Abstract
Purpose: Magnetic resonance-guided laser-induced thermal therapy (MRgLITT) is a minimally invasive procedure used to treat various intracranial pathologies. This study investigated the effects of variable power on maximal estimated thermal damage during ablation and duration required to reach maximal ablation.
Materials/Methods: All ablations were performed using the Visualase Thermal Therapy System (Medtronic Inc., Minneapolis, Minnesota), which uses a 980 nm diffusing tip diode laser. Cases were stratified into low, medium and high power. Maximal thermal damage estimate (TDEmax) achieved in a single plane and time to reach maximal damage (ttdemax) were measured and compared between groups using a 2×3 Fixed Factor Analysis of Covariance. Ablation area change for cases in which an initial thermal dose was followed by a subsequent dose, with increased power, was also assessed.
Results: We used real-time ablation data from 93 patients across various intracranial pathologies. ttdemax (mean ± SEM) decreased linearly as power increased (low: 139.2 ± 10.4 s, medium: 127.5 ± 4.3 s, high: 103.7 ± 5.8 s). In cases where a second thermal dose was delivered at higher power, the TDE expanded an average of 51.4 mm2 beyond the initial TDE generated by the first ablation, with the second ablation approaching TDEmax at a higher rate than the initial ablation.
Conclusion: Increased power results in a larger TDEmax and an increased ablation rate. In cases where an initial thermal dose does not fully ablate the target lesion, a second ablation at higher power can increase the area of ablation with an increased ablation rate.
Introduction
Magnetic resonance-guided laser-induced thermal therapy (MRgLITT) is a minimally invasive surgical technique that delivers laser energy through a fiberoptic catheter to achieve focal ablation of brain tissue in the treatment of various intracranial pathologies [Citation1]. This technique utilises magnetic resonance imaging (MRI) to visualise lesions pre- and post-operatively, as well as magnetic resonance thermal imaging (MRTI) to monitor thermal energy delivery intra-operatively [Citation1,Citation2]. The ability to monitor therapy in real time allows for strict control of the ablation area, and many previously inoperable lesions are now treatable with MRgLITT [Citation3–5]. To date, MRgLITT has been successfully applied in the treatment of many intracranial pathologies, including glioblastoma multiforme (GBM) [Citation6], ependymomas [Citation6], epilepsy [Citation7–10], metastatic tumours [Citation6,Citation11,Citation12] and intractable pain [Citation13]. Currently, there are two laser ablation systems approved for intracranial soft-tissue ablation: the Medtronic Visualase system (Medtronic Inc, Minneapolis, MN) and the Monteris NeuroBlate system (Monteris Medical, Plymouth, MN). The Visualase system utilises a 980 nm diffusing tip diode laser, while the NeuroBlate System utilises a 1064 nm diffusing tip diode laser [Citation14]. The data reviewed within this paper were collected using the Visualase system.
The understanding of ablation dynamics remains limited in the context of human intracranial treatments. Expanded use of MRgLITT requires a deeper understanding of how varying operative parameters can affect the dynamics of tissue damage during the delivery of thermal ablation. Important operative parameters subject to variation by the surgeon include laser power, temperature boundaries and ablation duration [Citation15]. Previous studies have evaluated the temporal profile of tissue damage by assessing the relationship between estimated thermal damage (TDE) and time, finding that expansion of the ablation area can either follow logistic or negative exponential growth as it approaches a finite maximum [Citation15]. However, the effects of laser power as an independent variable on this relationship remain largely unexplored. To date, authors have utilised laser powers ranging from 7.5 to 15 W with sufficient energy to achieve tissue ablation [Citation6,Citation12,Citation16]. Reported diameter of the ablated region ranges from 1.5 to over 3 cm, with the duration of reported total ablation time ranging from 0.5 to 26 min [Citation6,Citation9,Citation12,Citation16,Citation17]. These variations in laser power, maximum ablated area and duration of ablation underscore the need for relational analysis between these variables, as existing literature provides no indication for what power settings are most appropriate given a specific target. Rather, determination of laser intensity is left at the discretion of the laser operator based on prior experience and estimated size of the target. This uncertainty highlights the need to understand the role of power variations in laser ablation. To our knowledge, this is the first report in the literature that considers the effects of power on ablation dynamics when using a 980 nm diffusing tip diode laser during treatment of intracranial pathologies.
Methods
Patient selection
We retrospectively reviewed and selected a total of 93 patients who underwent MRgLITT for various intracranial pathologies at our institution. Pathology and patient demographics were not part of the selection criteria for this study. All patients underwent the procedure as part of the routine clinical care algorithm at our institution and were enrolled in an institutional review board-approved protocol.
