Abstract
Objective: To evaluate the safety of thermal ablation for hepatocellular carcinoma (HCC) in patients with abnormal coagulation function.
Methods: Fifty-seven HCC tumours in 50 patients were treated with thermal ablation. All patients had a meted platelet count <50 × 109/L or international normalised ratio (INR) ≥ 1.7. Gastroscopy before ablation, platelet concentrate or fresh frozen plasma transfusion during ablation and contrast enhanced ultrasoundgraphy (CEUS)-guided ablation to cease needle tract bleeding were performed to reduce haemorrhage. The incidences of haemorrhage and other major complications were recorded and patients were followed up to observe the local tumour progression (LTP), intrahepatic distant recurrence (IDR), overall survival (OS) and recurrence-free survival (RFS) rates.
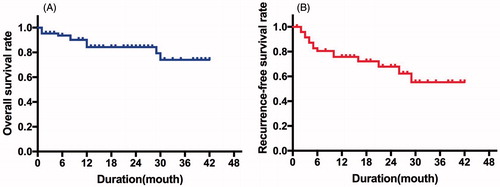
Results: Two incidences of needle tract bleeding and one needle tract bleeding together with bleeding at the suture of the spleen fossa were found. Three needle tract bleeding events were detected by CEUS and ceased after CEUS-guided complementary ablation. CEUS failed to detect bleeding at the suture of the spleen fossa. Therefore, a laparotomy was conducted for haemostasis. No other major complications were found after ablation. The median follow-up periods were 18.7 ± 12.0 months (range 1 ∼ 42 months) and 1 LTP and 15 IDRs occurred. The 1-, 2- and 3-year OS rates were 84.8%, 82.7% and 82.7%, and RFS rates were 67.9%, 64.0% and 64.0%, respectively.
Conclusion: With gastroscopy before ablation, platelet concentrate or fresh frozen plasma transfusion during ablation and CEUS-guided ablation to cease needle tract bleeding, thermal ablation is a safe treatment for HCC in patients with abnormal coagulation function.
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy and the second highest cause of tumour-related mortality worldwide [Citation1]. Currently, surgical resection, liver transplantation and thermal ablation are recognised as curative modalities for early-stage HCC, which can prolong a patient's overall survival (OS) and recurrence-free survival (RFS) times [Citation2,Citation3]. More than 90% of HCC patients develop within an established chronic liver disease, namely cirrhosis [Citation3]. With the progress of cirrhosis, some patients suffer abnormal coagulation function, including reduced synthesis of the coagulation factors caused by declining liver function [Citation4] and decreased platelet count because of hypersplenism [Citation5]. Abnormal coagulation function will increase the risk of surgery [Citation6,Citation7] and, in turn, confuse patients in the choice of appropriate treatments.
Liver transplantation has been considered the first-choice treatment for patient with HCC and declining liver function [Citation8]. However, the shortage of liver donors and high cost of surgery are major limitations. In addition, many of these patients cannot endure surgical resection due to insufficient hepatic functional reserve [Citation9]. For patients with thrombocytopenia caused by hypersplenism, splenectomy and splenic embolisation are effective procedures [Citation10,Citation11]. However, splenectomy may lead to the loss of immune function of spleen and the incidence of portal vein thrombosis increases after splenectomy [Citation10]. Splenic embolisation has limitations, however, including splenic abscess, splenic rupture, gastrointestinal bleeding, and high recurrence and mortality rates [Citation12]. Thus, some patients refuse to undergo these treatments.
