT parameters were as follows: 120 kVp, 70 mAs (effective tube current) and 2 mm slice thickness. All patients were positioned prone and placed under general anaesthesia by an attending anaesthesiologist. A single radiologist performed all RFA procedures consisting of four phases: planning, targeting, monitoring and survey [Citation8].
During the planning phase, unenhanced and contrast-enhanced CT scans were performed in 28 patients to visualise a tumour and neighbouring organs (). The skin entry site, insertion angle of electrode and tumour depth were determined during the planning phase. Three patients did not undergo contrast-enhanced CT due to chronic kidney disease or an extremely exophytic RCC.
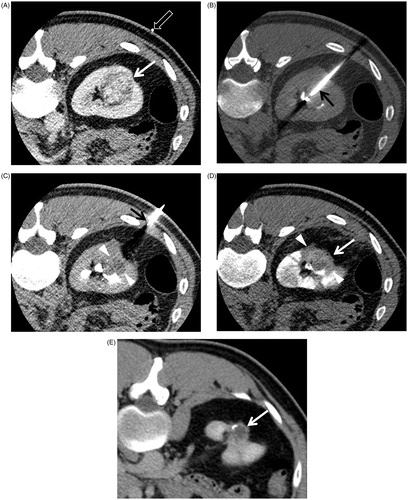
Figure 1. Low-dose CT protocols for guiding RFA in a 34-year-old man. (A) Contrast-enhanced axial CT image in the planning phase shows the location, size and number of RCC (white solid arrow) before lesion targeting. A white blank arrow indicates a grid for localising a tumour. (B) Unenhanced axial CT image in the targeting phase shows the direction and location of an electrode (black arrow) that is placed within the tumour. (C) Unenhanced axial CT image for monitoring phase shows the ablation area (arrowheads). No complication occurred following each ablation cycle. A black arrow indicates an electrode. (D) Unenhanced axial CT image for survey phase shows that the tumour size (white solid arrow) and tumour margin (arrowheads) are markedly shrunken. Overall CT image quality shown in (A–D) is not good but acceptable for RFA procedures. (E) Contrast-enhanced axial CT image, which is obtained 51 months after RFA, shows no local tumour progression but a post-ablation scar tissue (white solid arrow).
During the targeting phase, unenhanced CT scans were repeatedly performed to place an electrode (Proteus RF Electrode, STARmed, Goyang, Korea) within the tumour (). CT fluoroscopy was not performed, but an electrode was gradually advanced until an appropriate RCC region was targeted. CT-guided biopsy was performed in two patients prior to RFA procedures, while hydrodissection was performed in two patients after lesion targeting using a single electrode [Citation14,Citation15]. The median number of electrode reposition was two (range 0–9) to completely ablate 5 mm or more of the tumour margin as well as the tumour [Citation16].
During the monitoring phase, unenhanced CT scans were performed to determine any heat injury to neighbouring organs and if the ablation area was adequate to cover the tumour and tumour margin (). The targeting and monitoring phases were repeated immediately after each electrode repositioning.
During the survey phase, an unenhanced CT scan was performed to confirm complete ablation of the tumour and tumour margin and to identify any complications that had not been detected during the monitoring phase (). An electrode for RFA was internally cooled with pump-circulated saline. The length of the noninsulated electrode was controlled every 0.5 cm (range 0.5–3 cm) (. A generator (STARmed) was used to monitor tissue impedance and automatically adjust the maximum energy delivered. RFA was performed using only a single electrode. Generator power was kept running at 50 W for the first 30 s and gradually increased to 150–180 W thereafter until the electrical power peaked and then dropped to 0 W.
Data analysis
The effective dose was estimated by multiplying the dose length product (DLP) by a normalised conversion factor for the abdomen (0.015 mSv/mGy-cm) [Citation8]. The effective dose of each RFA phase was calculated by adding the effective doses for each scan performed during the corresponding phase. Meanwhile, the total effective dose of CT-guided RFA procedures was calculated by adding the effective doses of each RFA phases, which were then compared to determine which phase contributed most to the radiation dose. CT dose index volume (CTDIvol) and DLP were also obtained for comparison between RFA phases.
