Abstract
Background: High-intensity focussed ultrasound (HIFU) is a non-invasive ablative technique utilising the application of high frequency ultrasound (US) pressure waves to cause tissue necrosis. This emerging technology is currently limited by prolonged treatment times. The aim of the HIFU-F trial was to perform circumferential HIFU treatment as a means of shortening treatment times.
Methods: A prospective trial was set up to treat 50 consecutive patients ≥18 years of age. Eligible patients possessed symptomatic fibroadenomata, visible on US. Patients ≥25 years of age required histological confirmation of the diagnosis. Primary outcome measures were reduction in treatment time, reduction in volume on US after 12 months and complication rates.
Results: HIFU treatment was performed in 51 patients (53 treatments) with a mean age of 29.8 years (SD 7.2 years) and a diameter of 2.6 cm (SD 1.4 cm). Circumferential ablation reduced treatment times by an estimated 19.9 min (SD 25.1 min), which is a 29.4% (SD 15.2%) reduction compared with whole lesion ablation. Volume reduction of 43.2% (SD 35.4%; p < 0.005, paired t-test) was observed on US at 12 months post-treatment. Local complications completely resolved at 1 month apart from skin hyper-pigmentation, which persisted in nine cases at three months, six cases at 6 months and six at 12 months.
Conclusion: Circumferential HIFU treatment for breast fibroadenomata is feasible to reduce both lesion size and treatment time. HIFU is a non-invasive alternative technique for the treatment of breast fibroadenomata.
ISRCTN registration: 76622747
Introduction
Fibroadenomata represent the most common benign breast lumps in women [Citation1,Citation2]. Fibroadenomata are diagnosed clinically as a palpable lump or incidentally during breast imaging [Citation2,Citation3]. The diagnosis is based upon triple assessment (clinical examination, ultrasound (US) and a core needle biopsy (CNB)) [Citation1–3]. Patients with asymptomatic, biopsy-proven breast fibroadenomata are generally managed by reassurance and no follow-up is deemed necessary. Symptomatic or rapidly growing lesions are suitable for excision, either surgically or radiologically (e.g. by vacuum-assisted mammotomy), although these techniques have limitations associated with invasive procedures [Citation1–3].
High-intensity focussed ultrasound (HIFU) is a non-invasive ablative technique. During HIFU treatment, an US beam generated by a transducer propagates through soft tissue as a high-frequency pressure wave [Citation4,Citation5]. The beam is focussed onto the target tissue and the energy from the beam elevates the temperature of the targeted area to about 60–95 °C within seconds, leading to localised protein denaturation and coagulative necrosis – sparing the surrounding tissues from thermal damage [Citation4–6]. HIFU provides a completely non-invasive treatment, avoiding the potential complications associated with general anaesthesia and surgical excision [Citation7].
Data from systematic reviews [Citation8,Citation9] suggest that pilot studies of the treatment of breast cancer possess prolonged treatment times – of up to 3 h – as a significant disadvantage with the current HIFU technique. The aim of the HIFU-F trial was to perform circumferential HIFU treatment in order to isolate the fibroadenoma from its blood supply, resulting in necrosis of the fibroadenoma and a reduced treatment time. In a recently published article [Citation10], a protocol planned analysis of the first 20 patients included in this trial were compared with a control group of 20 patients who underwent only an US scan at 6 months after their initial diagnosis to determine the natural change in fibroadenoma volume. The study demonstrated that circumferential ablation reduced the mean treatment time by 37.5% (SD 20.1%) compared with whole lesion ablation. In addition, a significant mean reduction in fibroadenoma volume of 43.5% (SD 38.8%; p = 0.016, paired t-test) in the HIFU group compared with 4.6% (SD 46.0%; p = 0.530) in the control group was demonstrated on US after 6 months. This mean reduction in volume between the two groups was statistically significant in favour of the HIFU group (p = 0.002, grouped t-test). This article reports on HIFU treatment of all 51 patients who participated in the HIFU-F trial with a minimum follow-up of 12 months.
Materials and methods
A prospective trial was set up to treat 50 consecutive patients with circumferential HIFU. Written informed consent was obtained from all patients prior to inclusion. This study was approved by the National Research Ethics Committee (13/LO/1221).
