Abstract
The neural responses during hyperthermia, once thought of as simple physiological processes (e.g. thermal sensation and regulation), have now been recognised involving more cognitive processes, which would be of high importance to the management of those occupations during heavy heat exposure. Previous studies have demonstrated altered activity in localised subcortical clusters for thermal sensation and regulation, as well as cortical–cortical activity for behavioural tasks during hyperthermia. However, the involvement of cortical–subcortical activity during hyperthermia has not been investigated. In this study, we performed exploratory analyses comparing thalamocortical functional connectivity during whole body hyperthermic condition for an hour at 50 °C and normothermic condition at 25 °C. We found weakened functional connectivity of cortical fronto-polar/anterior cingulate cortex and prefrontal areas with the corresponding thalamic nuclei during hyperthermic versus normothermic comparisons. On the contrary, the motor/premotor, somatosensory and temporal cortical subdivisions showed increased connectivity with thalamic nuclei during hyperthermia. Thalamocortical connectivity changes in the prefrontal were identified to be correlated with the behavioural reaction time during psychomotor vigilance test after controlling for physiological variables. These distinct thalamocortical pathway alterations might reflect physiologically thermal sensation and regulation, as well as psychologically neural behaviour changes underlying cortical–subcortical activity during hyperthermia.
Introduction
Human body has an impeccable thermal sensation and regulation system with countless thermal receptors in the superficial skin, spinal cord and central nervous system. Thermal sensation and regulation systems have sufficient afferent and efferent neural pathways that contribute to monitoring internal temperature, superficial temperature, regulating internal heat production and heat dissipation [Citation1]. Once thought of as simple physiological processes (e.g. thermal sensation and regulation), the neural responses during hyperthermia have now been recognised involving more cognitive processes, such as emotions and behaviours, which would be highly probable to cause safety accidents [Citation2,Citation3]. It is of high importance to investigate the neural mechanisms of cognitive dysfunctions during hyperthermia, as it could be potentially beneficial to the management for those occupations during heavy heat exposure [Citation4].
Using functional and structural imaging approaches, the neuroanatomy of thermal sensation and two distinct thermoregulations (autonomic and behavioural thermoregulation) have been progressively revealed out [Citation5,Citation6]. Diverse neural clusters including amygdala, orbital frontal cortex, cingulate cortex, somatosensory cortex and insular cortex in the high-level cortical cortex contribute to thermal sensation and regulation [Citation6–8]. Recent behavioural and neuroimaging studies showed that the hyperthermia-induced abnormal activity in these clusters was involved in brain functions during resting state, visual attention network task and working memory task performing [Citation9–11]. Using an attention network test, Liu et al. [Citation10] reported regional enhanced activations in fronto-parietal cortex, which were correlated with aberrant executive control performance and core temperature. Regarding resting-state coritical–cortical functional connectivity, Qian et al. [Citation12] found that decreased brain connectivity was mainly associated with the medial orbital frontal cortex, temporal lobe and occipital lobe, whereas increased brain connectivity was mainly located within the limbic system. These findings of altered activity in high-level cortex implicated that the neural responses of hyperthermia on human brain might be physiological- and psychological-related process. However, these previous studies focussed on the cortical activity related to high-level cognition and behaviour; the relationships with subcortical activity that was mainly related to low-level thermal sensation and regulation were neglected.
With unique cytoarchitecture and firing patterns in human brain, the thalamus acts as a core structure that contains wide-spread connections with distinct zones of the cerebral cortex, named thalamocortical network previously [Citation13,Citation14], providing a valuable approach for imaging cortical–subcortical neural activity [Citation15]. The thalamus acts as a key hub of spinothalamic tract and thalamocortical radiations, involving with both sensory information transmission from bottom neural activity and cognition processing from top neural activity [Citation16]. The former one supports the information transmission of pain, temperature, touch and pressure, whereas the latter one contains a large number of fibres that extend from different nuclei of the thalamus and projects to visual cortex, somatosensory cortex, auditory cortex and prefrontal cortex, supporting high-level functions, such as consciousness regulation, alertness, executive control and so on. During recent years, emerging studies using functional connectivity and structural diffusion tracts have demonstrated that thalamocortical connectivity acts as a remarkable indicator for altered cortical–subcortical brain activity in schizophrenia, epilepsy and attention-deficit/hyperactivity disorder [Citation14,Citation17–19].
To this end, the current study was undertaken to explore whether the specific patterns of thalamocortical functional connectivity are differentially disrupted during whole body hyperthermia. Methodologically, using the similar research strategy by Woodward and Heckers [Citation15], we performed exploratory analyses comparing thalamocortical dysconnectivity during hyperthermia and the physiological and cognitive correlates of thalamocortical connectivity.