The number of scans, scan range, tube current and exposure time of CT-guided RFA procedures per session were recorded. These variables were also obtained from each RFA phase for comparison. Subset analysis was performed in terms of tumour size and location in order to assess the effect of radiation dose. Local tumour progression, primary effectiveness, distant metastasis, 3-year recurrence-free survival rate and complications were recorded to evaluate oncologic outcomes.
Statistical analysis
A nonparametric repeated-measures ANOVA (Freidman test) was used to compare CTDIvol, scan ranges, total mAs and exposure times in RFA phases because these data did not follow Gaussian distribution. Dunn’s multiple comparisons were the post-test used to compare all pairs of columns if a p value was less than 0.05.
Meanwhile, a parametric repeated-measures ANOVA was used to compare DLP, effective dose and scan range because these data followed Gaussian distribution. Tukey–Kramer multiple comparisons were used to compare all pairs of columns only if a p value was less than 0.05.
Unpaired t-test was used to compare radiation doses in terms of tumour size and location. RCCs were divided into two groups according to tumour size: 2 cm or less RCCs and more than 2 cm RCCs. RCCs were also divided into two groups according to tumour location: exophytic RCCs and endophytic RCCs. Radiation doses were not compared in terms of biopsy and hydrodissection because both CT-guided procedures were performed in two cases alone.
Kaplan–Meier analysis was used to calculate and compare the 3-year recurrence-free survival rate. Statistical analysis was performed with commercially available software (SAS version 9.4, SAS Institute, Cary, NC). A two-sided p value less than 0.05 was considered statistically significant.
Results
The median total DLP of CT-guided RFA per session was 1238.8 mGym (range 517.4–3391.7 mGym). The median DLP of the targeting phase (548.4 mGym) was higher than those of the other phases (148.0–296.0 mGym) (p < 0.001) (). The median total CTDIvol of CT-guided RFA per session was 259.7 mGy (range 88.0–714.5 mGy). The median CTDIvol (165.6 mGy) of the targeting phase was higher than those of the other phases (10.7–67.9 mGy) (p < 0.001) (). The median total effective dose of CT-guided RFA per a session was 18.6 mSv (range 7.8–50.9 mSv). The median effective dose of the targeting phase (8.2 mSv) was higher than those of the other phases (2.2–4.4 mSv) (p < 0.001) ().
Table 1. CTDIvol, DLP and effective dose of each RFA phase per session.
The median of the total number of scans of CT-guided RFA per session was 22 (range 13–67). The median number of scans of the targeting phase (14) was greater than those of the other phases (1–5) (p < 0.001) (). The median total scan range of CT-guided RFA per session was 10.3 cm (range 4.1–12.9 cm). The median scan range of the targeting phase (3.3 cm) was not different from that of the monitoring phase (3.3 cm) (p > 0.05), while it was shorter than those of the planning and post-survey phases (13.9–20.6 cm) (p < 0.001) (). The median total tube current of CT-guided RFA per session was 8973 mAs (range 5016–28725 mAs). The median tube current of the targeting phase was 4320 mAs, which was higher than those of the other phases (886–1800 mAs) (p < 0.001). The median total exposure time of CT-guided RFA per session was 96.9 s (range 59.7–287.2 s). The median exposure time of the targeting phase was 54.0 s, which was higher than those of the other phases (9.5–20.2 s) (p < 0.001) ().
Table 2. CT parameters of RFA phases.
The effective dose for 2 cm or less RCCs was 17.2 mSv, which was lower than that for more than 2 cm RCCs at 24.6 mSv (p = 0.0322) (). The effective dose for exophytic RCCs at 19.0 mSv was not significantly different from that for endophytic RCCs at 23.5 mSv (p = 0.1998).
