Abstract
Purpose: To retrospectively compare the efficacy and safety of combined radiofrequency ablation and percutaneous ethanol injection (RFA–PEI) with repeat hepatectomy for elderly patients with initial recurrent hepatocellular carcinoma (HCC) after hepatic surgery.
Methods: From January 2009 to June 2015, 105 elderly patients (≥70 years) who underwent RFA–PEI (n = 57) or repeated hepatectomy (n = 48) for recurrent HCC ≤ 5.0 cm were included in the study. The overall survival (OS) and recurrence-free survival (RFS) were analysed with the Kaplan–Meier method and compared by the log-rank test. Non-tumour-related death, complications and hospital stays were assessed. Univariate and multivariate analyses were performed to identify the prognostic significance of the variables in predicting the OS and RFS.
Results: OS rates were 78.2%, 40.8% and 36.7% at 1, 3 and 5 years after RFA–PEI and 76.3%, 52.5% and 42.6% after repeat hepatectomy, respectively (p = 0.413). Correspondingly, the 1-, 3- and 5-year RFS rates after RFA–PEI and repeat hepatectomy were 69.5%, 37.8%, 33.1% and 73.1%, 49.7%, 40.7%, respectively (p = 0.465). Non-tumour-related deaths in the RFA–PEI group (2/57) were significantly fewer than those in the repeat hepatectomy group (10/48) (p = 0.016). RFA–PEI was superior to repeat hepatectomy regarding the major complication rates and length of in-hospital stay (both p < 0.001). Multivariate analysis showed that the tumour number was the significant prognostic factor for the OS (hazard ratio (HR) = 1.961, 95% CI = 1.043–3.686, p = 0.037) and RFS (HR = 1.866, 95% CI = 1.064–3.274, p = 0.030).
Conclusion: RFA–PEI provides comparable OS and RFS to repeat hepatectomy for elderly patients with small recurrent HCC after hepatectomy but with fewer non-tumour-related deaths, major complications and shorter hospital stays.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies worldwide [Citation1]. Partial hepatectomy and radiofrequency ablation (RFA) are the two main curative treatments for early HCC when liver transplantation is not feasible due to a lack of donors [Citation2]. However, intrahepatic recurrence after hepatectomy was common with an incidence of more than 70% within 5 years [Citation3]. With the longer life expectancy of the general population and the improved treatment efficacy of HCC, the number of elderly patients with recurrent HCC is expected to increase [Citation4,Citation5]. However, the elderly are often excluded from clinical trials, which makes the best clinical evidence frequently not applicable to the elderly patients. The treatment management for elderly patients with recurrent HCC remains undefined.
Currently, the available treatment options for recurrent HCC are not particularly different from the treatment options for primary HCC. Repeat hepatectomy remains the first option for patients with preserved liver function. Nevertheless, postoperative adhesion, change in the intrahepatic structures and decreased liver function reserve make repeat hepatectomy more risky and limited for its wide use. Thus, effective and minimally invasive treatments are required urgently for recurrent HCC, especially for elderly patients who generally have a poor performance status with comorbid diseases.
As a micro-invasive treatment, RFA has been reported to achieve comparable efficacy to repeat hepatectomy for recurrent HCC after initial hepatectomy [Citation6–9], but RFA is inferior to surgery for local tumour control. Recently, the combination of RFA and percutaneous ethanol injection (RFA–PEI) was reported to obtain a complete ablation rate of 94% after one session for HCC measuring 3.0–7.0 cm [Citation10]. Higher complete ablation rates and better survival outcomes than those of RFA alone for primary HCC were shown [Citation11,Citation12]. RFA–PEI with a confirmed capability for creating a larger ablation zone than RFA alone might theoretically produce more satisfactory local tumour control for recurrent HCC. Moreover, the combined modality has the advantage of minimal invasiveness and fast recovery, from which the elderly patients hypothetically would benefit a great deal. However, there is no evidence so far to support whether RFA–PEI or repeat hepatectomy is the better treatment for elderly patients with recurrent HCC. Thus, we performed this retrospective study to directly compare these two treatments for elderly patients with recurrent HCC within the Milan criteria.