Laser ablation procedure
The laser ablation procedure has previously been described [Citation2,Citation6,Citation18]. Briefly, each patient undergoes pre-operative CT and MRI. The MRI and CT images are loaded onto stereotactic planning software and fused (Medtronic S7; Medtronic Inc, Minneapolis, MN), allowing for registration and trajectory planning [Citation6]. Laser placement is accomplished via frameless stereotaxy using bone-implanted fiducials. Using intraoperative neuro-navigation, a small burr hole is created using a 3.2-mm twist drill. A Visualase Thermal Therapy System bone anchor is secured onto the calvarium. The laser catheter is introduced through the fixed bone anchor to its pre-determined trajectory length. The patient is transferred to the MRI suite where the ablation procedure is performed.
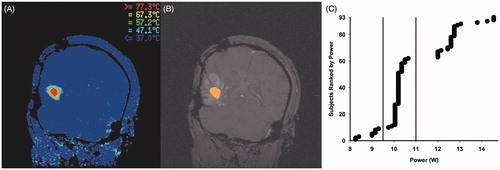
Magnetic resonance thermal imaging () provides real-time thermal monitoring of the procedure. Using the Visualase software, the operator identifies safety margins at the periphery and centre of the intended target such that the peripheral healthy tissue temperature does not surpass a predetermined temperature (50 °C) and the lesion centre does not exceed 90 °C to avoid steam and consequent undesired pressure. Following a short low-power test dose to confirm accurate laser placement, laser treatment is initiated by the operator at the user-determined power with the goal of achieving maximal target destruction. Cooling of the laser throughout the procedure is achieved via an enclosed irrigating catheter system, which promotes a clean dispersal of thermal energy [Citation6]. Ten possible irrigation rate settings are available to the operator. Irrigation rate was kept between levels 1 and 2 in each of the 93 cases. As heating commences, the laser creates an ellipsoid ablation, with the rate of growth in any one direction varying based on a number of factors, such as target morphology [Citation19]. The operator monitors the ablation area through interpretation of the TDE () on the control screen. The system software generates the real-time TDE using the Arrhenius rate model, which uses both the tissue temperature and the duration maintained at a specific temperature as primary variables [Citation15,Citation20]. This thermal imaging information is processed every 5 s, allowing for real-time monitoring of ablation area expansion throughout the procedure. In cases where a lesion requires more than one ablation, the laser catheter can be retracted along the longitudinal axis of the lesion by the surgeon between laser doses to expand the ablation area.
Data analysis
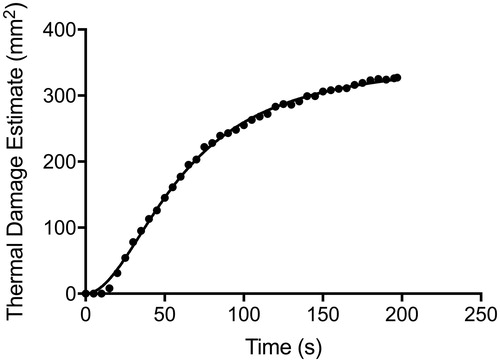
Cases were stratified into three groups by power: low (n = 9; range, 8.25–9.30 W), medium (n = 53; range, 9.75–10.65 W) and high power (n = 26, range, 12.00–14.55 W) as determined by their clustered distribution (). In certain cases, multiple doses were delivered at the target lesion; for analyses across the three groups, only the initial ablation from each case was considered in this study. The real-time TDE is measured as an area within a two-dimensional slice. The TDE zone is colour-coded in orange and is superimposed on the reference image. To estimate the area of ablation in each successive TDE image, we counted the number of superimposed colour-coded pixels in each frame (JPG format) using an image-processing software, GNU Image Manipulation Program version 2.8.4 (Free Software Foundation, Boston, MA). The field of view was set at 240 × 240 mm2 in each of the 93 cases analysed. Each pixel corresponds to 0.9 mm2. The TDE values derived from each successive 5 s frame are then plotted versus time to generate an ablation curve ().