Table 3. Subset analysis in terms of RCC size and location.
The median follow-up period was 31.4 months (range 4–51 months). Local tumour progression was 0% (0/31), and primary effectiveness was 100% (31/31) (). However, lymph node metastasis was detected in one patient. The 3-year recurrence-free survival rate was 96.7%. Only one minor complication occurred in which one patient had a perirenal haematoma that did not require transfusion. No major complications developed during or following the RFA procedures.
Discussion
Our results showed that the effective dose of CT protocols to guide RFA was relatively low. The dose was the highest during lesion targeting because the number of scans was the highest during that phase. The 3-year recurrence-free survival rate was good even when low-dose CT protocols were used.
The targeting phase requires more scans compared with other phases because an electrode must be accurately placed within a tumour for adequate treatment. Accurate electrode positioning and repositioning are essential, which increase the number of necessary scans. Park et al. reported that the number of scans was the most significant factor that increased the radiation dose [Citation8]. Accordingly, CTDIvol, DLP, tube current and exposure time are highest during the targeting phase. We tried to minimise the scan range during the targeting phase, which helped to reduce the radiation dose.
The radiation dose in our study was lower than that in other studies examining CT-guided ablation therapies, even though CT fluoroscopy was not used during the targeting phase [Citation8–12,Citation17]. CT fluoroscopy is useful for lesion targeting but may not contribute to a reduction in radiation dose [Citation8,Citation18]. We advanced an electrode in increments to a target lesion, and repeat CT scans were performed to determine whether the electrode was properly placed after each repositioning. This targeting technique increased the number of scans, but the radiation dose was smaller than that from using CT fluoroscopy.
Our low-dose CT protocols adopted a minimised scan range and used a low effective tube current (70 mAs), which can degrade CT image quality due to increased noise. Poor oncologic outcomes would negate the benefits of decreased radiation dose, but our study achieved a good 3-year recurrence-free survival rate. Intravenous injection of contrast material may contribute to an extended capability to visualise a tumour more clearly and enables easy identification of the ablation area during targeting and monitoring. Moreover, contrast enhancement increased the capability to detect and identify endophytic RCCs, which accounted for more than half of all tumours in our study. Previous studies that examined the radiation dose of cryoablation or RFA have not reported the oncologic outcomes of the procedure [Citation9–12].
Our study showed that the effective dose is the highest during the targeting phase; thus, the number of scans during this phase should be minimised. Appropriate electrode positioning is essential to achieve complete ablation and avoid major complications. Repeat CT scans are often performed to determine the position of an electrode when locating a tumour, increasing the radiation dose. As such, many guiding techniques that use US combinations, image fusion or navigation have been developed to minimise the number of scans necessary for targeting [Citation19,Citation20]. Penzkofer et al. reported that the DLP with electromagnetic tracking navigation (732 ± 481 mGycm) was significantly lower than that with CT-guided nonablation procedures (1343 ± 1054 mGycm) [Citation21]. CT fluoroscopic guidance incurs a high dose despite a reduced number of targeting scans, and it delivered a dose of 3.9 mGy to the interventional radiologists [Citation18]. Minimising the number of scans will be important for reducing the radiation dose if a narrow scan range and low tube current are used for RFA.
The median effective dose in our study was 18.6 mSv, which seems to be much lower than that of previous reports (31.5 mSv) [Citation9–12]. Park et al. [Citation8] reported that the effective dose of a single-phase contrast-enhanced abdomen and pelvis CT is 8 mSv. Tsalafoutas et al. [Citation9] reported that those of nonfluoroscopic CT-guided biopsy, abscess drainage and nephrostomy are 23 mSv, 16.2 mSv and 11.5 mSv, respectively. Nawfel et al. [Citation22] reported that the mean effective dose of CT urography was 14.8 mSv without using iterative reconstruction. Therefore, the effective dose of our low-dose CT protocols guiding RFA is only slightly higher or slightly lower than those of diagnostic and nonablative interventional CT scans. The radiation dose of CT scans following RFA must be considered. The radiation dose in many follow-up CT scans performed may influence life expectancy [Citation11]. Further investigation is necessary to assess how often follow-up CT should be performed.