Compared with surgical resection and liver transplantation, thermal ablation has the advantages of minimal invasiveness, high therapeutic efficacy, and relatively low incidence of complications [Citation13,Citation14]. Haemorrhage, including haemoperitoneum, haemothorax and haemophilia, is one of the most common complications of thermal ablation in HCC [Citation15,Citation16]. The most common reason for haemorrhage in thermal ablation is needle tract bleeding caused by electrode injury to intercostal vessels, tumour vessels, liver vessels or the biliary tract [Citation17]. Besides, the results of some studies showed that gastrointestinal variceal bleeding was related to death after ablation for HCC [Citation16,Citation18]. Some research has demonstrated that the risk of haemorrhage complications increased significantly in patients with abnormal coagulation function [Citation6,Citation19]. The Chinese guidelines suggest that severe coagulation disorders and the tendency towards severe bleeding should be contraindications for thermal ablation in HCC [Citation20]. Patients with abnormal coagulation function did, indeed, have increased the rate of haemorrhage [Citation6,Citation19]. Platelet count <50 × 109/L and international normalised ratio (INR) ≥ 1.7 were usually used to define abnormal coagulation function and listed as exclusion criteria in some studies [Citation18,Citation21]. Nevertheless, since ablation treatment is a microinvasive method, if proper interventional procedures are adhered to, the patients could receive ablation without an increased complication rate, thereby achieving a better prognosis. However, there are no standards for HCC patients with abnormal coagulation function to receive ablation. Therefore, this prospective study enrolled patients with abnormal coagulation function whose platelet count was <50 × 109/L or INR ≥1.7, and aimed to ascertain whether, with proper interventional procedures before, during, and after ablation, these HCC patients could safely receive ultrasound-guided thermal ablation.
Materials and methods
Patients
This study followed the Declaration of Helsinki, and was approved by the Ethical Review Board of our hospital. Informed consent was obtained from all subjects. All of the patients enrolled in this prospective study received thermal ablation for curative therapy in our hospital between July 2013 and July 2016. The inclusive criteria were (1) a diagnosis of HCC, confirmed on the basis of pathology or clinical manifestations; (2) a solitary HCC tumour <5 cm in maximum diameter, or multiple (≤3 in number) HCC tumours, each <3 cm in maximum diameter; (3) platelet count <50 × 109/L or INR ≥1.7; 4) Child-Pugh A or B; and 5) no anticoagulant use for over 1 week prior to the thermal ablation. The exclusion criteria were (1) evidence of haematemesis, hemafecia, or other forms of haemorrhage symptoms during the last month before hospitalisation; (2) severe infection or septicaemia; and (3) allergy to the ultrasound contrast agent.
Instruments
Radiofrequency ablation (RFA) was conducted using the cool-tip (Valleylab Corp, USA). Microwave ablation (MWA) was conducted using the Kangyou MWA system (Nanjing, China). The Mylab Twice (Esaote, Italy) equipment with an abdominal probe CA541 (1–8 MHz) was used for ultrasound examination. Contrast enhanced ultrasoundgraphy (CEUS) was performed using the real-time contrast-enhanced imaging technique with a mechanical index less than 0.05. SonoVue (Bracco, Italy) was used as the ultrasound contrast agent, which was infused through a peripheral vein at a dose of 2.4 ml and washed with 5 ml sterile saline.
Preoperative preparation
All patients underwent a routine gastroscopy examination. Endoscopic therapy, including endoscopic variceal ligation, sclerotherapy, or variceal sclerotherapy, was performed for patients who had bleeding tendencies with gastrointestinal varices prior to the ablation. All patients received an intravenous infusion of Omeprazole Sodium for Injection (40 mg) 1–2 days before the ablation.
Ablation
During ablation, 1 U platelet concentrate was transfused to patients whose platelet count was <50 × 109/L and 200 ml fresh frozen plasma and 2 U cryoprecipitate were transfused to patients whose INR ≥1.7. The ablation was performed under tracheal general anaesthesia in the operating room. MWA was preferred for tumours with feeding vessels, which were ablated first if detected and then the entire tumour was then ablated. For tumours with a rich blood supply, ablation was initially performed from the peripheral part of the tumour, and then in the central part of the tumour. If multiple ablations were required, the placement of the electrode in the liver parenchyma was optimised to reduce the number of needle entrances into the liver capsule. The needle channel was fully burnt before the electrode was pulled out.