Materials and methods
Participants
Twenty-eight healthy right-handed young male participants (24.4 ± 1.5 years, ranging from 20 to 27 years) took part in the present study. All the participants had no history of neurological or psychiatric disorders and never participated in similar fMRI experiments before. The protocol in accordance with the Declaration of Helsinki was approved by the Jinan Military General Hospital. Written informed consents were obtained from all the participants before the experiment.
Experiment procedure
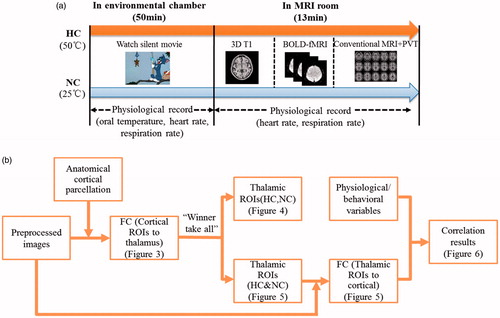
depicts the flow chart of the experiment design. Two thermal conditions were performed: a normothermic condition (NC, 25 °C and relative humidity (rH) of 60%) and a hyperthermic condition (HC, 50 °C and 60% rH) in a counterbalanced order with 3–7 d apart and at the same time of day, between 4:00 pm and 6:00 pm. The participants first underwent the heat exposure in an environmental chamber for 50 min, watching a silent movie. The temperature and humidity inside the chamber could be set arbitrarily using two temperature controllers (NTFA-18, Gree, Zhuhai, Guangdong, China) and two humidifiers (LH8809, Weiran, Foshan, Guangdong, China). After the 50-min heat exposure inside the chamber, the participants were taken to the MRI room for scanning. During the scanning, the heat exposure was continued. The heat was produce by a thermal-lab suit that was designed with a soft embedded pipeline. During MRI scanning, temperature-controlled hot water was pumped into the pipeline to simulate environmental heat. To minimise the time interval of both stages, the participants wore the thermal suits in the environmental chamber before the MRI scanning. The scanning sequence was designed in the order of 3D T1, blood oxygen level-dependent (BOLD) fMRI and conventional MRI, and lasted about 13 min.
During the last five minutes of conventional MRI scanning, a highly attention demanding task, psychomotor vigilance test (PVT), was performed to evaluate the sustained attention performance at the same time. Stimuli were projected from a computer via a projector to a screen at the foot of the scanner. Participants could see the stimuli through a mirror above the head coil and respond with a button press, after which their reaction time would be displayed for 1 s. Inter-stimulus intervals were randomised from 2 to 8 s. In the data analysis, we extracted the mean reaction time as a measure of overall level of attention performance.
Additionally, during the heating exposure in the environmental chamber and MRI scanner, physiological variables were measured intermittently once every 5 min, including oral temperature, heart rate and respiration rate. The oral temperature was collected from a sublingual thermocouple. And participants were asked to maintain their mouths closed during scanning to prevent artefactual cooling of the probe. Due to the unavailability of the equipment in the magnetic field, the temperature was not recorded during the scanning. The heart rate and respiration rate were still collected using the MRI scanner built-in ECG leads and breathing belt.
Data acquisition and preprocessing
The resting-state fMRI images were obtained using a GE MR750 3.0 T scanner (General Electric, Milwaukee, WI), with the parameters as follows: TR = 2000 ms, TE = 30 ms, flip angle (FA) = 90°, number of slices = 33, matrix = 64 × 64, field of view (FOV) = 24 × 24 cm2 and thickness/gap = 4/0 mm. Each scan consisted 210 EPI functional volumes. Additionally, after fMRI scanning, a high resolution T1-weighted sequence was obtained: 115 slices, TR = 11.1 ms, TE = 4.9 ms, slice thickness = 1.4 mm, FOV = 24 × 24 cm2 and FA = 20°.
Preprocessing was carried out using Statistical Parametric Mapping 8 package. The first 10 volumes were removed. The remaining 200 images were processed involving serial steps: slice timing correction, motion correction and spatially coregistration to structural images, normalisation into the standard Montreal Neurological Institute space. Consistent with prior studies [Citation14,Citation15], no spatial smoothing was applied. To remove signal drift and high-frequency noise, linear trend removing and bandpass filtering between 0.01 and 0.1 Hz were administered using custom-made software. Several sources of spurious variance were removed from the data via linear regression: signals from white matter, global mean signal, cerebrospinal fluid and head motion. Additionally, considering that the physiological differences between both thermal conditions might disturb the functional connectivity, we also regressed out the physiological variables including the mean rectal temperature, heart rate and respiration rate as covariate factors. Head motion correction results showed that images of three participants should be discarded due to displacement greater than 1 mm or rotation greater than 1°; thus, the images of remaining 25 participants would come into following data analysis.