During delivery of a thermal dose, the TDE gradually expands and asymptotically approaches a maximum (TDEmax) [Citation15]. The duration required to reach TDEmax is defined as ttdemax. Based on the ttdemax, we also calculated the time required to reach 50% (t50%) and 97% (t97%) of the maximal TDE after applying a least squares regression polynomial-smoothing filter for each TDE curve. To evaluate the rate of ablation independently of final ablation area, we measured the time to 100 mm2 of ablated area (t100mm) in each case and compared the results between power groups. One hundred square millimetre was chosen as the endpoint to evaluate because all 93 cases reached a TDE of at least 100 mm2. Finally, to further evaluate if increasing power resulted in a greater TDEmax, we identified 18 cases in which a single thermal dose was delivered at a target and allowed to reach maximal thermal damage, followed by a second thermal dose with increased power at the same site. Upon the second ablation reaching maximum thermal damage, we recorded the increase in ablated area between the two doses.
Statistical analysis
Ablation profiles were analysed using GraphPad Prism 7 (GraphPad Software, Inc, San Diego, CA). All statistical analyses were performed using SPSS version 24 (IBM, Armonk, NY). Multifactorial analysis of covariance (ANCOVA) was used to initially assess the impact of power among other factors upon laser ablation dynamics. Stepwise multiple regression analyses that paralleled the ANCOVAs were used to further characterise and separate out the impact of power upon the laser ablation dynamics. A p < 0.05 value was used to determine statistical significance.
Determination of statistical covariates
In order to utilise the full data set, several relationships between different endpoint conditions and measures were evaluated so as to determine whether they needed to be accounted for in the assessment of laser power. Because the area to be ablated is determined before the operation by the operator based on the size of the lesion, we constructed a generalised linear model (GLM) that accounts for these differences in final ablation area and allows us to evaluate how laser power affects the rate of ablation independent of intended target size. Thus, the ttdemax, t97% and t50% were used as dependent measures instead of the TDEmax, as the area to be ablated is determined before the start of the procedure by the operator based on the size of the target, whereas the operator is blind as to the exact duration it will take to achieve the intended ablation.
First, to assess whether each curve had achieved steady state (the point at which the ablation area reaches a maximum and no longer expands) at termination, the proportional time to achieve 97% of the maximal lesion estimate was calculated. It was assumed that if it took a proportionally long interval to cover the last 3% of volume lesioned, then the curve was near asymptotic values and considered complete. Conversely, if the proportional interval to climb the remaining 3% of the volume lesioned was fairly short, then the 97% was assumed to be much earlier in the curve with a much steeper slope (and thus had not achieved steady state). Based on these criteria, each ablation was designated as either complete, meaning the ablation had reached steady state, or incomplete, meaning that the ablation was terminated while the TDE was still expanding and had not reached steady state. It was determined that the proportional time to reach 97% of the ablation was correlated with ttdemax. Therefore, the proportional time to reach 97% of the maximal ablation estimate was included as a covariate in the final analysis of laser power, since the completeness of the ablation function impacts the influence of power. Additionally, whether the operator terminated the ablation manually or whether the tissue temperature limit was reached and automatically terminated the ablation was also analysed as a potential bias by contrasting the two conditions. Finally, although ttdemax, t97% and t50% were being used as dependent variables, TDEmax was still examined as a potential covariate in the final analyses of laser power, since it was assumed that larger lesions would take longer to ablate. Ultimately, both the proportional time to achieve 97% of the maximal lesion estimate and the TDEmax were included as continuous covariates. Additionally, mechanism of laser deactivation (manual termination or automatic termination due to temperature) along with the main fixed factor of interest (low, medium and high levels of power) were included as secondary fixed factors.
Results
Patient demographics
A total of 93 cases were analysed in this study. Patient pathology and demographics are summarised in .
Table 1. Patient information and operative indications.
Analysis of statistical covariates
The proportional time to achieve 97% of the maximal lesion estimate was the first factor considered as a covariate, as this indicates whether an ablation was able to achieve steady state. Steady state was achieved in 61% of ablations in the low-power group, 51% in the medium-power group and 31% in the high-power group. It was determined that the proportional time to reach 97% of the ablation was correlated with ttdemax (r= −0.605; p < 0.001) and was therefore included as a covariate in the final analysis of power. The mechanism by which the ablation was terminated (manual termination by the operator vs. automatic termination due to temperature limit breach) was also analysed as a potential covariate. Results indicated that laser deactivation by reaching the temperature tissue limits (93.4 ± 4.9 s; n = 53) occurred statistically sooner (F(1,91)=42.9; p < 0.001) than procedures deactivated manually by the operator (150.7 ± 7.1 s; n = 40), thereby qualifying mechanism of ablation termination as a fixed covariate. Finally, TDEmax was examined as a potential covariate, as it was assumed that larger lesions would require more time to ablate. A correlation verified this assumption (r(91)=0.249; p = 0.02), and as such, TDEmax was included as a continuous covariate.
