Abstract
Aim: The neutrophil–lymphocyte ratio (NLR) and other inflammation-based scores have been used as a prognostic tool to predict survival in solid tumours including pseudomyxoma peritonei (PMP). The aim was to evaluate the prognostic value of this marker and risk stratify PMP patients undergoing cytoreductive surgery (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC).
Methods: Retrospective analysis was conducted of a prospectively collected database of patients with PMP who underwent CRS and HIPEC between 1994 and 2015. The NLR was calculated by dividing the pre-operative neutrophil count by lymphocyte count. Predicted overall survival (OS) and disease-free interval (DFI) were calculated using a Kaplan–Meier survival model.
Results: The study included 699 patients, stratified into four groups as defined by their NLR. Group A: 200 (28.6%) patients (NLR = 0.10–2.00), Group B: 160 (22.8%) patients (NLR = 2.10–2.78), Group C: 184 (26.3%) patients (NLR = 2.79–4.31) and Group D: 155 (22.2%) patients (NLR ≥ 4.32). The median follow-up for this cohort was 36 months. The predicted DFI was 132.2, 113.1, 84.4 and 47.9 months and the OS was 141.1, 117.6, 88.7 and 51.2 months for Groups A, B, C and D, respectively. As the NLR increases, there is a reduction in long-term survival.
Conclusion: The pre-operative NLR is cost effective and has equivalent prognostic value to pre-operative tumour markers for patients with PMP treated with CRS and HIPEC. The NLR is a reliable tool that may have a role in predicting outcomes following CRS and HIPEC for patients with PMP of appendiceal origin.
Introduction
Pseudomyxoma peritonei (PMP) is an unusual and rare clinical condition typified by mucinous peritoneal deposits, generally originating from a perforated appendiceal tumour [Citation1]. The optimal treatment for PMP was established by Sugarbaker in the 1990s and is a combination strategy of cytoreductive surgery (CRS), and hyperthermic intraperitoneal chemotherapy (HIPEC) [Citation2].
Despite the low grade histological nature of PMP, tumour recurrence following complete cytoreduction remains a challenge [Citation3,Citation4]. There has been significant progress in identifying patients at high risk of disease recurrence over the last decade, including the extent of prior surgery, gender, age, completeness of cytoreduction, histological subtype and postoperative complications as predictors of outcome [Citation5,Citation6]. More recently, cancer-associated inflammation by means of baseline serum tumour markers and inflammation-based scores have been suggested as predictors of disease progression and overall survival [Citation6–11]. Increasing evidence supports the role of inflammatory mediators circulating in the tumour microenvironment in the propagation of malignant cells. Many studies have demonstrated the use of acute phase reactants and biomarkers in risk stratifying prognosis and outcome in cancer patients [Citation12]. Recently, The Glasgow Prognostic Score (GPS) based on several acute phase proteins has been shown to predict survival following CRS and HIPEC [Citation13]. A number of studies have also evaluated the neutrophil–lymphocyte ratio (NLR) as an indicator of systemic inflammation and predictor of survival and outcome, in a range of cancers including pancreatic, gastric, colorectal and renal cancer [Citation14,Citation15].
Aim
Based on the hypothesis that tumour development and metastasis are associated with systemic inflammation [Citation16], the aim of this study was to evaluate the prognostic value of the pre-operative NLR following complete cytoreductive surgery (CCRS) and HIPEC in patients with PMP.
Patients and method
The Peritoneal Malignancy Institute, Basingstoke, is one of two national referral centres in the UK for PMP. The present study was a retrospective analysis of prospectively collected data in a dedicated peritoneal malignancy database between March 1994 and February 2015. This study was viewed as a service development by the local research and development committee, thus formal ethical approval was not required.
Study population
The study population included all patients who underwent CCRS and HIPEC for peritoneal malignancy of appendiceal origin with complete relevant data.
CRS and HIPEC
All patients were treated in accordance with standard PMP treatment protocols aiming for complete macroscopic tumour removal (CCRS). The chemotherapy agent used for HIPEC was mitomycin C (dose 10 mg/meter-squared body surface area) perfused for 60 min at 42–43 °C using the open abdomen technique. This dose was reduced by 33% in patients with a body mass index of >30, over 60 years of age, severe abdominal distension and recent chemotherapy (last 3 months). Patients had early postoperative intraperitoneal 5-FU (15 mg/kg) for 3–5 days unless they were considered unfit in which case the postoperative chemotherapy was withheld or stopped if not tolerated or complications developed. Patients were routinely admitted to the Intensive Therapy Unit (ITU) postoperatively.