Data analysis
As described in prior studies [Citation13,Citation20,Citation21], investigations of thalamocortical connectivity were mainly implemented in two ways: first, using the entire thalamus as the seed ROI with the cortical cortex in a voxel-wise manner; second, parcelling the cortex into large, anatomically divided ROIs with the thalamus in a voxel-wise manner. The first method averaged BOLD signals from the entire thalamus, precluding the analysis of connectivity between nuclei within thalamus and cortical cortex. The second method might limit spatial specificity within the cortex with the use of large cortical ROIs. To overcome these limitations, we combined elements of both methods. depicts the flow chart of the data processing. First, the cortical cortex was divided into seven ROIs based on anatomical landmarks using WFU_PickAtlas software. Similar with the approaches used in the previous studies [Citation13,Citation14,Citation21], the cortical ROIs were defined as: (1) the somatosensory cortex (BA1,2,3,5), (2) the motor and premotor areas (BA4,6), (3) the posterior parietal cortex (BA7, 39, 40, posterior cingulate cortex, precuneus, supramarginal and angular gyrus), (4) the prefrontal cortex excluding frontal polar and anterior cingulate cortex (ACC) (BA 8, 9, 32, 45, 46, 47), (5) the fronto-polar cortex and ACC (BA 10, 11, 24, 25), (6) the occipital lobe (BA 17, 18, 19, 37) and (7) the temporal lobe (BA 20, 21, 22, 34, 36, 38). We calculated functional connectivity mapping from cortical ROIs to thalamus in a voxel-wise manner. Then, paired t comparison on the functional connectivity mapping during both thermal conditions was performed to investigate the thalamocortical connectivity alterations with the thalamic nuclei. Besides, the functional connectivity strength was compared for each cortical ROI within each voxel in the thalamus. The cortical ROI that correlates strongest with the voxel would “win.” And the voxel would be colour coded with the corresponding cortical ROI. This was called “winner take all” strategy [Citation13–15,Citation20,Citation21]. Using this method, we parcelled the thalamus into functional ROIs for HC group and NC group. And also we calculated the thalamic ROIs combining the images of HC and NC groups. Using these ROIs, functional connectivity from thalamic ROIs to cortical cortex in a voxel-wise manner was calculated and paired compared in both thermal conditions. Given that the functional connectivity might be affected by physiological disturbance during hyperthermia, especially, in the HC session of fMRI, the participant would move more and have different physiological responses, we performed covariate regression involving the physiological factors and head motion during paired comparison on the functional connectivity between both sessions. The physiological variables, including the rectal temperature, heart rate and respiration rate, were calculated by averaging the recording data during the experiment. The head motion was obtained by averaging volume-to-volume motion as Utevsky et al. [Citation22] did. The paired comparisons thresholded at cluster-level corrected q = 0.05 (false discovery rate, FDR correction) for voxel-level corrected p = 0.05. Lastly, to evaluate the possible contributions of neural variables to behavioural performances, we should perform correlation analysis for them after controlling non-neural variables. Therefore, two-tailed partial correlation analysis was performed between the reaction time of PVT and mean functional connectivity from thalamic ROIs to cortical areas, controlling for physiological parameters including oral temperature, heart rate and respiration rate, as well as head motion.
Results
Physiological changes
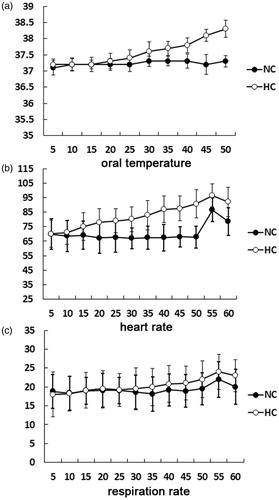
As expected, heat exposure for approximately one hour caused continuous physiological changes. All the parameters in the NC condition exhibited stability throughout the measurement, whereas the oral temperature and heart rate progressively increased during the hyperthermia. As depicted in , the oral temperature progressively increased from 37.1 ± 0.2 °C in the beginning to 38.3 ± 0.26 °C in the end. The heart rate increased from 70.2 ± 8.5 in the beginning to 93.2 ± 9.5 in the end. The respiration rate showed a little progressive rise during the experiment, but did not reach statistically significance.