Power versus time to TDEmax
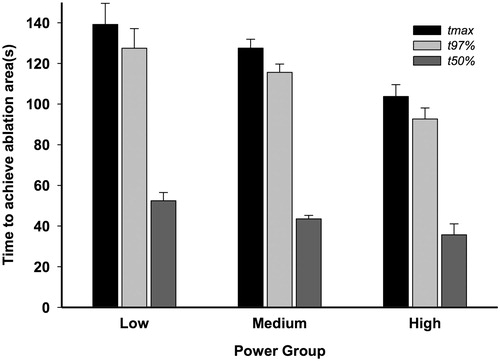
Results from a 2 × 3 Fixed Factor, ANCOVA, using both the proportional time to achieve 97% of the maximal lesion estimate and the TDEmax as continuous covariates and with ttdemax as the dependent variable, revealed a linear decrease in adjusted time as power increased (mean ± SEM) (low: 139.2 ± 10.4 s, medium: 127.5 ± 4.3 s and high: 103.7 ± 5.8 s, F(2,85)=6.935; p = 0.002). The fixed factor of laser termination mechanism was also found to be statistically significant (F(1,85)=17.510; p < 0.001). However, power and termination mechanism did not interact with each other, allowing for simple main effects (F(2,85)=0.733; p = 0.48).
Power versus time to t97% and t50%
Parallel ANCOVAs were run with both t97%, and t50% as dependent variables, with parallel results (t97%: low: 127.5 ± 9.6 s, medium: 115.6 ± 4.0 s and high: 92.7 ± 5.4 s, F(2,85) = 7.488; p = 0.001) and (t50%: low: 52.4 ± 4.1 s, medium: 43.5 ± 1.7 s and high: 35.7 ± 5.4 s, F(2,85) = 7.488; p = 0.001), respectively. Statistical interactions between the two fixed factors of ablation termination method and power were not found for the remaining dependent time measures either (t97%: F(2,85)=0.676; p = 0.51, t50%: F(2,85) = 0.487; p = 0.62).
Average adjusted time across the three laser power levels for the dependent variables of ttdemax, t97%, and t50% are depicted in .
Power versus time to TDE100mm
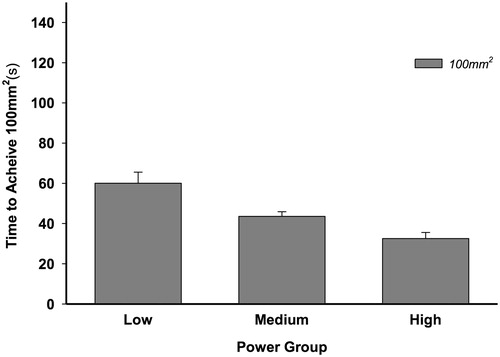
In evaluating time to achieve TDE100mm (t100mm), the continuous variables of proportional time to achieve 97% of the maximal lesion estimate and the TDEmax itself were no longer necessary to account for and thus were excluded from the model as covariates. A 2 × 3 fixed-factor analysis of variance (ANOVA) was performed parallel to the ANCOVA analyses above, with t100mm as the dependent variable. Results revealed a linear decrease in adjusted time as power increased (low: 60.1 ± 5.5 s, medium: 43.6 ± 2.3 s and high: 32.5 ± 3.0 s, F(2,84) = 7.068; p = 0.001) (). The fixed factor of termination type (manual or automatic) was found to approach statistical significance at (F(1,84) = 3.523; p = 0.06). As before, power and termination type did not interact with each other, allowing for simple main effects (F(2,84) = 0.719; p = 0.49).
Multiple regression models
Stepwise multiple regression models were constructed to mimic the ANCOVA analyses. The full models accounted for R2= 0.624 of ttdemax, R2 = 0.551 of t97% and R2 = 0.228 of t50%, respectively. For these models, power was coded similar to ANCOVA analyses as a fixed factor of one to three values matching low, medium and high values. Power coded in this way accounted for an R2 Change = 5.1% of ttdemax and an R2 Change = 6.7% of t97% while in the stepwise multiple regression model for t50%, power as a predictor accounted for insufficient statistical variance to be included. The models were then re-run, implementing power as a continuous variable using the full distribution of the 93 values (range: 8.25–14.55 W) to see if predictability improved. When run using continuous power variables, the full regression models accounted for near identical proportions of variance as before (R2 = 0.625 of ttdemax, R2 = 0.554 of t97% and R2 = 0.224 of t50%). Similarly, power coded as a continuous variable accounted for an R2 Change = 5.2% of ttdemax but accounted for more of the other two remaining dependent times, as power was computationally selected earlier in the regression models, resulting in an R2 Change = 10.5% of t97% and an R2 Change = 8.4% of t50%, respectively.