Detection and treatment of needle tract bleeding
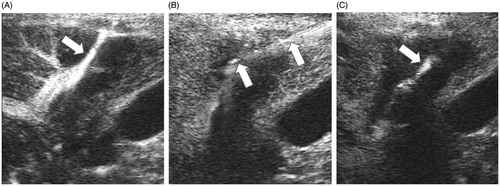
After ablation, CEUS was conducted to check needle tract bleeding. The diagnostic criteria of needle tract bleeding by CEUS based on movements of contrast agent microbubbles along the needle tract into the abdomen, chest or bile duct cavity. The slow movement of contrast agent microbubbles through the needle tract suggests slow bleeding. Continuous linear microbubbles of contrast agent suggest rapid bleeding (). Patients with slow bleeding received intravenous or intramuscular injections of 0.5ku Bangting (haemocoagulase for injection). In patients with rapid needle tract bleeding, CEUS-guided ablation at the needle tract was conducted to stop bleeding. Electrode was inserted into outer parts of needle tract which was near the capsule under the guidance of CEUS (). The RFA was conducted with 200 W of power for 3 min, and the MWA was 70 W for 3 min. After complementary ablation, CEUS was conducted again to evaluate the bleeding (). Repeated ablation at needle tract was conducted if necessary until the above-mentioned ultrasound images of bleeding were not detected. The patients were observed for 10–20 min after the complementary ablation at the needle tract. The observation was terminated if no progressive abdominal, pleural or bile duct haemorrhagic drainage was detected and the patient vital signs remained stable. Transcatheter arterial embolisation (TAE), laparoscopic surgery or laparotomy was conducted if patients showed obvious clinical signs of bleeding that could not be detected with CEUS, or if bleeding was not successfully controlled after three complementary ablation attempts to stop bleeding.
Figure 1. CEUS-guided ablation at needle tract. (A) CEUS showed continuous linear microbubble extravasated along the needle tract (white arrow). (B) Electrode was inserted into outer parts of needle tract which was near the capsule under the guidance of CEUS (white arrow). (C) CEUS showed no microbubble extravasation along the needle tract after thermal ablation. The high echo showed by white arrow was caused by the carbonisation of electrode, not needle tract bleeding.
Postoperative observation
Vital signs and clinical symptoms were closely observed after ablation, especially right abdominal pain, abdominal uplift, drop of blood pressure, etc. Amount, colour and quality of drainage were recorded if a drainage tube was present. Laboratory examination and ultrasound were repeated 1 day after ablation to check for haemorrhage complications. After 3–7 days of observation, patients were discharged if no severe complications were identified.
Follow-up
All patients received contrast-enhanced computed tomography (CT)/magnetic resonance imaging (MRI) or CEUS as the standard for complete ablation one month after ablation. Complications related to ablation were recorded. The follow-up was then performed using CEUS, a blood biochemical and serum alpha fetoprotein (AFP) examination at 3-month intervals, and CT/MRI at 6-month intervals. OS, RFS, LTP, and IDR were also recorded.
Statistical analyses
Statistical analyses were conducted using SPSS 20.0 software. Measurement data were presented as mean ± standard deviation. The survival curves were constructed using the Kaplan-Meier method. A significant difference was considered to be a p value less than 0.05.
Results
A total of 50 patients with 57 tumour lesions were consecutively enrolled in this study. The demographic information and tumours are listed in . No case was excluded during the research.
Table 1. The demographic and tumour information of patients included in this study.
Haemorrhage complications were observed in 3 cases (6.0%, 3/50), all of which were haemoperitoneum related to needle tract bleeding. Two (4.0%, 2/50) of these were found during ablation, which presented with rapid bleeding, received CEUS-guided complementary ablation, resulting in the successful termination of bleeding. The other case of bleeding was found 4 h after ablation (2.0%, 1/50). In this case, the patient received ablation together with splenectomy. CEUS-guided complementary ablation was conducted and showed that bleeding ceased after complementary ablation. However, the patient’s clinical symptoms failed to improve, which suggested that active bleeding still existed. Therefore, a laparotomy was conducted and bleeding was found at the suture of spleen fossa. In addition, a conjunctival haemorrhage occurred in one case on the second day after ablation, but the symptoms disappeared spontaneously. Other patients showed stable vital signs and no severe complications were found. All patients were discharged within 7–10 days after ablation.