Materials and methods
This retrospective comparative study was performed at two tertiary referral centres (the First Affiliated Hospital of Sun Yat-sen University and the Cancer Center of Sun Yat-sen University, Guangzhou, China). The institutional review boards of these two centres approved this study, and written informed consent was obtained from all patients before treatment. Our study followed the principles of the Declaration of Helsinki.
Patients
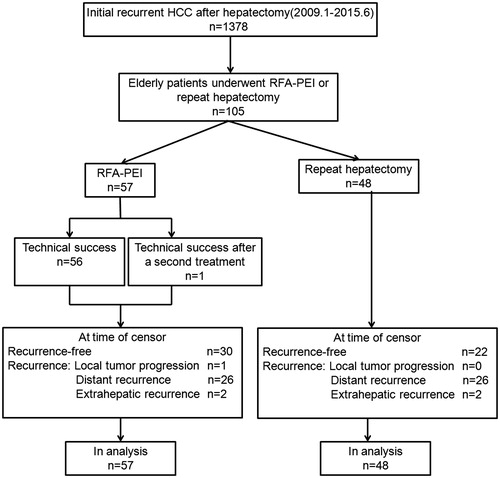
From January 2009 to June 2015, there were 1378 patients with initial recurrent HCC after hepatectomy. Among them, 105 consecutive elderly patients (age ≥70 years) (92 men, 13 women; median age: 75 years; range: 70–88 years) that underwent either RFA–PEI (n = 57) or repeat hepatectomy (n = 48) were included in this study according to the following criteria: (1) initial recurrent HCC after partial hepatectomy with R0 resection; (2) a single nodule ≤ 5 cm or 2–3 nodules, each nodule ≤ 3 cm; (3) no radiologic evidence of vascular invasion or extrahepatic metastases; (4) lesions visible on ultrasound with an acceptable and safe path to allow interventions in the RFA–PEI group; (5) Child-Pugh class A; (6) normal coagulation status; (7) no history of encephalopathy, ascites refractory to diuretics or variceal bleeding; (8) an Eastern Co-operative Oncology Group performance status of 0 and (9) no history of any other concurrent malignancies.
Recurrent HCC was diagnosed according to the most current clinical guidelines at the time of treatment [Citation13,Citation14]. The diagnosis was made histologically in four (7%) patients in the RFA–PEI group by means of a percutaneous biopsy before treatment, and histologic diagnoses were all confirmed for the repeat hepatectomy group after treatment.
The patient selection process is shown in . The choice of RFA–PEI or repeat hepatectomy for each patient was at the discretion of the physician in charge, who explained the potential benefits and procedural risks of RFA–PEI and surgery in detail and took the preferences of the individual patients into account.
Treatment protocol
RFA–PEI procedure
At each centre, RFA–PEI was performed percutaneously by the interventional radiologists with at least 10 years of experience in RF ablation. The detailed methods of RFA–PEI were described in previous studies [Citation10,Citation11]. Treatments were performed with conscious analgesic sedation (intravenous administration of 0.1 mg of fentanyl, 5 mg of droperidol and 0.1 mg of tramadol hydrochloride) and local anaesthesia (5 ml of 1% lidocaine). The whole procedure was performed under the real-time guidance of ultrasound. For PEI, an 18-gauge needle (Hakko Co., Ltd, Japan) was used. For RFA, cool-tip electrodes (Valleylab, Boulder, CO) were used.
First, the RFA needle was inserted into the low-centre of the target tumour. Then, the 18-gauge needle was placed immediately adjacent to the radiofrequency needle in another access path, with the needle tip positioned at the bottom of the tumour. The ethanol was slowly injected in the form of injection–rotation–injection, and until resistance was felt or the whole tumour appeared completely hyperechoic. The amount of ethanol was determined by the size of the tumour and was always kept below the estimated volume of the tumours and was calculated by: (V): V= (4/3)[π(r + 0.5)3], where r is the tumour radius, in centimetres. The mean volume of ethanol used was 9.0 ± 2.0 ml (range: 5–20). After the completion of ethanol injection, the needle was left in place for 1–2 min before it was withdrawn.