Thalamocortical connectivity: cortical ROIs to thalamus analysis
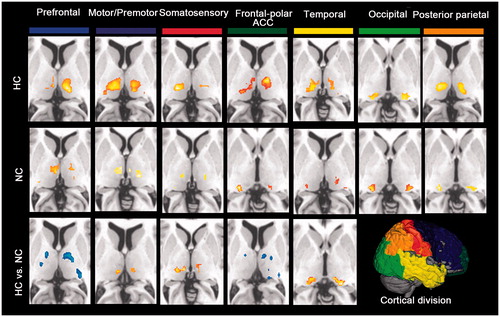
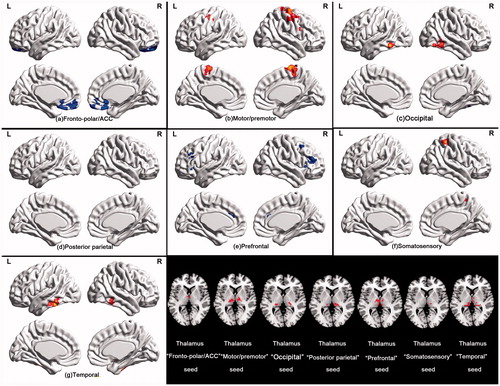
Consistent with previous studies, each cortical region was specifically connected to distinct nuclei within the thalamus (). Specifically, thalamic connectivity with the somatosensory and motor/premotor subdivisions was mainly located in the ventral posterior lateral nucleus in both groups. Paired comparison showed increased thalamic connectivity in the ventral lateral nucleus with the cortical somatosensory and motor/premotor areas during hyperthermia. The prefrontal subdivision was robustly connected to medial dorsal nucleus in both groups. Between-group comparison revealed that decreased thalamic connectivity in the bilateral medial dorsal nucleus and left ventral posterior lateral nucleus with the prefrontal ROI was found in HC group. The frontal polar and ACC showed strong connections to ventral medial and lateral nucleus in the hyperthermic condition, whereas to ventral medical nucleus and pulvinar in the normal condition. Paired comparison revealed decreased connectivity of the frontal-polar and ACC with bilateral ventral anterior nucleus and left pulvinar during hyperthermia. The temporal cortex in both conditions showed strong correlation with posterior medial nucleus. But increased correlation with the bilateral pulvinar in the HC condition during the paired comparison. The posterior parietal cortex correlated strongly with lateral posterior nucleus and pulvinar in the HC group, whereas with ventral pulvinar in the NC group. The occipital ROIs showed strong connections to ventral lateral nucleus. But no significant differences were found by posterior parietal cortex and occipital ROIs during group-paired comparisons.
Figure 3. Functional connectivity mapping of the thalamus for each cortical ROI in each thermal condition and the paired comparison. The most prominent differences between both conditions showed decreased frontal-thalamic (including cortical fronto-polar/ACC and prefrontal areas), increased sensorimotor-thalamic (including somatosensory and motor/premotor areas) and temporal-thalamic connectivity during hyperthermia.
Dynamical functional thalamic ROIs and thalamocortical connectivity mapping within cortical ROIs
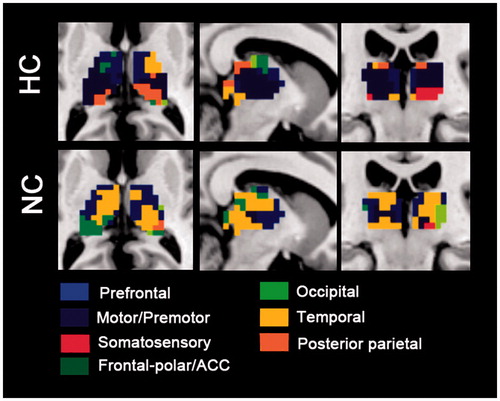
Using the winner take all approach, we found altered spatial extent and intensity of functional connectivity from cortical ROIs to thalamus (). The spatial extent of fronto-polar/ACC and prefrontal and temporal ROIs with the thalamus were decreased during hyperthermia, whereas the spatial extent of motor/premotor, posterior parietal and somatosensory ROIs to the thalamus were enlarged. No spatial extent alteration was found in the occipital ROI.
Figure 4. “Winner take all” displays the altered spatial extent functional thalamic ROIs between both thermal conditions. In HC group, sensorimotor-thalamic connectivities encompass a greater portion of the thalamus, whereas prefrontal and temporal areas encompass much less of the portions of the thalamus.
To characterise the functional connectivity from thalamic ROIs to cortical areas, seed-based functional connectivity analysis of thalamic ROIs with the cortical cortex in a voxel wise manner was performed. Considering the dynamical thalamic ROIs between thermal conditions, we got thalamic ROIs based on the dataset of all the individuals using winner take all strategy (). Taking these ROIs as seeds, both hyperconnectivity and hypoconnectivity were exhibited with their corresponding cortical ROIs. As depicted in , participants exhibited hypoconnectivity between the thalamic “prefrontal” seed and right superior frontal gyrus, left medial superior frontal gyrus and left middle frontal gyrus in the hyperthermic condition. Furthermore, thalamic “fronto-polar/ACC” seed connectivity with orbital medial superior frontal gyrus and ACC was also reduced, whereas thalamic “motor/premotor” and “somatosensory” seeds showed hyperconnectivity with cortical areas. The former one exhibited greater connectivity with bilateral precentral gyrus, paracentral lobule and right middle frontal gyrus. The latter one showed greater connectivity with bilateral postcentral gyrus. The thalamic “temporal” seed showed increased connectivity with bilateral posterior inferior temporal gyrus. No significant altered connectivity was found on the thalamus “occipital” and “posterior parietal” seeds. In a more relaxed threshold with voxel-level threshold p = 0.01 but no FDR correction, the thalamus “occipital” seed showed greater connectivity with bilateral fusiform gyrus and inferior occipital gyrus.