Study variables
The following clinicopathological variable were included in the analysis: age, gender, baseline serum tumour markers (CA-125, CA 19-9 and CEA), baseline NLR. The NLR was calculated from the baseline blood sample collected within the 5 days prior to surgery by dividing the absolute neutrophil count by the absolute lymphocyte count. The appendiceal pathology specimens were classified as either low grade or high grade mucinous carcinoma on pathological evaluation by experienced peritoneal malignancy pathologists. The primary end points were overall survival and disease-free interval.
Statistical analysis
Continuous variables were presented as median (interquartile range; IQR) and categorical data presented as frequency and percentages. Kaplan–Meier curves were plotted to determine the time to recurrence and survival outcomes and expressed in median and 95% confidence interval (CI). Statistical significance for the survival was analysed with the log-rank (Mantel–Cox) test and Cox proportional – hazard regression model – was used to evaluate the hazard ratios of the variables between the groups. The p values of less than 0.05 was considered significant. Data were analysed using SPSS software (version 24, IBM SPSS Inc., Chicago, IL).
Results
During the 20-year period 1076 patients with PMP of appendiceal origin underwent CRS. Seven hundred and fifty-six patients (70.2%) had CCRS and HIPEC, of which 699 patients were included. The relevant pre-operative blood reports were not available in 86 patients who were subsequently excluded. There were 444 (63.5%) females and 255 (36.5%) were males. The median age was 56 years (IQR: 46–65). The median NLR was 2.78 (IQR: 2.00–4.32) and patients were stratified into four groups as defined by their cut off points determined by the quartile values. Group A included 200 (28.6%) patients with NLR: 0.10–2.00, Group B comprised of 160 (22.8%) patients with NLR: 2.10–2.78, Group C included 184 (26.3%) patients with NLR: 2.79–4.31 and Group D had 155 (22.2%) patients with NLR ≥ 4.32. There were 201 (28.7%) patients with normal tumour markers, 155 (22.1%) had one tumour marker elevated, 189 (27.3%) had two elevated and 154 (23.0%) had all three tumour makers elevated (). The median follow-up was 36 months (IQR: 22–63).
Table 1. NLR and tumour markers.
Survival outcomes
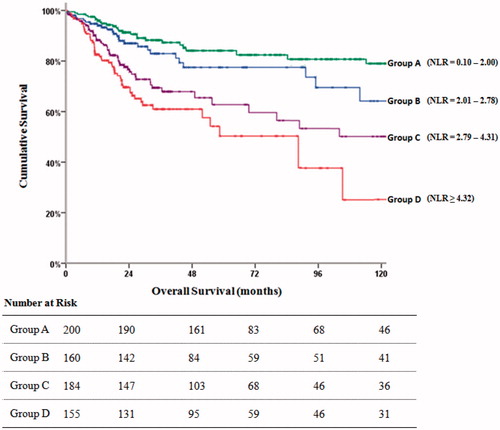
The median DFI was 132.2 months in patients in Group A (95% CI: 117.6–147.8), 113.1 months (95% CI: 100.8–127.6) for Group B, 84.4 months (95% CI: 71.8–98.7) for Group C and 47.9 months (95% CI: 39.8–56.1) for Group D (p = 0.001) (). The median DFI was 128.2 months (95% CI: 120.0–137.8) in patient with normal TM, 119.0 months (95% CI: 105.6–133.4) with one elevated TM, 68.8 months (95% CI; 58.1–79.5) for two elevated TMs, 53.2 months (95% CI: 44.9–61.5) for three elevated TMs (p = 0.001).
Figure 1. The Kaplan–Meier plot shows the disease free interval of the NLR groups and numbers at risk.

The median OS was 142.1 months (95% CI: 136.2–161.4) for Group A, 126.2 months (95% CI: 115.3–138.5) for Group B, 90.5 months (95% CI: 80.7–100.0) for C and 66.3 months for (95% CI: 49.7–70.6) Group D (). The median OS was 142.1 months (95% CI: 136.2–161.4) for patients with normal TM, 126.2 months (95% CI: 115.3–138.5) for one TM elevated, 90.5 (95% CI: 807–100.0) for two TMs elevated and 66.3 (95% CI: 49.7–60.1) for all three TM elevated ().
Table 2. Overall survival of grouped NLR and TMs.
The hazard ratio of the univariate and multivariate analyses for overall survival and disease free survival is given in and , respectively. In multivariate analyses elevated tumour markers, high tumour grade and elevated NLR over 2.78 were independent predictors of poor prognosis in overall survival and disease free survival. In addition, male gender was an independent predictor of worse prognosis in overall survival and age under 56 years was found to reduce the disease free survival.