Afterwards, RFA was started 3–5 min after PEI completion. The number of needle puncture and ablation points was determined by the number and diameter of the tumours with the aim of achieving an ablative margin of at least 0.5 cm beyond the tumour boundary. Ablations were finished when the echogenic zone induced by RFA at the US was large enough to cover the entire tumour and surrounding liver. The needle tract was ablated with the electrode at the end of the procedure to prevent bleeding and tumour seeding. Hydrodissection by using artificial ascites or pleural effusion was used for tumours in high-risk locations.
Repeat hepatectomy
Open surgery was performed under general anaesthesia using the incision for the initial hepatectomy. One of three surgeons with 10–21 years of experience in hepatic resection performed the repeat hepatectomy. Intra-operative ultrasound was routinely used to evaluate the tumour burden, the liver remnant and the possibility of a negative resection margin. The type of hepatectomy was defined according to the current guidelines [Citation15]. Anatomic resection was defined as the complete removal of at least one Couinaud segment containing the tumour and the corresponding hepatic territory. Other types of resection, such as wedge resection or tumour enucleation, were classified as non-anatomic resection. The surgical approach was chosen based on the hepatic functional reserve, tumour location and preference of the operator. Generally, anatomic resection was performed if the patient’s liver functional reserve permitted.
Treatment assessment and follow up
In the RFA–PEI group, contrast-enhanced ultrasound (CEUS) was performed the morning after treatment to evaluate the technical success of the ablation [Citation16]. An additional RFA–PEI was given if the residual viable tumour tissue was found. If incomplete ablation was still observed after the additional RFA–PEI, the treatment was defined as a failure and these patients were referred to other therapies [Citation17]. In the repeat hepatectomy group, frozen section investigation was used to evaluate the resection margins and status (R0 vs. R1 resection) for all the detected lesions [Citation18].
Contrast-enhanced computed tomography (CECT) and CEUS were done 4 weeks after the treatment for both the groups [Citation16]. Thereafter, the patients were followed-up once every 3 months for the first 2 years, once every 6 months from 2 to 5 years and once every 12 months after 5 years. At each follow-up visit, CEUS and blood tests, including liver function tests and AFP, were determined. CECT was performed once every 6 months. Chest radiography, magnetic resonance imaging and bone scintigraphy were performed when necessary.
Re-recurrence included local tumour progression (defined as the appearance of tumour enhancement around the ablation zone or resection margin) [Citation16], intrahepatic distant recurrence and extrahepatic recurrence. The treatment choice for re-recurrence, such as resection, RFA, TACE, sorafenib or conservative treatment, was determined by the consensus opinion of our multidisciplinary treatment team, according to the characteristics of the recurrent tumour, the patients’ liver function and the patient’s request.
The American Society of Anesthesiologists (ASA) physical status classification score was applied to determine the pre-treatment risk related to the comorbidities and general health status. The ASA classification divides patients into the following five classes: ASA score 1: normal health; ASA score 2: mild systemic disease, without functional limitations; ASA score 3: severe systemic disease with some functional limitation and anaesthetic risk; ASA score 4: incapacitating systemic disease that is a constant threat to life; ASA score 5: not expected to survive 24 h with or without operation [Citation19,Citation20].
Complications were stratified according to the National Cancer Institute Common Toxicity Criteria grading version 4.0 [Citation21]. Major complications were defined as clinical events leading to additional therapeutic interventions or prolonged hospitalisation [Citation22].
The overall survival (OS) was defined as the interval between the time of recurrent HCC observed after initial treatment and the time of death or last follow-up. RFS was defined as the interval between the time that initial recurrent HCC was diagnosed and the time of recurrence or last follow-up. This study was censored on 30 June 2016.
Statistical analysis
The following statistical analysis was performed using SPSS 20.0 (SPSS Inc., Chicago, IL) and the R programme (R Foundation for Statistical Computing, Vienna, Austria). Continuous variables were presented as the means ± SD and categorical variables as numbers and percentages. The χ2 test was performed to compare the categorical variables, and the unpaired t-test was used to compare the continuous variables. Survival curves were depicted using the Kaplan–Meier method and compared using the log-rank test. The potential predictors for survival were analysed by univariate and multivariate analysis in the Cox’s proportional hazards regression model. A p values <0.05 was considered significant.