Patient selection
As described in the article by Peek et al. [Citation10], patients were eligible for the HIFU-F trial if they were at least 18 years of age and had been referred to the one-stop Breast Clinic at Guy’s Hospital with a symptomatic fibroadenomata – defined as either a palpable lesion with or without pain developing from this lesion – which was visible on US. Patients of 25 years of age or older required confirmation of the diagnosis by US guided core needle biopsy, as per local Unit protocol. Patients were excluded if they had a fibroadenoma of less than 1 cm, had previous breast implants, were pregnant or lactating, had received any laser or radiation therapy to the ipsilateral breast, if epithelial atypia was seen on histopathology or if there was any suspicion of a phyllodes tumour. No other exclusions were applied.
A control group including 20 patients were consecutively recruited, without matching with the HIFU group, to determine the natural course in volume change of their fibroadenoma as assessed by US, 6 months after initial presentation. Patients were recruited using the same inclusion and exclusion criteria as the HIFU treatment group, demonstrating a definite treatment response in the HIFU group [Citation10].
All eligible patients were identified: (1) at the multi-disciplinary meeting (MDM), (2) as scheduled for surgical excision of a fibroadenoma or (3) as referred to the breast clinic with a symptomatic breast lump requesting treatment. All eligible patients were approached and received a patient information sheet if interested in participating in the HIFU-F trial.
Primary outcome measures were the change in treatment time compared with whole lesion ablation (estimation based on the computerised treatment plan) and the volume reduction on US after 12 months. Secondary outcomes were the complication rate and patient recorded outcome measures (palpability of the lump and pain pre- and post-treatment measured with visual analogue scale (VAS)).
HIFU treatment
Patients were treated using the US-guided-Echopulse device (Theraclion Ltd, Malakoff, France), which is CE marked for the treatment of breast fibroadenomata and thyroid nodules. The device contains a cooling and coupling disposable unit to cool the skin during treatment. Treatment was performed under real-time US guidance using a 7.5–12 MHz diagnostic transducer and a 3.0 MHz therapeutic transducer with a central hole measuring 1.1 cm for the coaxial imaging transducer. Oval tissue volumes of approximately 0.9 cm in length and 0.2 cm in height and width were ablated. No updates in software or hardware were used during this study.
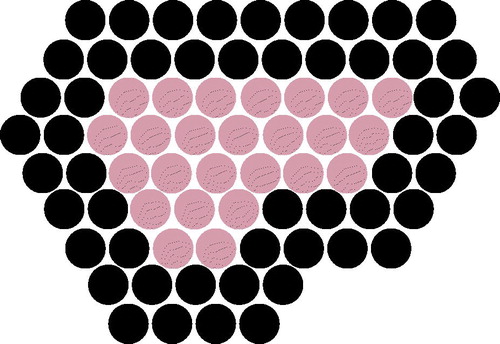
All patients received treatment as a day-case procedure using local anaesthesia (1.0% lidocaine with adrenaline with or without 0.25–0.5% Bbupivacaine, ratio 1:1). In cases where the fibroadenoma was located close (<0.5 cm) to the skin or pectoralis major muscle, local anaesthesia was injected between the fibroadenoma and the skin or muscle. Depending on the size of the breast and the position of the fibroadenoma, the patient was placed in either a supine or lateral position and the breast was fixed using an immobilisation system. Retro-areolar FAD were treated with the patient placed in a lateral position. After an US scan with a handheld probe was performed to locate the fibroadenoma, the device head was positioned on top of the fibroadenoma to outline the fibroadenoma and the skin in the radial and anti-radial view. For every radial slice, all treatment pulses were visualised and adjusted if required. The procedure started with a single pulse in the centre of the fibroadenoma in order to determine the correct energy level, identified by a hyper-echoic mark (HEM) visible during administration of the pulse. The HIFU device calculated the energy and power level of each pulse during treatment. In this study, only the circumference of the fibroadenoma was ablated; two circumferential rings were treated and the centre was deselected (). The Echopulse device treats only one central, top or bottom disc shaped target volume (curved or horizontal) during one treatment session. The optimal target volume was selected in order to avoid thermal damage to skin and pectoral muscle and to cover the most central part of the fibroadenoma. After the treatment, the patient’s skin was observed for any treatment related changes. Patients were asked to provide a pain score after the procedure for pre-, intra- and post-treatment pain using a VAS scoring system between 0 and 10. Patients followed nurse-led discharge policies in accordance with local protocol.