A final pair of stepwise multiple regression were run using t100mm as the dependent variable, which mimicked the ANOVA analyses performed earlier. The full model accounted for R2=0.232 of t100mm, with power coded as a fixed factor, matching one of low, medium or high power levels. Power coded in this way accounted for an R2 Change = 19.7% of t100mm. The final stepwise multiple regression coded power as a continuous variable, resulting in an overall model R2 = 0.208 of t100mm, and an R2 Change = 17.3% of t100mm, for power.
Second ablation, higher power
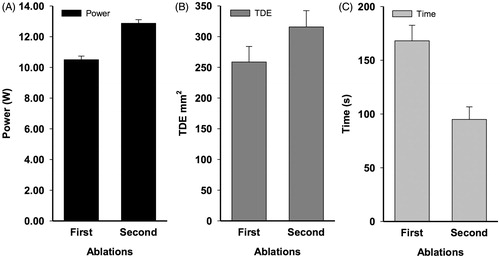
To evaluate the effect of increasing power on tissue that had already been ablated, we identified 18 cases where an initial ablation of a target lesion was followed with a subsequent thermal dose at higher power at the same site. Unlike the previous analyses, where a number of the ablations did not reach asymptote, all 18 initial ablations included in this analysis had reached steady state but had not achieved the ablation size desired by the operator. As such, the correlation between TDEmax and ttdemax was much stronger (r(18) = 0.514; p = 0.03). The mean power of the initial ablation was 10.50 W, while the average power of the second ablation was 12.88 W, with the average difference in power = 2.37 (). In all 18 cases, the second higher powered ablation resulted in an increase in the TDE area (F(1,18) = 94.717; p < 0.001) (). The second higher power thermal dose resulted in an average TDE expansion of 51.4 mm2 beyond the TDE generated by the initial ablation (range: 13.5–99.0 mm2). The second ablation with the higher thermal power dose also took a shorter time to accomplish than the initial ablation at the lower thermal power dose (F(1,18)=31.250; p < 0.001) ().
Figure 5. Average power of first and second ablations in cases where a single thermal dose was delivered and reached a maximum thermal damage estimate, followed by a second thermal dose delivered at the same site at higher power (A). Average TDEmax of first and second ablation (B). Average duration required to reach TDEmax in first and second ablation (C).
Discussion
Little has been reported on the effects of varying intraoperative parameters on the thermal dynamics seen during laser ablation. We performed MRgLITT using the Visualase system, which is one of the two FDA approved systems to perform this procedure for the treatment of various focal intracranial pathologies [Citation1,Citation6,Citation9,Citation12,Citation21–23]. In this study, we evaluated and compared the effects of variable power on maximal thermal damage estimate and the duration required to reach maximum ablation (ttdemax) between three groups; low, medium and high power. We evaluated and compared t50%, t97% and t100mm between power groups as well. Finally, we evaluated whether further TDE expansion occurs when a second thermal dose at higher power is administered to the same target following an initial lower power thermal dose. The GLMs and linear regression models constructed were able to account for more than half of the variance of the time measures that were near the end of the ablation curves (ttdemax and t97%), with power accounting for up to 10% of the variance, particularly when power was coded as original continuous values. For the time values drawn from earlier in the ablation curve (t50% and TDE100mm), the statistical models accounted for less variance overall. However, since the measures were not as complicated by the finishing dynamics (asymptotic completeness) of the ablation curves, power was able, in some instances, to account for nearly a fifth (19.7%) of the variance. Thus, overall, the degree of power delivered to the laser emitting tip can both hasten and enlarge a given ablation and can account for up to 10–20% of the given ablation dynamics. Additionally, the observation that power as a continuous variable in our regression model accounted for nearly an identical proportion of variance as when it was coded as a categorical variable strengthens our conclusions regarding the relationship between power and ablation rate.