Results
Baseline characteristics
The baseline characteristics between the two groups were comparable (). The average age of the patients was 73.7 ± 2.9 years (range: 70–88) in the RFA–PEI group and 73.5 ± 3.5 years (range: 70–87) in the repeat hepatectomy group (p = 0.587). The ASA scores of all the patients were no larger than 3, and we divided them into two subgroups (ASA score 1–2 and ASA score 3). There was no significant difference in the distribution of the ASA scores between the two groups (p = 0.078). The mean size of recurrent tumours was 2.5 cm ±1.2 for the RFA–PEI group and 2.6 cm ±1.1 for the repeat hepatectomy group (p = 0.568). The proportion of solitary tumours was higher than that of two or three tumours in both the groups. The features of the primary tumours, including BCLC stage and micro-vessel invasion, were without significant differences (p = 0.769, p = 0.959, respectively). The number of liver segments resected in the initial hepatic resection were not significantly different between the two groups either (1, 2, >2 segments: p = 0.974, p = 0.908, p = 0.883, respectively).
Table 1. Clinical characteristics of patients.
Technical success of RFA–PEI and outcomes of repeat hepatectomy
Complete ablation was first achieved in 56 patients. For the remaining patient with a viable residual tumour, an additional session of RFA–PEI was performed with technical success.
Table S1 lists the operative and perioperative outcomes for the repeat hepatectomy group. Thirty patients had anatomic liver resection, including 25 with one liver segment resected, five with more than one liver segments resected and 18 patients who received non-anatomic wedge resection. R0 resection was achieved in all patients.
Survival analysis
During follow-up, 27 patients in the RFA–PEI group and 26 patients in the repeat hepatectomy group developed recurrence (p = 0.691) (). There was no significant difference between the two groups in local tumour progression (LTP) (1/57 vs. 0/48, p = 0.361), intrahepatic distant recurrence (26/57 vs. 26/48, p = 0.631) or extrahepatic metastasis (2/57 vs. 2/48, p = 0.866) (). The treatments used to treat re-recurrent HCC are shown in .
Table 2. Recurrence of treatment.
Table 3. Treatment of re-recurrent HCC.
The median follow-up durations were 37.2 months (range: 2–78) and 36.9 months (range: 2–78) for the RFA–PEI and repeat hepatectomy groups, respectively. At the time of censoring, a total of 30 patients and 25 patients died in the RFA–PEI group and the repeat hepatectomy group, respectively. The causes of death are reported in . There were 15 tumour-related deaths in the repeat hepatectomy group and 28 in the RFA–PEI group. The tumour-related deaths between the two groups were without significant difference (p = 0.226) in either the subgroup of ASA score 1–2 (p = 0.556) or ASA score 3 (p = 0.135). However, non-tumour-related deaths in the RFA–PEI group (2/57) were significantly fewer than that in the repeat hepatectomy group (10/48) (p = 0.016). There was no significant difference in non-tumour-related deaths between the two groups in the subgroup with an ASA score of 3 (p = 0.253) whereas the lower proportion of the non-tumour-related deaths were found in the RFA–PEI group than that in the repeat hepatectomy group (p = 0.023) in the subgroup with an ASA score of 1–2. The OS rates at 1, 3 and 5 years after RFA–PEI were 84.2%, 42.5%, 39.0%, and after repeat hepatectomy were 77.1%, 54.1% and 46.5%, respectively (p = 0.612; ). Correspondingly, the 1-, 3- and 5-year RFS rates after RFA–PEI and repeat hepatectomy were 63.9%, 39.8%, 39.8% and 79.2%,47.4%, 41.1%, respectively (p = 0.549; ).
Figure 2. Kaplan–Meier survival curves of patients who underwent combined radiofrequency ablation and percutaneous ethanol injection (RFA–PEI) or repeat hepatectomy for elderly patients with recurrent hepatocellular carcinoma after hepatic resection. (a) Cumulative overall survival curves and (b) cumulative recurrence-free survival curves.
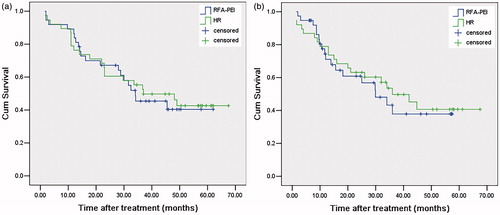
Table 4. Causes of death on follow-up.