Figure 5. Paired comparison for functional connectivity mapping of the functional thalamic seeds with cortical cortex in both thermal conditions. Functional connectivity of the thalamic “fronto-polar/ACC” and “prefrontal” seeds was markedly attenuated during hyperthermia, whereas functional connectivity of the thalamic “motor/premotor”, “occipital”, “temporal” and “somatosensory” seeds appeared higher during hyperthermia.
Neuro-behavioural correlations
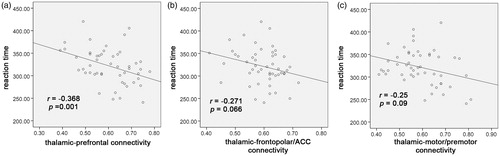
To further assess the potential behavioural implications of thalamocortical connectivity, we performed partial correlation between the averaged functional connectivity from thalamic ROIs to cortical areas and the behavioural performance including data in both conditions (). The results showed that thalamic-prefrontal connectivity significantly predicted with the reaction time of PVT (r = –0.368, p = 0.001). However, the thalamic-frontopolar/ACC connectivity (r = –0.271, p = 0.066) and thalamic-motor/premotor connectivity (r = –0.25, p = 0.09) showed a trend of correlation with the behavioural performance but did not reach statistical significance. The other thalamic-cortical connectivity, including thalamic-occipital connectivity (r = –0.071, p = 0.634), thalamic-temporal connectivity (r = –0.067, p = 0.653), thalamic-posterior parietal connectivity (r = –0.161, p = 0.28) and thalamic-somatosensory connectivity (r = –0.17, p = 0.253), showed no correlations with behavioural performance after controlling for non-neural variables.
Figure 6. Partial correlation analysis on the thalamocortical functional connectivity with the behavioural performance revealed significant correlations of the reaction time with the thalamic-prefrontal connectivity, but a trend of correlations with the thalamic-frontopolar/ACC connectivity and thalamic-motor/premotor connectivity.
Discussion
In this study, we replicated previous findings about spatially distinct thalamic correlations with cortical cortex. Considering wide-spread connections from thalamus to distinct zones of cortical cortex, thalamocortical functional connectivity provides a valuable approach to investigate cortical–subcortical activity during hyperthermia. The current study demonstrated cortical–subcortical disruptions that were characterised by both thalamocortical hypoconnectivity and hyperconnectivity during hyperthermia. Specifically, the combination of decreased frontal-thalamic (including cortical fronto-polar/ACC and prefrontal areas) and increased sensorimotor-thalamic (including somatosensory and motor/premotor areas) connectivity was the most striking aspect of our study. These alterations in thalamocortical pathways were related to slower reaction time in PVT task, suggesting hyperthermia-induced thermal sensation, regulation and neural behaviour changes underlying cortical–subcortical activity.
Paired comparisons on thalamocortical functional connectivity revealed weakened thalamic-prefrontal connectivity, specifically between the medial dorsal nucleus in the thalamus and right superior frontal gyrus, left medial superior frontal gyrus and left middle frontal gyrus in the prefrontal area during hyperthermia. The medial dorsal thalamus has been previously reported to bear a striking resemblance to fronto-parietal or executive control networks [Citation23]. This cortical–subcortical network supports human high-level cognitive functions, including working memory, executive control, vigilance, flexibility and planning. Anatomically, the medial dorsal thalamus acts as primary nucleus exchanging excitatory activity with the prefrontal cortex, which is responsible for carrying out these high-level cognitive functions [Citation24,Citation25]. The network has been proved to be based on solid intrinsic brain activity and white matter fibre [Citation13]. Deficits of prefrontal-dorsomedial thalamus connectivity in both human and animals could cause damaged performance of working memory and executive control functions [Citation15,Citation26]. Supporting that, the PVT reaction time was significantly correlated with the prefrontal-thalamic connectivity. During attention task, heat exposure exerts its detrimental effects on performance by competing for and eventually draining attentional resources, especially, for high cognition-demanding task [Citation3,Citation27]. Weakened thalamic-prefrontal connectivity provided more convinced evidence for deteriorated working memory [Citation28,Citation29], executive control [Citation30], electrophysiological responses [Citation2] and neuroimaging findings [Citation10,Citation11].
Considering prior findings of specific functions of orbital frontal cortex on thermal sensation, regulation and emotions during heat stress [Citation6,Citation9], the frontopolar/ACC was divided as a single cortical ROI from the prefrontal subdivision. We found weakened thalamic-frontopolar/ACC connectivity, specifically, between the ventral anterior nucleus and left pulvinar in the thalamus and the medial superior frontal gyrus and ACC. The orbitofrontal cortex and ACC associated with the behavioural thermal regulation have wide-spread connectivity with the ventral part of the thalamus. The orbital frontal cortex and ACC usually respond to discomfort sensation of thermal stimuli [Citation6]. Given the evidence that the orbital frontal cortex and ACC contribute to some vegetative behaviours and widespread fibres with thalamus, the decreased functional connectivity may relate to an affective component of thermal sensation and regulation responses [Citation31]. Passive hyperthermia caused negative affectivity for thermal sensation and comfort, which might drive the participant to trigger conscious decisions to preserve thermal balance or escape from the noxious thermal stimuli when it is possible (e.g. remove the body from the thermal lab suit or expect the experiment to end earlier).