Figure 1. HIFU treatment planning showing the two circumferential treatment rings (black) and the deselected centre of the fibroadenoma (grey).
Circumferential treatment time was recorded from the beginning of the first pulse to the end of the last pulse, along with the number of pulses delivered. The average time to deliver a treatment pulse was calculated (including any delays between pulses due to prolonged treatment pauses) and used to estimate the total treatment time required to treat the whole lesion. In addition, total procedure time – including treatment planning and administration of local anaesthesia – was recorded.
Follow-up
Patients were followed up at 2 weeks, 3, 6 and 12 months – with a physical examination and US scan. Clinicians were blinded from the US results during physical examination but ultra-sonographers performing the scans were not blinded from previous scan results. The fibroadenoma volume was calculated using standard formulae in which V is the volume and A, B and C are the maximum diameters measured on US [Citation11]
Statistical analysis
Statistical analysis was performed using IBM SPSS statistics version 23 (SPSS Inc., Chicago, IL). A paired t-test and Wilcoxon signed rank test were used to determine the significance of the reduction in fibroadenomata volumes. The logarithms of the initial and final volumes were calculated and a Pitman–Morgan test was used to determine if the percentage volume reduction varies with initial fibroadenomata volumes. An independent t-test was used to determine if the patients lost to follow-up had different initial volumes compared with those who completed follow-up and if there was a significant difference in volume reduction after 12 months between patients with two successfully treated rings and patients with less than two successfully treated rings.
A Mann–Whitney U-test was used to determine if there were any statistically significant differences in distribution between the patients with or without post-treatment hyper-pigmentation in terms of distance of fibroadenomata to the skin, distance of fibroadenomata to the pectoralis major muscle, mean power and mean energy used during the treatment.
Results
Patient screening
A total of 262 patients with fibroadenomata were screened between December 2013 and September 2015. About 46.6% of these patients (122 of 262) were potentially eligible for HIFU treatment and were approached by our team of which 19.5% (51 of 262) were included in the trial and treated with HIFU and an additional 20 patients were included in the HIFU-F trial as control patients (7.6%, 20 of 262). Another 19.5% (51 of 262) were excluded as they were not contactable or did not want to participate in the trial, 18.3% (48 of 262) had incidental fibroadenomata, 23.7% (62 of 262) had fibroadenomata smaller than 1 cm, 3.1% (8 of 262) had giant fibroadenomata and 8.4% (22 of 262) were excluded due to our exclusion criteria.
Patient characteristics
Circumferential HIFU treatment was performed in 51 patients (53 treatments) with a mean age of 29.8 years (SD 7.2 years) and a mean fibroadenomata size of 2.6 cm (SD 1.4 cm). The mean distance between the skin and the fibroadenomata was 0.4 cm (SD 0.2 cm) and between the pectoralis major and the fibroadenomata 0.5 cm (SD 0.3 cm). About 45.3% of lesions (24 of 53) were located in the left and 54.7% (29 of 53) in the right breast. About 35.8% of lesions (19 of 53) were located in the upper outer quadrant, 18.9% (10 of 53) in the lower outer quadrant, 28.3% (15 of 53) in the upper inner quadrant and 17.0% (9 of 53) in the lower inner quadrant ().
Table 1. (a) Patient and (b) HIFU treatment characteristics (SD: standard deviation).
HIFU treatment
Two circumferential rings were completed in 67.9% (36 of 53) of treatments, one ring in 30.2% (16 of 53; only one or two treatment pulses were missing to complete the second ring in 22.6% (12 of 53)) and in 1.9% (1 of 53) no circumferential ring was completed and treatment had to be abandoned (). The central layer was treated in 94.3% (50 of 53) of treatments, the bottom in 3.8% (2 of 53) and the top in 1.9% (1 of 53).
A mean of 16.6 ml (SD 8.3 ml) local anaesthesia was administered prior to the treatment. HIFU treatment was performed with a mean of 117.2 Joule per pulse (SD 29.6 Joule per pulse; 52 treatments) and 29.1 Watt per pulse (SD 7.3 Watt per pulse; 52 treatments). A mean volume of 0.9 cm3 (SD 0.3 cm3; 52 treatments) was treated during each treatment session.
Out of 29 patients who were asked if they would have HIFU treatment for fibroadenomata again, 19 patients replied “yes“, seven patients replied “no” (because of pain in all cases) and three patients were indecisive.