Univariate analysis showed that prothrombin activity and the number of tumours were the two factors significantly associated with OS (). Multivariate analysis further revealed the number of tumours to be the only potential predictor for OS (hazard ratio (HR) = 1.961; 95% CI: 1.043–3.686; p = 0.037). Of all the included variables, the number of tumours was found to be the only potential predictor of RFS in both the univariate and multivariate analysis (HR = 1.866; 95% CI: 1.064–3.274; p = 0.030).
Table 5. Univariate and multivariate analysis of predictors of overall survival and recurrence-free survival after treatment.
Complications
There was one treatment-related death in the repeat hepatectomy group and zero in the RFA–PEI group (). Major complications were observed in 0 of the 57 (0.0%) patients in the RFA–PEI group and 12 of the 48 patients (25.0%) in the repeat hepatectomy group (p < 0.001). The major complications included cardiac disorder, hepatic failure, lung infection and postoperative haemorrhage. Regarding minor complications, pain, fever and mild ascites were more common in the repeat hepatectomy group than those in the RFA–PEI group (p = 0.007, p = 0.025, p = 0.049, respectively). The hospital stay was significantly shorter in the RFA–PEI group (average: 5.3 days; range: 4–10 days) than in the repeat hepatectomy group (average: 12.3 days; range: 8–42 days) (p < 0.001).
Table 6. Complications after treatment.
Discussion
Our study demonstrated that RFA–PEI achieved equivalent survival outcomes to repeat hepatectomy for elderly patients with recurrent HCC. Compared to repeat hepatectomy, RFA–PEI yielded comparable tumour-related deaths, much fewer non-tumour-related deaths, fewer major complications and shorter hospital stays.
The reasons we chose elderly patients (≥70 years) with recurrent HCC as the study subjects were as follows. First, research for elderly patients has been underprioritised, underrepresented and underfunded historically [Citation23]. The elderly are generally not candidates for clinical trials, which makes the best clinical evidence often not applicable to this population. However, the global population is ageing, and their increased need in healthcare inspires us to conduct such a retrospective study to pave the way for better studies for elderly patients in the future. Second, a study has justified repeat hepatectomy for recurrent HCC in elderly patients (≥75 years old) because they found that advanced age by itself does not have a negative effect on postoperative complications and long-term survival [Citation24]. Cucchetti et al. showed that HCC patients aged >70 years benefited most from surgery with the fewest years of life lost among patients of different age groups, supporting the choice of hepatectomy for HCC in elderly patients [Citation25]. Although some studies favouring hepatectomy for elderly patients have been reported [Citation24,Citation25], clinicians are still worried about referring elderly patients to invasive surgery. Instead, they may choose other less invasive treatments, such as ablation. By far, comparative study for the efficacy of micro-invasive modalities (e.g. ablation) and surgery in elderly patients with recurrent HCC is rare. Third, in China, 70-year-old men and women have average life expectancies of approximately 74 years and 81 years, respectively [Citation26]. This provides an observation window of 4–7 years for conduction of a clinical study.
The OS and RFS were not significantly different between the RFA–PEI and repeat hepatectomy groups. The underlying reasons might be as follows. First, the LTP rate (1.8%) of RFA–PEI in our study was relatively low and not significantly different from that of repeat hepatectomy, which reflected the good efficacy of RFA–PEI in local tumour control. One session of RFA produces a necrotic zone of 3.0–5.0 cm in diameter, and its combination with PEI could induce an enlarged ablation zone to completely ablate tumours no more than 5 cm in diameter, with an adequate safety margin taken into consideration [Citation10–12]. With the larger safety ablation margin, the chance of clearance of micrometastasis increases and the chance of distant recurrence could be reduced. In our study, the intrahepatic distant recurrence rate in the RFA–PEI group was comparable to that in the repeat hepatectomy group. The contributing factors to favourable efficacy of RFA–PEI included the reduction of the heat-sink effect by the destruction of vessels within or around the tumours with ethanol, the diffusion of ethanol into the area not reached by RF power and the better thermal conduction by the lower extent of carbonisation of the tissue around the electrode with ethanol [Citation27,Citation28]. Second, the status of the background liver parenchyma considerably affects tumour recurrence (intrahepatic distant recurrence or de novo tumours) and OS during the follow-up period, as well as the efficacy of treatment for initial recurrent tumours [Citation29–31]. Nearly half of our study subjects experienced tumour recurrence. Thus, the treatment choice for re-recurrent HCC is critical in the patients’ prognosis. In our study, the treatment types (curative or not) for re-recurrence were not significantly different between the two groups.