Hyperconnectivity in somatomotor-thalamic pathway was mainly located in the ventral nuclei of the thalamus with the precentral and postcentral gyrus. Both functional and structural thalamocortical connectivity mappings have demonstrated that cortical somatosensory had solid connectivity with the ventral posterior part of thalamus and motor/premotor area with the ventral anterior and lateral parts of thalamus. These nuclei work as the key hubs for sensory and motor information processing from spinal cord, brain stem and basal ganglia to cortical cortex [Citation32]. The hyperconnectivity in present study showed an enhanced interaction between cortical somatomotor area and thalamic nuclei during hyperthermia. Somatosensory and motor/premotor areas were reported to be associated with behavioural thermoregulation. The activation of somatosensory cortex was correlated with the intensity of the thermal stimuli [Citation5]. Motor/premotor plays a vital role in behavioural thermoregulation as cortical controller of behaviour. Thermal sensation and regulation is a multidimensional experience involving sensory, affective, cognitive, as well as vegetative and motor components. The immediate consequence of thermal stimuli is to withdraw the body from the heat environment. This might be the reason for enhanced motor and premotor connectivity to thalamus. The neuronal information processes necessary for motor control were proposed as a network within which the thalamus appears to be a subcortical motor centre [Citation33]. Investigations of the pathways of the cerebellum to thalamus and multiple motor cortices suggested that the role of thalamus fulfils a key function in the modulation of movement processing based on cognitive requirements [Citation34]. Alterations in the somatomotor-thalamic pathways would cause distorted somatosensory functions in patients with multiple sclerosis and schizophrenia [Citation14,Citation35].
It is worth mentioning that thalamic-prefrontal hypoconnectivity and somatomotor-thalamic hyperconnectivity were inversely correlated in previous studies [Citation15,Citation20], suggesting that functional connectivity alterations in both pathways might be related. Disrupted function of mediodorsal thalamus was reported to be related to the thalamic-prefrontal hypoconnectivity and corresponding somatomotor-thalamic hyperconnectivity. Inhibiting the projections from mediodorsal thalamus to prefrontal cortex using the microinjection of gamma-aminobutyric acid agonists could cause raised motor responses in rats [Citation36]. Recent studies found that mediodorsal thalamus hypofunction disrupts thalamic-prefrontal connectivity and causes cognitive deficits [Citation37,Citation38]. Therefore, the alterations of thalamic-prefrontal and somatomotor-thalamic connectivity in present study may, in part, account for the alteration of each other during hyperthermia. This awaits further confirmation on the activity in the mediodorsal thalamus.
Additionally, the temporal-thalamic connectivity was increased in the thalamic pulvinar with the posterior inferior temporal gyrus. This finding might reflect neural activity redistribution within temporal lobe. The inferior temporal gyrus processes visual stimuli of objects in our field of vision and is associated with memory and memory recall to identify that object. It is associated with the processing and perception created by visual stimuli, comparing that processed information to stored memories of objects to identify the object [Citation39]. Disrupted connections in the pathways might result in altered visual information perception and processing. This finding might provide potential explanations for our previous study, which declared impaired early stage of face recognition during hyperthermia [Citation40]. Additionally, compared with the frontal-thalamic and somatomotor-thalamic connectivity, the temporal-thalamic connectivity was relatively preserved during hyperthermia. The diversity indicated hyperthermia had selective impact on cortical-thalamic pathways.