Treatment times
The mean procedural circumferential treatment time was 32.9 min (SD 9.8 min). Circumferential ablation shortened the estimated procedural treatment time by 19.9 min (SD 25.1 min), which is a mean reduction of 29.4% (SD 15.2%) compared with whole lesion ablation. Total procedure time – including treatment planning and administration of local anaesthesia – was 66.0 min (SD 16.5 min).
Response to treatment
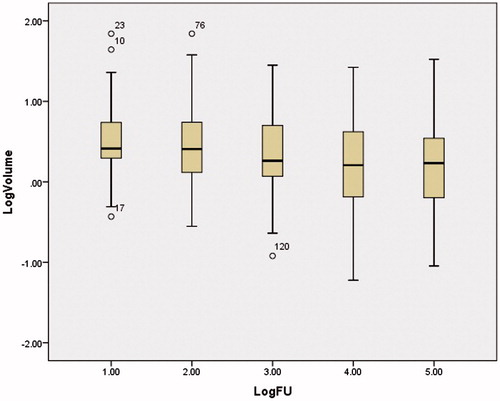
Prior to HIFU treatment the mean fibroadenoma volume was 6.1 cm3 (SD 12.1 cm3). At two weeks (49 patients (n)), the fibroadenomata showed a slight decrease in volume of 12.3% (SD 27.1%; paired t-test p = 0.007; Wilcoxon signed ranks test Z= −2.91, p = 0.004) to 5.7 cm3 (SD 11.0 cm3). At 3 months (n = 48), the decrease was 12.5% (SD 123.7%; p = 0.003; Z= −3.59, p < 0.005) to 3.6 cm3 (SD 4.5 cm3). At 6 months (n = 42), a reduction of 35.0% (SD 102.8%; p < 0.005; Z= −4.47, p < 0.005) to 2.8 cm3 (SD 4.3 cm3) was seen. At 12 months (n = 40), a reduction in volume of 43.2% (SD 35.4%; p < 0.005; Z= −4.65, p < 0.005) to 3.0 cm3 (SD 5.3 cm3) was seen (and ).
Figure 2. Change in fibroadenomata volume on ultrasound over time by patient (n) in percentage reduction.
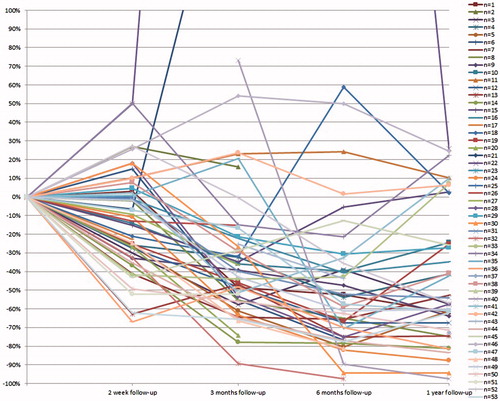
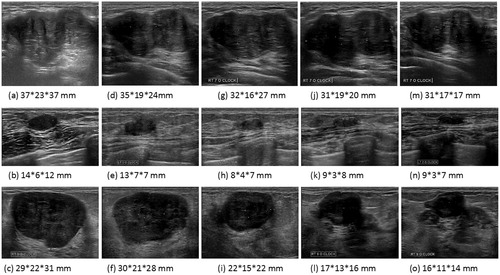
Figure 3. Ultrasound images of three patients showing change in volume over time; pre-treatment (a–c), 2 weeks (d–f), 3 months (g–i), 6 months (j–l) and 12 months (m–o) follow-up.
A Pitman–Morgan test showed that large fibroadenomata do not decrease by as large a percentage of volume as small fibroadenomata (). In addition, comparing the patients who completed follow-up with those who were lost to follow-up, it was seen that there was no statistical difference in initial fibroadenoma volume (independent t-test, unequal variances, t(18) = 0.282, p = 0.781). No significant difference was seen between the reductions in fibroadenoma volumes after 12 months for patients with one or two treatment rings treated (independent t-test, unequal variances, t(26) = 0.152, p = 0.880).
Figure 4. Boxplot of the logarithm of the fibroadenoma volume as seen on ultrasound at each follow-up moment (1: pre-treatment, 2: 2 weeks, 3: 3 months, 4: 6 months and 5: 12 months follow-up, °: outliers).