Notably, non-tumour-related death was less frequent in the RFA–PEI group than in the repeat hepatectomy group. This result was supported by the fact that fewer non-tumour-related deaths were found in the RFA–PEI group for patients with relatively good health status (ASA score 1–2) after stratifying the results by ASA score. Considering the background of comparable baseline characteristic, such as age and general health status (assessed by associated comorbidities and ASA score), between the groups, the above finding suggests that the features of RFA–PEI, including micro-invasiveness, high safety and fast recovery, might have less negative impact on the general health of elderly patients. In addition, the activating effect of RFA–PEI on the immune system might also play part of the role in improving non-tumour-related survival [Citation32]. These advantages of RFA–PEI were especially meaningful for elderly patients who were likely to suffer from or even die from other diseases besides the tumour itself.
In the multivariate analysis, tumour number was the only significant prognostic factor for the OS and RFS. First, the possibility of incomplete removal of the tumours increases as the number of tumours increases. Second, micro-invasion might be very likely to occur in patients with multiple tumours, resulting in recurrence and thus affecting the RFS and OS. Third, patients with multiple tumours have more tumour burden than those with solitary ones, in which curative treatment is less likely to be performed when tumours recur.
Some studies have suggested that RFA–PEI is safe in primary HCC with low rates of major complications (range: 0.0–4.6%) [Citation10–12]. No major complication observed after RFA–PEI even for elderly patients with recurrent HCC in our study strongly confirmed the safety of this technique. For repeat hepatectomy, there was only one treatment-related death in our study, which is consistent with the results of recent studies with low mortality rates (range: 0.0–1.22%) for recurrent HCC [Citation33–35]. However, for recurrent HCC patients with limited reserve liver function and changes of intrahepatic structures after initial hepatectomy, a second hepatectomy may bring a higher risk of major complications, especially in elderly patients with comorbidities as seen in our study. Moreover, lower morbidity and a shorter hospital stay in the RFA–PEI group indicates the micro-invasiveness of RFA–PEI. For such a special group of elderly patients, RFA–PEI seems to maintain its curative feature with good local tumour control, boosted by the prominent advantage of low invasiveness, which is exclusively important for elderly patients.
Recently, microwave ablation (MVA) has been shown to be a promising prospect in the treatment of HCC for its favourable efficacy due to larger ablation zones and less influence by the heat-sink effect [Citation36–39]. However, MVA might be accompanied by relatively high complication rates caused by increased energy application and tissue destruction, thus limiting its use for the tumours close to critical structures. In contrast, RFA–PEI might have fewer major complications and is especially suitable to treat tumours adjacent to critical structures, such as the subcapsular region and areas near large vessels or the gallbladder, whereas the RFA is likely to be incomplete [Citation10–12]. Thus, for elderly patients with recurrent hepatectomy, RFA–PEI might still have its advantages, such as high safety with acceptable efficacy. However, since no study has been performed to directly compare RFA–PEI with MVA so far, future studies are needed to investigate this aspect.
To our knowledge, this is the first study comparing RFA–PEI with repeat hepatectomy in the treatment of recurrent HCC for elderly patients. For such a special study population, RFA–PEI showed its equivalent efficacy with fewer non-tumour-related deaths and less invasiveness to repeat hepatectomy. Based on this, we suggest that RFA–PEI is an efficient treatment, which may be considered as a first-line choice in elderly patients with recurrent HCC due to the following reasons: small-sized recurrent HCC is usually detected in routine follow-up, there are fewer non-tumour-related deaths of RFA–PEI, the high invasiveness and complication rate of repeat hepatectomy, as well as more comorbidities and slower recovery of elderly patients from surgery. On the other hand, repeat surgery remains the first choice in cases where RFA–PEI is infeasible, such as tumours that are invisible on ultrasound or difficult to puncture owing to high-risk locations.