Our study has several limitations. First, we investigated the thalamocortical connectivity based on BOLD signals that might be affected by physiological changes, such as cerebral blood perfusion or blood redistribution during hyperthermia. Several transcranial Doppler sonography studies confirmed decreased mean blood velocity in the middle cerebral artery after heat exposure for 2–3 h, indicating reduced blood supply. However, more liberal intensity of heat exposure (1 h) was conducted in present study. Our previous study has confirmed that global cerebral blood flow (CBF) remained stable under such intensity of heat exposure [Citation41]. We held the opinion that CBF fluctuations during such liberal heat intensity may have little influence on BOLD signals. Still, hyperthermia might cause some regional CBF alterations [Citation41,Citation42], as well as accelerated heat rate, respiration rate and metabolism, which potentially interfere with the BOLD signals. Furthermore, the functional connectivity might suffer from important pitfalls that limit its insight into neuronal coupling [Citation43]. Changes in functional connectivity could reflect changes in another connection, observational noise or neuronal fluctuations [Citation44]. Unlike functional connectivity, employing computational approaches that estimate effective connectivity (e.g. psychophysiological interactions analysis, PPI) can quantify directed relationships between brain regions and control for confounds that limit functional connectivity [Citation45]. Especially, in a meta-analysis study by Smith et al. [Citation45], different types of PPI studies that used similar seeds and contexts would consistently converge on similar target regions, supporting the robustness of PPI as a tool for studying functional integration. In future, we would address the thalamocortical connectivity during a designed task using an effective connectivity model to avoid the pitfalls in current study. It is worth mentioning that the “winner take tall” approach that based on functional connectivity comparisons should also be interpreted cautiously. Second, the reversibility or dynamics of thalamocortical connectivity under heat exposure merits further consideration. The weakened prefrontal-thalamic and increased somatomotor-thalamic connectivity during hyperthermia might return to the normal state after removing heat for some time. This issue awaits much more group comparisons. Third, the heat exposure procedures in current study were inhomogenous, including two ways: environmental chamber and thermal-lab suit, which might produce different thermal intensity and sensation for participants. The methodological discrepancies might make it difficult to repeat the heat exposure in other studies. However, wearing a thermal-lab suit lying in the MRI room for an hour would produce a high experiment cost, as well as impatience of participants. Therefore, the 50-min heat exposure in the chamber was a compensatory method for unavailability of long-time heat exposure in the MRI scanner.
In conclusion, to explore the cortical–subcortical activity during hyperthermia by thalamocortical connectivity analysis, the present study obtained decreased frontal-thalamic, temporal-thalamic and increased sensorimotor-thalamic functional connectivity. Altogether, our results reflect hyperthermia-induced thermal sensation, regulation and neural behaviour changes underlying cortical–subcortical activity. These distinct thalamocortical connectivity changes revealed that the different thalamocortical pathways exist for hyperthermia-induced physiological and psychological disruptions.
Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Romanovsky AA. (2007). Thermoregulation: some concepts have changed. Functional architecture of the thermoregulatory system. Am J Physiol Regul Integr Comp Physiol 292:R37–46.
- Hocking C, Silberstein RB, Lau WM, et al. (2001). Evaluation of cognitive performance in the heat by functional brain imaging and psychometric testing. Comp Biochem Physiol A Mol Integr Physiol 128:719–34.
- Hancock PA, Vasmatzidis I. (2003). Effects of heat stress on cognitive performance: the current state of knowledge. Int J Hyperthermia 19:355–72.
- Smith DL, Petruzzello SJ, Kramer JM, Misner JE. (1997). The effects of different thermal environments on the physiological and psychological responses of firefighters to a training drill. Ergonomics 40:500–10.
- Rolls ET, Grabenhorst F, Parris BA. (2008). Warm pleasant feelings in the brain. Neuroimage 41:1504–13.
- Flouris AD. (2011). Functional architecture of behavioural thermoregulation. Eur J Appl Physiol 111:1–8.
- Dimicco JA, Zaretsky DV. (2007). The dorsomedial hypothalamus: a new player in thermoregulation. Am J Physiol Regul Integr Comp Physiol 292:R47–63.
- McAllen RM, Farrell M, Johnson JM, et al. (2006). Human medullary responses to cooling and rewarming the skin: a functional MRI study. Proc Natl Acad Sci U S A 103:809–13.
- Sun G, Qian S, Jiang Q, et al. (2013). Hyperthermia-induced disruption of functional connectivity in the human brain network. PLoS One 8:e61157.
- Liu K, Sun G, Li B, et al. (2013). The impact of passive hyperthermia on human attention networks: an fMRI study. Behav Brain Res 243:220–30.
- Jiang Q, Yang X, Liu K, et al. (2013). Hyperthermia impaired human visual short-term memory: an fMRI study. Int J Hyperthermia 29:219–24.
- Qian S, Sun G, Jiang Q, et al. (2013). Altered topological patterns of large-scale brain functional networks during passive hyperthermia. Brain Cogn 83:121–31.
- Zhang D, Snyder AZ, Shimony JS, et al. (2010). Noninvasive functional and structural connectivity mapping of the human thalamocortical system. Cereb Cortex 20:1187–94.
- Woodward ND, Karbasforoushan H, Heckers S. (2012). Thalamocortical dysconnectivity in schizophrenia. Am J Psychiatry 169:1092–9.
- Woodward ND, Heckers S. (2016). Mapping thalamocortical functional connectivity in chronic and early stages of psychotic disorders. Biol Psychiatry 79:1016–25.
- Yuan R, Di X, Taylor PA, Gohel S, Tsai YH, et al. (2015). Functional topography of the thalamocortical system in human. Brain Struct Funct 221:1971–84.
- Welsh RC, Chen AC, Taylor SF. (2010). Low-frequency BOLD fluctuations demonstrate altered thalamocortical connectivity in schizophrenia. Schizophr Bull 36:713–22.