The first report [Citation15] in the literature to quantitate ablation dynamics during treatment of intracranial pathologies demonstrated that TDE expansion increases at a decreasing rate as it approaches an asymptotic maximum, establishing that prolonged ablation yields diminishing increases in the volume of target tissue damage. In this study, our first goal was to determine whether increased laser intensity would result in a corresponding increase in maximal thermal damage. Intuitively, we hypothesised that higher power would result in a larger maximum area of ablation. However, this relationship proved difficult to evaluate, as naturally, higher powers are selected for ablations with larger target sizes, while lower powers are selected for smaller targets and for targets in close proximity to critical areas of the brain. Additionally, in evaluating our data set, we found that determining the effect of power on the final ablation size would be problematic, as a true steady state (the point at which the ablation area ceases to expand while the laser is still activated) was only achieved in approximately half of the ablations analysed (low: 61%, medium; 51%; high: 31%). This decreasing proportion of ablations reaching steady state across groups as power increased is itself noteworthy, as it suggests that at higher powers, complete target lesion ablation will likely be achieved before the TDE reaches a true maximum, or, that the temperature limit will be reached and trigger automatic deactivation of the laser. This is an important factor for the operator to consider, as higher power increases the risk of damage to healthy tissue beyond the boundaries of the target if left unmonitored. Nonetheless, evaluating the effects of power on true final ablation size would not be possible with the inclusion of cases that did not reach steady state, as the laser was deactivated in these cases before the ablation area stopped expanding. As such, we created a statistical model that allowed us to account for several different operative variables including final ablation size so that we could evaluate how power affected the time to reach 50%, 97% and 100% of TDEmax. Thus, our statistical model serves as an evaluator for ablation rate in the face of a constellation of intraoperative variables independent of the total area that the surgeon intends to ablate.
The ttdemax decreased linearly as power increased across the three groups, suggesting that at higher power, a shorter duration of laser-on time is required to fully ablate the target. An important factor that we considered that may have affected the total ablation duration (ttdemax) was whether the laser was deactivated manually by the operator or automatically due to breach of temperature limits. In cases where the operator manually deactivates the laser, it is possible that the ttdemax values observed are artificially elevated due to the operator’s belief that prolonged laser-on time would result in TDE expansion when in reality, the TDEmax had already been reached. This is more likely to have inflated the ttdemax of the low-power group, as more ablations at low power were permitted to reach steady state, and thus, have had prolonged laser-on time without further expansion. Nonetheless, by including mechanism of laser termination as a covariate in our statistical model, we were able to adequately account for this factor and its effect on ablation dynamics, finding that increasing power results in an increased ablation rate regardless of how the laser is deactivated. A previous study also noted the bias of prolonged laser-on time and its effect on ttdemax and suggested that t97% is a more accurate approximation of the optimal ablation time [Citation15]. For this reason, we also evaluated t97%, as t97% is robust to bias created by possible unnecessary prolonged ablation time seen in ttdemax. Our results showed that the t97% was reached in approximately 90 s in the high-power group, compared to just over two minutes in the low-power group. Coupling this with the fact that increased power was positively correlated with a larger TDEmax, we see that high power results in a larger TDE in a shorter duration of time. In other words, higher power results in an increased ablation rate and a greater potential final ablation area. These are especially important factors for the operator to consider when ablating regions in high-risk areas of the brain, as higher power settings will expand the ablation area more rapidly with increased ablation area potential, thereby augmenting the risk of damage to surrounding healthy tissue.
The results of the t50% parallel that seen across ttdemax and t97%. It is important to consider that there is still inherent bias within t50%, as larger lesions will result in a larger TDE, and thus, the final ablation area will have some effect on the duration required to reach 50% of maximal ablation. For this reason, our evaluation of t100mm provides a clearer assessment of rate. This is because unlike t50%, t100mm is a measure truly independent of final ablation area. Because the TDEmax was greater than 100 mm2 in all 93 cases, t100mm illustrates the time required to reach a fixed point while the TDE is still expanding in all cases. Our results showed that the t100mm decreased linearly as power increased, providing further evidence that higher power results in an increased ablation rate.
Our final analysis aimed to evaluate whether a second thermal dose delivered at higher power following an initial thermal dose at lower power would result in an increased TDEmax. Our analyses comparing the low-, medium- and high-power groups suggest that higher-power results in a larger area of ablation. However, because these analyses only consider the initial thermal dose delivered in each of the 93 cases, this conclusion is limited by variance that may be attributable to differences in pathology or other unknown factors. By considering cases where an initial thermal dose was delivered and allowed to reach steady state followed by a second thermal dose at higher power, we were able to directly evaluate whether increased power results in expansion of the ablation area within a single patient’s treated pathology. This is most relevant in the setting where the ablation at an initial power setting does not result in the desired ablation size. Our analyses show that increasing power at the target site does indeed result in expansion of the TDE, thereby resulting in a larger possible maximum ablation area. Furthermore, a second ablation at higher power results in a larger area of ablation in a shorter amount of time, providing further evidence that higher power results in a faster ablation rate. Because the second ablation is superimposed over previously ablated tissue, this may also suggest that subsequent ablation of previously damaged tissue will burn faster than undamaged tissue, though this observation should be investigated further in future studies. Nonetheless, operators can assume that increasing power in a subsequent ablation without adjusting the position of the laser will expand the TDE area should additional target destruction be necessary.