There are several limitations in our study. First, it is a retrospective study without randomisation. However, baseline characteristics of patients in the two groups were well matched. We believe that this occurred partly by chance and partly because of the large number of patients with HCC we treat every year. Second, the sample size of our study is relatively small. Third, our results may lack universality to patients in other regions because of differences in demographics and the underlying aetiologies of HCC. Thus, a multi-centre study with larger sample size should be performed to provide more solid evidence for the management of recurrent HCC in elderly patients. Fourth, we just tried to control the flow and pressure of the injection of ethanol with the operators’ experience but not to apply a technique such as controlled pressure-pulses.
In conclusion, RFA–PEI provides comparable long-term survival outcomes to repeat hepatectomy for elderly patients with recurrent HCC after initial hepatectomy. RFA–PEI is superior to repeat hepatectomy for fewer non-tumour-related deaths, fewer major complications and shorter hospital stays.
Supplemental Material
Download PDF (91.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Siegel RL, Miller KD, Jemal A. (2015). Cancer statistics, 2015. CA Cancer J Clin 65:5–29.
- Bruix J, Sherman M. American Association for the Study of Liver Diseases. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Poon RT, Fan ST, Lo CM, et al. (2000). Long-term prognosis after resection of hepatocellular carcinoma associated with hepatitis B-related cirrhosis. J Clin Oncol 18:1094–101.
- US Department of Health and Human Services. Global Health and Aging. Available from: http://www.nia.nih.gov/research/publication/global-health-and-aging/preface [last accessed 1 Jul 2015].
- El-Serag HB. (2012). Epidemiology of viral hepatitis and hepatocellular carcinoma. Gastroenterology 142:1264–73.
- Camma C, Di Marco V, Orlando A, et al. (2005). Treatment of hepatocellular carcinoma in compensated cirrhosis with radio-frequency thermal ablation (RFTA): a prospective study. J Hepatol 42:535–40.
- Poon RTP, Fan ST, Tsang FHF, Wong J. (2002). Locoregional therapies for hepatocellular carcinoma: a critical review from the surgeon's perspective. Ann Surg 235:466–86.
- Tateishi R, Shiina S, Teratani T, et al. (2005). Percutaneous radiofrequency ablation for hepatocellular carcinoma. An analysis of 1000 cases. Cancer 103:1201–9.
- Song KD, Lim HK, Rhim H, et al. (2015). Repeated hepatic resection versus radiofrequency ablation for recurrent hepatocellular carcinoma after hepatic resection: a propensity score matching study. Radiology 275:599–608.
- Huang GL, Lin MX, Xie XY, et al. (2014). Combined radiofrequency ablation and ethanol injection with amultipronged needle for the treatment of medium and large hepatocellular carcinoma. Eur Radiol 24:1565–71.
- Zhang YJ, Liang HH, Chen MS, et al. (2007). Hepatocellular carcinoma treated with radiofrequency ablation with or without ethanol injection: a prospective randomized trial. Radiology 244:599–607.
- Vallone P, Catalano O, Izzo F, Siani A. (2006). Combined ethanol injection therapy and radiofrequency ablation therapy in percutaneous treatment of hepatocellular carcinoma larger than 4 cm. Cardiovasc Intervent Radiol 29:544–51.
- Bruix J, Sherman M, Llovet JM, et al. (2001). Clinical management of hepatocellular carcinoma. Conclusions of the Barcelona-2000 EASL conference. European Association for the Study of the Liver. J Hepatol 35:421–30.
- Bruix J, Sherman M. Practice Guidelines Committee, American Association for the Study of Liver Diseases. (2005). Management of hepatocellular carcinoma. Hepatology 42:1208–36.
- Strasberg SM. (2005). Nomenclature of hepatic anatomy and resections: a review of the Brisbane 2000 system. J Hepatobiliary Pancreat Surg 12:351–5.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology 273:241–60.
- Peng ZW, Zhang YJ, Chen MS, et al. (2013). Radiofrequency ablation with or without transcatheter arterial chemoembolization in the treatment of hepatocellular carcinoma: a prospective randomized trial. J Clin Oncol 31:426–32.
- Sobin LH, Wittekind C. (2002). TNM classification of malignant tumours. 6th ed. Hoboken, NJ: John Wiley & Sons.