Increasing tumour size indicates increasing ablation area due to a high number of tumour cells [Citation16]. Our results showed that patients with tumours greater than 2 cm were exposed to higher radiation dose than those with tumours 2 cm or less. Exophytic RCC has smaller tumour margin volume than the same-sized endophytic RCC [Citation16]. Subsequently, the effective radiation dose for endophytic RCCs is likely to be higher than those for exophytic RCCs. However, our results did not show a significant difference between exophytic and endophytic RCCs in terms of effective radiation dose even if the mean effective doses for endophytic RCCs were higher than those for exophytic RCCs. This result might be because of the small number of cases compared in the present study.
Recently, low-dose CT protocols have been used in clinical practice to improve probe visualisation during targeting scans. Other than the first planning scan to visualise the lesion, low-dose CT for subsequent scans is useful for probe visualisation.
Our study had some limitations. First, the estimated effective dose was used to calculate the radiation dose; the actual dose delivered to the patient’s skin or internal organs was not measured. Age, gender and specific organ-weighted radiosensitivity were also not considered in the current study. The DLP-to-effective dose conversion factor was 0.015 mSv/mGy-cm in the present study [Citation8], while it may range from 0.016 to 0.018 [Citation23]. Second, general anaesthesia was used to control patient pain, while many investigators have used conscious sedation during percutaneous RFA [Citation1,Citation24–26]. However, their recurrence-free survival rates are not as high as in our study, even when differences in the follow-up period are considered. Future studies should investigate how general anaesthesia influences radiation dose and oncologic outcome. Third, the number of patients that underwent CT-guided RFA was relatively small. Fourth, the median body mass indices of the study population were within normal range; an increased number of overweight or obese patients would also require increased radiation dose. Fifth, the follow-up period for evaluation of oncologic outcomes was relatively short. Finally, our study used a retrospective design.
In conclusion, low-dose CT protocols for guiding RFA may reduce radiation dose administered to patients while preserving good oncologic outcomes. The effective dose is higher during the targeting phase than during the planning, monitoring or survey phases because the number of scans is the highest for lesion targeting. Therefore, decreasing the number of scans decreases the dose. To decrease the radiation dose, minimising the number of scans is necessary unless it compromises the survival rate of the patients.
Acknowledgments
Our study was conducted without any financial support.
Disclosure statement
No potential conflict of interest was reported by the author.
References
- Zagoria RJ, Pettus JA, Rogers M, et al. (2011). Long-term outcomes after percutaneous radiofrequency ablation for renal cell carcinoma. Urology 77:1393–7.
- Psutka SP, Feldman AS, McDougal WS, et al. (2013). Long-term oncologic outcomes after radiofrequency ablation for T1 renal cell carcinoma. Eur Urol 63:486–92.
- Wah TM, Irving HC, Gregory W, et al. (2014). Radiofrequency ablation (RFA) of renal cell carcinoma (RCC): experience in 200 tumours. BJU Int 113:416–28.
- Veltri A, Calvo A, Tosetti I, et al. (2006). Experiences in US-guided percutaneous radiofrequency ablation of 44 renal tumors in 31 patients: analysis of predictors for complications and technical success. Cardiovasc Intervent Radiol 29:811–18.
- Park BK, Kim CK, Choi HY, et al. (2010). Limitation for performing ultrasound-guided radiofrequency ablation of small renal masses. Eur J Radiol 75:248–52.
- Lewin JS, Nour SG, Connell CF, et al. (2004). Phase II clinical trial of interactive MR imaging-guided interstitial radiofrequency thermal ablation of primary kidney tumors: initial experience. Radiology 232:835–45.
- Boss A, Clasen S, Kuczyk M, et al. (2005). Magnetic resonance-guided percutaneous radiofrequency ablation of renal cell carcinomas: a pilot clinical study. Invest Radiol 40:583–90.