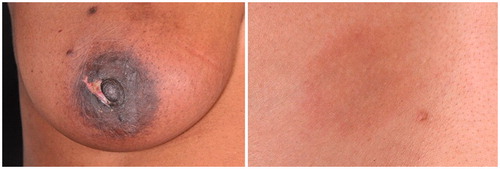
All patients were able to feel their fibroadenoma prior to treatment. A total of 24.5% of patients (13 of 53) could no longer feel the fibroadenoma after treatment, in 11.3% (6 of 53) this data was not available and 64.2% (34 of 53) were still able to feel a residual lump – although reduced in size. An increase in volume was seen in nine patients at 12 months follow-up. A total of three patients decided to undergo surgery after follow-up due to a lack of response on US. On histopathology, residual fibroadenoma tissue was seen with areas of fibrosis and fat necrosis ().
Figure 5. Ultrasound images (pre-treatment – 22*12*12 mm, 2 week follow-up – 19*8*10 mm and 3 months follow-up – 19*8*10 mm) showing no change in volume and histopathology outcome of a patient who underwent surgical excision 3 months after HIFU treatment, showing fat necrosis and fibrosis.
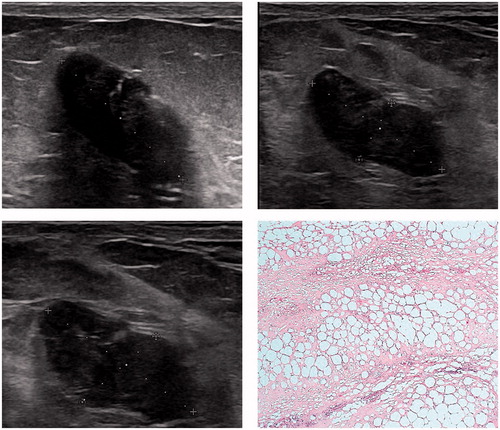
Table 2. Fibroadenomata volumes over time measured with ultrasound (mean (standard deviation)).
Pain symptoms
During treatment, the maximum pain felt by patients scored 7.1 (SD 2.6) on a 0–10 VAS score. If the application of a sonication was paused due to pain, the sonication and treatment were only completed with consent of the patient. In the single abandoned treatment, consent to continue treatment was not provided. Immediately after treatment pain scored 1.6 (SD 1.8).
Pre-treatment a total of 21 patients had pain at the site of the fibroadenoma (VAS score 1.5 (SD 2.7)). Two weeks post-treatment, 11 patients no longer experienced any pain and two patients developed pain from the area of the fibroadenoma. At 3 months pain was reduced in 15 patients, one patient had recurrent pain, an additional two patients developed pain and one of two patients who developed pain at 2 weeks follow-up no longer felt any discomfort. At 6 months, 15 patients were pain free, the patient with recurrent discomfort still had pain near the FAD and all but one patient who developed pain post-treatment were now pain free. At 12 months, 14 patients with pre-treatment pain were pain free, all but one patient who developed post-treatment pain were now pain free and the patient with recurrent discomfort still had pain near the FAD.
Local complications
At 2 weeks post-treatment, bruising of the skin (n = 15 patients), skin erythema (n = 12), skin numbness (n = 3) and single cases of hypo-pigmentation, hyper-pigmentation, skin burns, skin blisters, skin dimpling and pain in the ipsilateral axilla were reported. At 3 months post-treatment only one case of skin erythema persisted and at 12 months one case of skin numbness persisted.
Skin hyper-pigmentation was observed in nine and six cases at 3 and 12 months, respectively (). There was no significant difference between the distance from the fibroadenoma to the skin and pectoralis major and the occurrence of hyper-pigmentation (p = 0.748 and p = 0.969, respectively). No significant difference was identified between mean power and energy levels used during the treatments and the occurrence of hyper-pigmentation (p = 0.461 and p = 0.511, respectively).
Discussion
Our study has shown that with circumferential HIFU treatment, a treatment time reduction of 29.4% (SD 15.2%, 32.9 min (SD 9.8 min)) and a fibroadenoma volume reduction of 43.2% (SD 35.4%) after 12 months was achieved. The combination of circumferential treatment and performing this treatment under local anaesthesia resulted in a treatment, which reduced the fibroadenoma volume but did not make the lump non-palpable in the majority patients (64.2%; 34 of 53).