Table 3. Univariate and multivariate Cox regression analysis for overall survival (OS).
Table 4. Univariate and multivariate Cox regression analysis for disease free survival (DFS).
Discussion
This is the largest study, to our knowledge, studying the association between baseline NLR and survival following complete CRS and HIPEC in patients with PMP of appendiceal origin. Recent studies have demonstrated that the NLR can predict OS and DFI in a range of tumour types [Citation14,Citation17–22]. The current study shows that NLR provides an objective and simple approach to the routine clinical evaluation of patients with PMP undergoing surgical treatment.
The strongest determinant of long-term survival in PMP patients is complete removal of macroscopic tumour by means of complete cytoreduction [Citation5]. Nevertheless, even with complete tumour removal, a proportion of patients with low disease burden, or a histologically confirmed low grade tumour, go on to develop disease recurrence.
The NLR is inexpensive and easily calculated in the clinical setting making it an effective clinical evaluation tool. The prognostic ability makes the NLR a useful adjunct in patient management and helps in risk stratification. CCRS and HIPEC are a major procedure with risks of mortality and significant morbidity [Citation5]. Being able to predict poor post-operative long-term outcomes may help in preoperative patient selection and intraoperative surgical planning.
The majority of studies assessing the association between NLR and survival include an elderly patient population with multiple co-existing morbidities. The PMP population tends to be younger than patients with most other solid tumours which would suggest that NLR evaluation is less likely to be confounded by these co-morbid factors [Citation23].
Our results suggest that markers of inflammation are predictive of DFI and OS and could be used in conjunction with tumour markers which we have also shown to have predictive value in this group patients [Citation10].
Chua et al. [Citation9] also found a raised neutrophil count predicted reduced DFI and OS and a raised lymphocyte count was shown to demonstrate a trend toward improved OS.
The NLR may also help with pre-operative decision making, such as considering other options, for example systemic chemotherapy for high risk patients [Citation24] or allow for a period of optimisation and assessment in patients with co-morbidities driving an inflammatory response. Further to this, the results of the preoperative NLR may guide the judicious use of adjuvant systemic chemotherapy to delay, or prevent PMP recurrence in this high risk cohort. The clinical application of the NLR would influence not only surgical management but could also encourage a modified radiological surveillance strategy. Finally, the addition of the NLR to other available scores used to categorise for high-risk disease in PMP, such as the modified GPS [Citation25] may help to improve prediction of tumour behaviour and patient survival.
There are limitations in this study, primarily related to its retrospective nature. Furthermore, all patients were included in the analysis solely based on the availability of their pre-operative blood results and may not be entirely representative of all PMP patients treated with CCRS and HIPEC. In addition, some patients in the cohort included for analysis may have concurrent illnesses altering their baseline neutrophil and lymphocyte values. The results obtained in this study may not be applicable to other centres as this was a single-centre cohort study, although combined data from two further peritoneal malignancy centres show similar results to our institution [Citation11]. To gain further validity, the NLR should be tested in other centres to establish its usefulness in clinical practice.
Conclusion
The results from the current study conclude that in a large single centre patient cohort the NLR can predict OS and DFI in PMP patients treated by complete CRS and HIPEC. This data compares well to tumour marker status in terms of prognostic ability. The NLR, an inexpensive, reproducible marker, is able to stratify patients by risk profile to ultimately augment the perioperative management of PMP patients and improve outcomes following CCRS and HIPEC.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Smeenk RM, van Velthuysen ML, Verwaal VJ, Zoetmulder FA. (2008). Appendiceal neoplasms and pseudomyxoma peritonei: a population based study. Eur J Surg Oncol 34:196–201.
- Sugarbaker PH. (1995). Peritonectomy procedures. Ann Surg 221:29–42.
- Miner TJ, Shia J, Jaques DP, et al. (2005). Long-term survival following treatment of pseudomyxoma peritonei: an analysis of surgical therapy. Ann Surg 241:300–8.
- Smeenk RM, Verwaal VJ, Antonini N, Zoetmulder FA. (2007). Survival analysis of pseudomyxoma peritonei patients treated by cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. Ann Surg 245:104–9.
- Chua TC, Moran BJ, Sugarbaker PH, et al. (2012). Early- and long-term outcome data of patients with pseudomyxoma peritonei from appendiceal origin treated by a strategy of cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. J Clin Oncol 30:2449–56.