- Xia S, Li X, Kimball AE, et al. (2012). Thalamic shape and connectivity abnormalities in children with attention-deficit/hyperactivity disorder. Psychiatry Res 204:161–7.
- Kim JB, Suh SI, Seo WK, et al. (2014). Altered thalamocortical functional connectivity in idiopathic generalized epilepsy. Epilepsia 55:592–600.
- Fair DA, Bathula D, Mills KL, et al. (2010). Maturing thalamocortical functional connectivity across development. Front Syst Neurosci 4:10.
- Zhang D, Snyder AZ, Fox MD, et al. (2008). Intrinsic functional relations between human cerebral cortex and thalamus. J Neurophysiol 100:1740–8.
- Utevsky AV, Smith DV, Huettel SA. (2014). Precuneus is a functional core of the default-mode network. J Neurosci 34:932–40.
- Niendam TA, Laird AR, Ray KL, et al. (2012). Meta-analytic evidence for a superordinate cognitive control network subserving diverse executive functions. Cogn Affect Behav Neurosci 12:241–68.
- Alelú-Paz R, Giménez-Amaya JM. (2008). The mediodorsal thalamic nucleus and schizophrenia. J Psychiatry Neurosci 33:489–98.
- Goldman-Rakic PS, Porrino LJ. (1985). The primate mediodorsal (MD) nucleus and its projection to the frontal lobe. J Comp Neurol 242:535–60.
- De Witte L, Brouns R, Kavadias D, et al. (2011). Cognitive, affective and behavioural disturbances following vascular thalamic lesions: a review. Cortex 47:273–319.
- Hancock PA. (1986). Sustained attention under thermal stress. Psychol Bull 99:263–81.
- Gaoua N, Racinais S, Grantham J, El Massioui F. (2011). Alterations in cognitive performance during passive hyperthermia are task dependent. Int J Hyperthermia 27:1–9.
- Racinais S, Gaoua N, Grantham J. (2008). Hyperthermia impairs short-term memory and peripheral motor drive transmission. J Physiol (Lond.) 586:4751–62.
- Sun G, Yang X, Jiang Q, et al. (2012). Hyperthermia impairs the executive function using the attention network test. Int J Hyperthermia 28:621–626.
- Craig AD. (2002). how do you feel? Interoception: the sense of the physiological condition of the body. Nature Neurosci 3:655–66.
- Craig AD, Bushnell MC, Zhang ET, Blomqvist A. (1994). A thalamic nucleus specific for pain and temperature sensation. Nature 372:770–3.
- Moustafa AA, McMullan RD, Rostron B, Hewedi DH, Haladjian HH. (2017). The thalamus as a relay station and gatekeeper: relevance to brain disorders. Rev Neurosci 28:203–218.
- Prevosto V, Sommer MA. (2013). Cognitive control of movement via the cerebellar-recipient thalamus. Front Syst Neurosci 7:56.
- Dell'Acqua ML, Landi D, Zito G, et al. (2010). Thalamocortical sensorimotor circuit in multiple sclerosis: an integrated structural and electrophysiological assessment. Hum Brain Mapp 31:1588–600.
- Churchill L, Zahm DS, Duffy P, Kalivas PW. (1996). The mediodorsal nucleus of the thalamus in rats-II. Behavioral and neurochemical effects of GABA agonists. Neuroscience 70:103–12.
- Bolkan SS, Stujenske JM, Parnaudeau S, et al. (2017). Thalamic projections sustain prefrontal activity during working memory maintenance. Nat Neurosci 20:987–96.
- Parnaudeau S, O'Neill PK, Bolkan SS, et al. (2013). Inhibition of mediodorsal thalamus disrupts thalamofrontal connectivity and cognition. Neuron 77:1151–62.
- Denys K, Vanduffel W, Fize D, et al. (2004). The processing of visual shape in the cerebral cortex of human and nonhuman primates: a functional magnetic resonance imaging study. J Neurosci 24:2551–65.
- Sun G, Li M, Yang Z, et al. (2012). Hyperthermia exposure impaired the early stage of face recognition: an ERP study. Int J Hyperthermia 28:605–20.
- Qian S, Jiang Q, Liu K, et al. (2014). Effects of short-term environmental hyperthermia on patterns of cerebral blood flow. Physiol Behav 128:99–107.
- Nunneley SA, Martin CC, Slauson JW, et al. (2002). Changes in regional cerebral metabolism during systemic hyperthermia in humans. J Appl Physiol 92:846–51.
- Gerstein GL, Perkel DH. (1969). Simultaneously recorded trains of action potentials: analysis and functional interpretation. Science 164:828–30.
- Friston KJ. (2011). Functional and effective connectivity: a review. Brain Connect 1:13–36.
- Smith DV, Gseir M, Speer ME, Delgado MR. (2016). Toward a cumulative science of functional integration: a meta-analysis of psychophysiological interactions. Hum Brain Mapp 37:2904–17.