This study is the largest cohort of patients with benign breast lesions treated with HIFU. Circumferential HIFU treatment showed a mean volume reduction of 43.2% (SD 35.4%) after 12 months, an improvement compared with the 35.0% (SD 102.8%) reduction seen at 6 months, suggesting that the treatment effect continues up to 12 months post-treatment. Recently, several trials have been published treating fibroadenomata. Kovatcheva et al. [Citation12] published on 42 women with 51 fibroadenomata who underwent whole lesion ablation using US-guided HIFU and found a 72.5% reduction in volume after 12 months follow-up. The mean treatment time was 118 min and at 12 months, only one patient with asymptomatic subcutaneous induration was seen. Cavallo Marincola et al. [Citation13] performed whole lesion US guided HIFU on ten patients and showed a 50% reduction after 3 months follow-up and no adverse events. The mean treatment time from first to last pulse was 57.2 min and the mean procedure time was 136 min. These studies suggest that whole lesion ablation might be more effective than circumferential ablation but there are no comparative studies.
Treatment times were reduced by 29.4% and the total amount of time the patient spent in the treatment room (procedure time) was just over an hour. This treatment duration is more acceptable to patients than the 1–3 h treatment times described for whole tumour ablation, traditionally seen [Citation8,Citation9].
Circumferential HIFU treatment aims to cut off the blood supply and if this is not successful, the reduction might not be as significant as expected. An increase in volume was seen in 15 patients at 2 weeks, which can be explained by inflammation and oedema causing difficulty in determining the size of the fibroadenoma. An increase in volume was seen in nine patients at 12 months, which might be due to an incomplete ablation of two circumferential rings. In some patients, an increase was seen which reduced in volume by the next follow-up. This might be as a result of unclear borders caused by defragmentation of the fibroadenoma, leading to an over-estimation of the size. Due to this difference in response to treatment, patient selection is very important. Patients should be made aware of this gradual decrease in volume compared to the instant and complete removal of the lesion with surgical excision. Patients might otherwise become anxious about the persistence of a lesion.
HIFU treatment does seem particularly effective for pain management as only three out of 21 patients with pre-treatment pain still felt pain from the area of the lump after 12 months. This could be due to treatment pulses being administered to local sensory pain receptors, hence why locations where patients initially had pain were no longer painful following subsequent treatment pulses. Patients who presented with pre- or inter-treatment pain, however, would like to have the treatment again if they found another fibroadenoma.
The most common local complication found during this trial was altered skin pigmentation. In some cases, the altered pigmentation was not noticeable by the patients; nevertheless this is a complication, which was reported more often within our study than any other studies. This might be due to a higher level of cosmesis expected by the reviewing clinicians. The patients included had fibroadenomata closely located to the skin and this might be a cause for the hyperpigmentation due to the skin being heated more than initially expected even though the skin was cooled by the treatment probe. However, no correlation could be found between the distance of the fibroadenoma from the skin, distance from the pectoralis major muscle, energy level used during treatment and power level used during treatment and the incidence of skin hyper-pigmentation. Neither was a darker pigmented skin a risk factor for altered pigmentation due to HIFU treatment.
Our study adds to the existing published evidence confirming that HIFU is a viable alternative option for the treatment of fibroadenomata. Further clinical implementation and cost-effectiveness analysis is required. Also, aesthetic outcome in management of symptomatic fibroadenomata is very important in younger women. Therefore, patient reported outcome measures (PROMS) should also be considered in order to evaluate aesthetic benefits for patients.
The initial aim was to recruit 50 control patients in order to compare volume reduction within the HIFU group with the control group. However, initial assessment of 20 HIFU patients versus 20 control patients showed a statistically significant difference (p = 0.002, grouped t-test) between these two groups after 6 months, and, therefore, it was decided to stop recruitment of the control group [Citation10].
The immediate, future research evaluation of HIFU would be progression to the treatment of small (with a diameter of 1–3 cm), good prognostic, screen-detected breast cancers which should aim to demonstrate consistent and complete pathological ablation. These could be feasibly treated in short timeframes under local anaesthetic. The HIFU system could also potentially allow US-guided axillary management of suspicious nodes – in keeping with the emergence of increasing axillary conservatism. This application will be bolstered by continual technological advancements from manufacturers to enhance resolution and allow multiple treatment sites during the procedure. However, the first step should be the standardisation of HIFU treatment for fibroadenomata in the clinical setting before breast cancer treatment is evaluated within clinical studies.