There are several limitations in this study. The majority of patients in this study received multiple, and often topographically overlapping, thermal doses; our analyses of low-, medium- and high-power groups only consider initial ablations. For this reason, these data are only applicable to ablations of undamaged tissue. In cases where the laser position is adjusted and additional overlapping thermal doses are delivered, the ablation profile may be affected by previous heat damage. It is important to note that while we did evaluate cases where a second thermal dose was delivered following an initial dose, this analysis is limited to cases where the positioning of the laser constant or was unadjusted. Future studies should evaluate the characteristics of tissue damage in subsequent overlapping ablations following adjustment of laser position. The greatest limitation in this study is the assumption that the information obtained by 2 D data can be extrapolated to a 3 D target. Even in multiple planes, the ablation volume would be an estimate at best, and the true volume of ablation cannot currently be calculated during the procedure. For now, we will have to assume that the real-time TDE is somehow positively correlated with the volume.
Another important point to note is that the data reported in this paper were acquired using the Visualase MRI-Guided Laser Ablation Technology. The Monteris NeuroBlate ablation system is also approved for intracranial ablation: however, this system utilises a different catheter design, thermal damage imaging graphic interface and laser wavelength. Additionally, the Visualase system disperses heat in all directions in ellipsoidal volume and requires repositioning of the laser catheter for maximal lesion coverage. In contrast, the NeuroBlate system employs radial projection of the thermal dose and allows the operator to adjust laser intensity and direction in successive doses to achieve maximal lesion coverage [Citation24]. For these reasons, the reported parameters and conclusions of this paper may not be applicable to the NeuroBlate system.
Another limitation is the inability to quantitate the effect of irrigation speed and temperature on ablation dynamics in this setting. The system itself allows for a somewhat qualitative assessment of irrigation speed, which makes this evaluation currently difficult. As previously noted, the irrigation rate was set between levels 1 and 2 in each of the 93 cases analysed. A previous study investigated the flow rate through the cooling catheter at each pump setting available (levels l–10), finding that rate of flow increases non-linearly with pump setting [Citation13]. However, it is still presently unknown whether ablation dynamics are affected within this small range of irrigation rate variability. Moving forward, there will need to be some attention paid to this variable, as it can be adjusted by the user during the procedure [Citation13].
The lack of stratification by pathology may be considered a weakness. Previous studies have shown that untreated glioblastoma multiforme (GBM) requires a longer duration to reach maximal ablation compared with recurrent metastasis/radiation necrosis and hippocampal ablation [Citation15]. A likely explanation for this difference is that tissue in recurrent metastasis/radiation necrosis and epilepsy may be poorly perfused relative to that of GBM, which tends to be well vascularised [Citation15]. Blood vessels may potentially act as heat sinks and accelerate the dissipation of heat, thereby requiring prolonged laser-on time. Considering this, it is likely that the inclusion of multiple pathologies with different histological characteristics contributed to a large degree of variance observed in many of our statistics. It will be of future interest to consider the effects of power while also taking pathology into account. Future studies should also investigate the effects of procedural variation on clinical results, such as whether outcomes are affected when utilising higher power for less time compared with lower power for a longer duration.
Conclusions
Increased power results in a larger area of ablation and an increased ablation rate. In cases where an initial thermal dose does not fully ablate the target lesion, a second ablation at higher power can increase the area of ablation at an increased rate. Future studies are required to examine the effects of previous therapies on ablation dynamics, as well how ablation dynamics across different pathologies are affected by power.
Disclosure statement
Dr. Danish is a consultant for Medtronic, and has received educational honoraria.
References
- Norred SE, Johnson JA. (2014). Magnetic resonance-guided laser induced thermal therapy for glioblastoma multiforme: a review. Biomed Res Int 2014:761312.
- Medvid R, Ruiz A, Komotar RJ, et al. (2015). Current applications of MRI-guided laser interstitial thermal therapy in the treatment of brain neoplasms and epilepsy: a radiologic and neurosurgical overview. AJNR Am J Neuroradiol 36:1998–2006.
- Kahn T, Bettag M, Ulrich F, et al. (1994). MRI-guided laser-induced interstitial thermotherapy of cerebral neoplasms. J Comput Assist Tomogr 18:519–32.