- Elias D, Gilly F, Quenet F, et al. (2010). Pseudomyxoma peritonei: a French multicentric study of 301 patients treated with cytoreductive surgery and intraperitoneal chemotherapy. Eur J Surg Oncol 36:456–62.
- Kusamura S, Hutanu I, Baratti D, Deraco M. (2013). Circulating tumor markers: predictors of incomplete cytoreduction and powerful determinants of outcome in pseudomyxoma peritonei. J Surg Oncol 108:1–8.
- Canbay E, Ishibashi H, Sako S, et al. (2013). Preoperative carcinoembryonic antigen level predicts prognosis in patients with pseudomyxoma peritonei treated with cytoreductive surgery and hyperthermic intraperitoneal chemotherapy. World J Surg 37:1271–6.
- Chua TC, Chong CH, Liauw W, et al. (2012). Inflammatory markers in blood and serum tumor markers predict survival in patients with epithelial appendiceal neoplasms undergoing surgical cytoreduction and intraperitoneal chemotherapy. Ann Surg 256:342–9.
- Taflampas P, Dayal S, Chandrakumaran K, et al. (2014). Pre-operative tumour marker status predicts recurrence and survival after complete cytoreduction and hyperthermic intraperitoneal chemotherapy for appendiceal pseudomyxoma peritonei: analysis of 519 patients. Eur J Surg Oncol 40:515–20.
- Kusamura S, Baratti D, Hutanu I, et al. (2015). The role of baseline inflammatory-based scores and serum tumor markers to risk stratify pseudomyxoma peritonei patients treated with cytoreduction (CRS) and hyperthermic intraperitoneal chemotherapy (HIPEC). Eur J Surg Oncol 41:1097–105.
- Roxburgh CS, McMillan DC. (2010). Role of systemic inflammatory response in predicting survival in patients with primary operable cancer. Future Oncol 6:149–63.
- McMillan DC. (2012). The systemic inflammation-based Glasgow Prognostic Score: a decade of experience in patients with cancer. Cancer Treat Rev 39:534–40.
- Templeton AJ, Mcnamara MG, Seruga B, et al. (2014). Prognostic role of neutrophil-to-lymphocyte ratio in solid tumors: a systematic review and meta-analysis. J Natl Cancer Inst 106:dju124.
- Hanahann D, Weinberg RA. (2011). Hallmarks of cancer: the next generation. Cell 144:646–74.
- Gabay C, Kushner I. (1999). Acute-phase proteins and other systemic responses to inflammation. N Engl J Med 340:448–54.
- Zhang X, Zhang W, Feng LJ. (2014). Prognostic significance of neutrophil lymphocyte ratio in patients with gastric cancer: a meta-analysis. PLoS One 9:e111906.
- Cao J, Zhu X, Zhao X, et al. (2016). Neutrophil-to-lymphocyte ratio predicts PSA response and prognosis in prostate cancer: a systematic review and meta-analysis. PLoS One 11:e0158770.
- Xue TC, Zhang L, Xie X, et al. (2014). Prognostic significance of the neutrophil-to-lymphocyte ratioin primary liver cancer: a meta-analysis. PLoS one 9:e96072.
- Stotz M, Gerger A, Eisner F, et al. (2013). Increased neutrophil lymphocyte ratio is a poor prognostic factor in patients with primary operable and inoperable pancreatic cancer. Br J Cancer 109:416.
- Kang MH, Go SI, Song HN, et al. (2014). The prognostic impact of the neutrophil-to-lymphocyte ratio in patients with small-cell lung cancer. Br J Cancer 111:452–60.
- Ishizuka M, Nagata H, Takagi K, et al. (2013). Combination of platelet count and neutrophil to lymphocyte ratio is a useful predictor of postoperative survival in patients with colorectal cancer. Br J Cancer 109:401.
- Di Fabio F, Mehta A, Chandrakumaran K, et al. (2016). Advanced pseudomyxoma peritonei requiring gastrectomy to achieve complete cytoreduction results in good long-term oncologic outcomes. Ann Surg Oncol 23:4316–432.
- Farquharson AL, Pranesh N, Witham G, et al. (2008). A phase II study evaluating the use of concurrent mitomycin C and capecitabine in patients with advanced unresectable pseudomyxoma peritonei. Br J Cancer 99:591–6.
- Tan GHC, Novo CA, Dayal S, et al. (2016). The modified Glasgow prognosis score predicts for overall and disease-free survival following cytoreductive surgery and HIPEC in patients with pseudomyxoma peritonei of appendiceal origin. Eur J Surg Oncol 43:388–94.