- Monfardini L, Della Vigna P, Bonomo G, et al. (2013). Interventional oncology in the elderly: complications and early response in liver and kidney malignancies. J Geriatr Oncol 4:58–63.
- Mak PH, Campbell RC, Irwin MG. American Society of Anesthesiologists. (2002). The ASA physical status classification: inter-observer consistency. Anaesth Intensive Care 30:633–40.
- National Cancer Institute: Common Toxicity Criteria, Version 4.0: Cancer Therapy Evaluation Program, 2009. Available from: http://evs.nci.Nih.Gov/ftp1/CTCAE/CTCAE_4.03_2010-06-14_Quick-Reference_5x7.pdf.
- Omary RA, Bettmann MA, Cardella JF, et al. (2003). Quality improvement guidelines for the reporting and archiving of interventional radiology procedures. J Vasc Interv Radiol 14:S293–5.
- Søreide K, Wijnhoven BP. (2016). Surgery for an ageing population. Br J Surg 103:e7–9.
- Tsujita E, Utsunomiya T, Ohta M, et al. (2010). Outcome of repeat hepatectomy in patients with hepatocellular carcinoma aged 75 years and older. Surgery 147:696–703.
- Cucchetti A, Sposito C, Pinna AD, et al. (2016). Effect of age on survival in patients undergoing resection of hepatocellular carcinoma. Br J Surg 103:e93–9.
- Zhou M, Wang H, Zhu J, et al. (2016). Cause-specific mortality for 240 causes in China during 1990–2013: a systematic subnational analysis for the Global Burden of Disease Study 2013. Lancet 387:251–72.
- Goldberg SN, Kruskal JB, Oliver BS, et al. (2000). Percutaneous tumor ablation: increased coagulation by combining radio-frequency ablation and ethanol instillation in a rat breast tumor model. Radiology 217:827–31.
- Wong SN, Lin CJ, Lin CC, et al. (2008). Combined percutaneous radiofrequency ablation and ethanol injection for hepatocellular carcinoma in high-risk locations. Am J Roentgenol 190:W187–95.
- Hasegawa K, Kokudo N, Makuuchi M, et al. (2013). Comparison of resection and ablation for hepatocellular carcinoma: a cohort study based on a Japanese nationwide survey. J Hepatol 58:724–9.
- Kim YS, Lim HK, Rhim H, et al. (2013). Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 58:89–97.
- Feng K, Yan J, Li X, et al. (2012). A randomized controlled trial of radiofrequency ablation and surgical resection in the treatment of small hepatocellular carcinoma. J Hepatol 57:794–802.
- Zerbini A, Pilli M, Fagnoni F, et al. (2008). Increased immunostimulatory activity conferred to antigen-presenting cells by exposure to antigen extract from hepatocellular carcinoma after radiofrequency thermal ablation. J Immunother 31:271–82.
- Itamoto T, Nakahara H, Amano H, et al. (2007). Repeat hepatectomy for recurrent hepatocellular carcinoma. Surgery 141:589–97.
- Wu CC, Cheng SB, Yeh DC, et al. (2009). Second and third hepatectomies for recurrent hepatocellular carcinoma are justified. Br J Surg 96:1049–57.
- Huang ZY, Liang BY, Xiong M, et al. (2012). Long-term outcomes of repeat hepatic resection in patients with recurrent hepatocellular carcinoma and analysis of recurrent types and their prognosis: a single-center experience in China. Ann Surg Oncol 19:2515–25.
- Kuang M, Lu MD, Xie XY, et al. (2007). Liver cancer: increased microwave delivery to ablation zone with cooled-shaft antenna – experimental and clinical studies. Radiology 242:914–24.
- Liu Y, Zheng Y, Li S, et al. (2013). Percutaneous microwave ablation of larger hepatocellular carcinoma. Clin Radiol 68:21–6.
- Abdelaziz A, Elbaz T, Shousha HI, et al. (2014). Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 28:3429–34.
- Cornelis FH, Durack JC, Kimm SY, et al. (2017). A Comparative study of ablation boundary sharpness after percutaneous radiofrequency, cryo-, microwave, and irreversible electroporation ablation in normal swine liver and kidneys. Cardiovasc Intervent Radiol. [Epub ahead of print]. DOI: 10.1007/s00270-017-1692-3.