- Ascher PW, Justich E, Schrottner O. (1991). A new surgical but less invasive treatment of central brain tumours preliminary report. Acta Neurochir Suppl (Wien) 52:78–80.
- Bettag M, Ulrich F, Schober R, et al. (1991). Stereotactic laser therapy in cerebral gliomas. Acta Neurochir Suppl (Wien) 52:81–3.
- Jethwa PR, Barrese JC, Gowda A, et al. (2012). Magnetic resonance thermometry-guided laser-induced thermal therapy for intracranial neoplasms: initial experience. Neurosurgery 71(1 Suppl Operative):133–44.
- Curry DJ, Gowda A, McNichols RJ, Wilfong AA. (2012). MR-guided stereotactic laser ablation of epileptogenic foci in children. Epilepsy Behav 24:408–14.
- Esquenazi Y, Kalamangalam GP, Slater JD, et al. (2014). Stereotactic laser ablation of epileptogenic periventricular nodular heterotopia. Epilepsy Res 108:547–54.
- Willie JT, Laxpati NG, Drane DL, et al. (2014). Real-time magnetic resonance-guided stereotactic laser amygdalohippocampotomy for mesial temporal lobe epilepsy. Neurosurgery 74:569–84. discussion 84–5.
- Gonzalez-Martinez J, Vadera S, Mullin J, et al. (2014). Robot-assisted stereotactic laser ablation in medically intractable epilepsy: operative technique. Neurosurgery 10(Suppl 2):167–72. discussion 72–3.
- Rao MS, Hargreaves EL, Khan AJ, et al. (2014). Magnetic resonance-guided laser ablation improves local control for postradiosurgery recurrence and/or radiation necrosis. Neurosurgery 74:658–67. discussion 67.
- Torres-Reveron J, Tomasiewicz HC, Shetty A, et al. (2013). Stereotactic laser induced thermotherapy (LITT): a novel treatment for brain lesions regrowing after radiosurgery. J Neurooncol 113:495–503.
- Sinha S, Hargreaves E, Patel NV, Danish SF. (2015). Assessment of irrigation dynamics in magnetic-resonance guided laser induced thermal therapy (MRgLITT). Lasers Surg Med 47:273–80.
- Sloan AE, Ahluwalia MS, Valerio-Pascua J, et al. (2013). Results of the NeuroBlate System first-in-humans Phase I clinical trial for recurrent glioblastoma. J Neurosurg 118:1202–19.
- Sun XR, Patel NV, Danish SF. (2015). Tissue ablation dynamics during magnetic resonance-guided, laser-induced thermal therapy. Neurosurgery 77:51–8. discussion 8.
- Carpentier A, McNichols RJ, Stafford RJ, et al. (2011). Laser thermal therapy: real-time MRI-guided and computer-controlled procedures for metastatic brain tumors. Lasers Surg Med 43:943–50.
- Hawasli AH, Bagade S, Shimony JS, et al. (2013). Magnetic resonance imaging-guided focused laser interstitial thermal therapy for intracranial lesions: single-institution series. Neurosurgery 73:1007–17.
- Stafford RJ, Fuentes D, Elliott AA, et al. (2010). Laser-induced thermal therapy for tumor ablation. Crit Rev Biomed Eng 38:79–100.
- McGahan JP, Griffey SM, Budenz RW, Brock JM. (1995). Percutaneous ultrasound-guided radiofrequency electrocautery ablation of prostate tissue in dogs. Acad Radiol 2:61–5.
- Pearce JA. (2013). Comparative analysis of mathematical models of cell death and thermal damage processes. Int J Hyperthermia 29:262–80.
- Carpentier A, McNichols RJ, Stafford RJ, et al. (2008). Real-time magnetic resonance-guided laser thermal therapy for focal metastatic brain tumors. Neurosurgery 63(1 Suppl 1):ONS21–8. discussion ONS8–9.
- Carpentier A, Chauvet D, Reina V, et al. (2012). MR-guided laser-induced thermal therapy (LITT) for recurrent glioblastomas. Lasers Surg Med 44:361–8.
- Jethwa PR, Lee JH, Assina R, et al. (2011). Treatment of a supratentorial primitive neuroectodermal tumor using magnetic resonance-guided laser-induced thermal therapy. J Neurosurg Pediatr 8:468–75.
- Mohammadi AM, Hawasli AH, Rodriguez A, et al. (2014). The role of laser interstitial thermal therapy in enhancing progression‐free survival of difficult to access high-grade gliomas: a multicenter study. Cancer Med 3:971–9.