- Park BK, Morrison PR, Tatli S, et al. (2012). Estimated effective dose of CT-guided percutaneous cryoablation of liver tumors. Eur J Radiol 81:1702–6.
- Tsalafoutas IA, Tsapaki V, Triantopoulou C, et al. (2007). CT-guided interventional procedures without CT fluoroscopy assistance: patient effective dose and absorbed dose considerations. AJR Am J Roentgenol 188:1479–84.
- Arnold DC, 2nd, Schroeder G, Smith JC, et al. (2013). Comparing radiation exposure between ablative therapies for small renal masses. J Endourol 27:1435–9.
- Eisenberg JD, Gervais DA, Singh S, et al. (2015). Radiation exposure from CT-guided ablation of renal masses: effects on life expectancy. AJR Am J Roentgenol 204:335–42.
- McEachen JC, Leng S, Atwell TD, et al. (2016). Percutaneous renal tumor ablation: radiation exposure during cryoablation and radiofrequency ablation. Cardiovasc Intervent Radiol 39:233–8.
- Kim HJ, Park BK, Park JJ, Kim CK. (2016). CT-guided radiofrequency ablation of T1a renal cell carcinoma in Korea: mid-term outcomes. Korean J Radiol 17:763–70.
- Park BK, Kim CK. (2009). Complications of image-guided radiofrequency ablation of renal cell carcinoma: causes, imaging features and prevention methods. Eur Radiol 19:2180–90.
- Park BK, Kim CK, Park SY, Shen SH. (2013). Percutaneous radiofrequency ablation of renal cell carcinomas in patients with von Hippel Lindau disease: indications, techniques, complications, and outcomes. Acta Radiol 54:418–27.
- Park SY, Park BK, Kim CK. (2012). Thermal ablation in renal cell carcinoma: what affects renal function? Int J Hyperthermia 28:729–34.
- Levesque VM, Shyn PB, Tuncali K, et al. (2015). Radiation dose during CT-guided percutaneous cryoablation of renal tumors: effect of a dose reduction protocol. Eur J Radiol 84:2218–21.
- Stewart JK, Looney CB, Anderson-Evans CD, et al. (2015). Percutaneous cryoablation of renal masses under CT fluoroscopy: radiation doses to the patient and interventionalist. Abdom Imaging 40:2606–12.
- Krucker J, Xu S, Venkatesan A, et al. (2011). Clinical utility of real-time fusion guidance for biopsy and ablation. J Vasc Interv Radiol 22:515–24.
- Abi-Jaoudeh N, Kruecker J, Kadoury S, et al. (2012). Multimodality image fusion-guided procedures: technique, accuracy, and applications. Cardiovasc Intervent Radiol 35:986–98.
- Penzkofer T, Bruners P, Isfort P, et al. (2011). Free-hand CT-based electromagnetically guided interventions: accuracy, efficiency and dose usage. Minim Invasive Ther Allied Technol 20:226–33.
- Nawfel RD, Judy PF, Schleipman AR, Silverman SG. (2004). Patient radiation dose at CT urography and conventional urography. Radiology 232:126–32.
- Huda W, Ogden KM, Khorasani MR. (2008). Converting dose-length product to effective dose at CT. Radiology 248:995–1003.
- Park BK, Kim CK, Lee HM. (2008). Image-guided radiofrequency ablation of Bosniak category III or IV cystic renal tumors: initial clinical experience. Eur Radiol 18:1519–25.
- Sung HH, Park BK, Kim CK, et al. (2012). Comparison of percutaneous radiofrequency ablation and open partial nephrectomy for the treatment of size- and location-matched renal masses. Int J Hyperthermia 28:227–34.
- Park BK, Kim CK. (2010). Percutaneous radio frequency ablation of renal tumors in patients with von Hippel-Lindau disease: preliminary results. J Urol 183:1703–7.