Conclusion
Circumferential HIFU treatment for breast fibroadenomata is feasible to reduce both lesion size and treatment time. HIFU is a non-invasive alternative technique for the treatment of breast fibroadenomata.
HIFU-F trialists’ Collaborators
Prof. Michael Douek (King’s College London, Guy’s and St. Thomas’ NHS Foundation Trust), [email protected]; Ms Mirjam Peek (King’s College London, Guy’s and St. Thomas’ NHS Foundation Trust), [email protected]; Mr. Muneer Ahmed (King’s College London), [email protected]; Mrs Julie Scudder (Guy’s and St. Thomas’ NHS Foundation Trust), [email protected]; Prof. Rose Baker (University of Salford), [email protected]; Mr. Petros Charalampoudis (Guy’s and St. Thomas’ NHS Foundation Trust), [email protected]; Prof. Sarah Pinder (King’s College London, Guy’s and St. Thomas’ NHS Foundation Trust), [email protected]; Mr. Ashutosh Kothari (Guy’s and St. Thomas’ NHS Foundation Trust), [email protected]; Mr. Hisham Hamed (Guy’s and St. Thomas’ NHS Foundation Trust), [email protected]; Mr. Tibor Kovacs (Guy’s and St. Thomas’ NHS Foundation Trust), tibor.kovacs@gstt,nhs.uk; Mrs Sarah MacWilliams (St. Bartholomew’s Hospital); Mr. Bauke Anninga (King’s College London, Guy’s and St. Thomas’ NHS Foundation Trust), [email protected], Mrs Lorna Cook (King’s College London, Guy’s and St. Thomas’ NHS Foundation Trust), [email protected].
Disclosure statement
The authors report no conflicts of interest. Theraclion Ltd provided the Echopulse device at no costs and provided technical support during the treatments.
Additional information
Funding
References
- Cerrato F, Labow BI. (2013). Diagnosis and management of fibroadenomas in the adolescent breast. Semin Plast Surg 27:23–5.
- Sperber F, Blank A, Metser U, et al. (2003). Diagnosis and treatment of breast fibroadenomas by ultrasound-guided vacuum-assisted biopsy. Arch Surg (Chicago, Ill: 1960) 138:796–800.
- Greenberg R, Skornick Y, Kaplan O. (1998). Management of breast fibroadenomas. J Gen Intern Med 13:640–5.
- Schmitz AC, Gianfelice D, Daniel BL, et al. (2008). Image-guided focused ultrasound ablation of breast cancer: current status, challenges, and future directions. Eur Radiol 18:1431–41.
- Haar GT, Coussios C. (2007). High intensity focused ultrasound: physical principles and devices. Int J Hyperthermia 23:89–104.
- Kim SH, Jung SE, Kim HL, et al. (2010). The potential role of dynamic MRI in assessing the effectiveness of high-intensity focused ultrasound ablation of breast cancer. Int J Hyperthermia 26:594–603.
- Payne A, Todd N, Minalga E, et al. (2013). In vivo evaluation of a breast-specific magnetic resonance guided focused ultrasound system in a goat udder model. Med Phys 40:073302.
- Peek MC, Ahmed M, Napoli A, et al. (2017). Minimal invasive ablative techniques in the treatment of breast cancer: a systematic review. Int J Hyperthermia 33:191–202.
- Peek MC, Ahmed M, Napoli A, et al. (2015). Systematic review of high-intensity focused ultrasound ablation in the treatment of breast cancer. Br J Surg 102:873–82. Discussion 882.
- Peek MC, Ahmed M, Scudder J, et al. (2016). High intensity focused ultrasound in the treatment of breast fibroadenomata: results of the HIFU-F trial. Int J Hyperthermia 32:881–8.
- Krekel NM, Zonderhuis BM, Stockmann HB, et al. (2011). A comparison of three methods for nonpalpable breast cancer excision. Eur J Surg Oncol 37:109–15.
- Kovatcheva R, Guglielmina JN, Abehsera M, et al. (2015). Ultrasound-guided high-intensity focused ultrasound treatment of breast fibroadenoma-a multicenter experience. J Ther Ultrasound 3:1.
- Cavallo Marincola B, Pediconi F, Anzidei M, et al. (2015). High-intensity focused ultrasound in breast pathology: non-invasive treatment of benign and malignant lesions. Expert Rev Med Dev 12:191–9.