One month after ablation, all patients received contrast-enhanced CT/MRI and all tumours (100%) were completely ablated. The median follow-up periods were 18.7 ± 12.0 months (1 ∼ 42 months). During the follow-up, 1 LTP (2.0%, 1/50) was detected and the patient subsequently received transcatheter arterial chemoembolization (TACE). Fifteen cases of IDR (30.0%, 15/50) were detected; 8 received ablation, 2 received TACE, and 5 received supportive care. A total of 8 patients (16.0%, 8/50) died. Five of them died within 1 year after ablation, and the causes of death were HCC progression (n = 1), hepatic failure (n = 2) and variceal bleeding (n = 2); 2 died of hepatic failure within 2 years after ablation; 1 died of hepatic failure within 3 years after ablation. The 1-, 2- and 3-year OS () and RFS rates () were 84.8%, 82.7% and 82.7%, and 67.9%, 64.0% and 64.0%, respectively.
Discussion
Although the overall incidence of haemorrhage after thermal ablation is relatively low, these complications significantly increase the inpatient costs and hospitalised stays, and can even cause death [Citation17]. Abnormal coagulation was found to be a significant risk factor for haemorrhage [Citation19]. Indeed, the rate of needle tract bleeding in this study was 6.0% (3/50), which was higher than that of previous studies [Citation17,Citation22–25], indicating that abnormal coagulation had been associated with an increased risk of bleeding. In order to reduce the risk of haemorrhage, precautionary measures were taken before, during, and after the ablation.
Gastrointestinal varices are present in almost half of patients with cirrhosis and routine gastroscopy examination and endoscopic therapy was suggested to be conducted in patients with portal hypertension, even those without evidence of variceal bleeding, in order to reduce the risk of gastrointestinal variceal bleeding [Citation26]. In addition, studies have shown that the risk of short term re-bleeding was high in patients with variceal bleeding [Citation27], so we excluded patients who had evidence of variceal bleeding in the month preceding the ablation. These patients should first receive treatment for the variceal bleeding, then undergo the thermal ablation. Because thermal ablation is commonly used for early-stage HCC, which often grows slowly [Citation28], patients will not miss the optimal treatment opportunity. As a result, no variceal bleeding was found in this study. Besides, Omeprazole Sodium for Injection can be used to prevent upper gastrointestinal bleeding in people who are at high risk. Although abnormal coagulation was a significant risk factor for haemorrhage, fresh frozen plasma, cryoprecipitate, and platelet concentrates were commonly used to temporarily correct coagulopathy during ablation [Citation7], thereby reducing the risk of haemorrhage.
Because the most common reason for haemorrhage in thermal ablation is needle tract bleeding [Citation17], avoiding injury to blood vessels and biliary tract is very important to prevent bleeding. First, MWA is preferred for tumours with feeding vessels, due to its higher thermal efficiency over RFA for better haemostatic effects [Citation29]. Second, if multiple ablations are required, the placement of the electrode in the liver parenchyma should be optimised to reduce the number of needle entrances into liver capsule, and the needle channel should be fully burnt before the electrode was pulled out to decrease the risk of needle tract bleeding.
Among the currently used imaging methods, ultrasound is relatively more suitable for examination during ablation, and studies have shown that CEUS is sensitive to detect trauma-caused bleeding in solid organs with a similar accuracy as enhanced computerised tomography [Citation30,Citation31]. Thus, CEUS is effective for detecting needle tract bleeding during ablation. Although most bleedings after thermal ablation ceased spontaneously, measures may be necessary in cases of severe haemorrhagic complications. TAE or surgery was thought to be treatment for large arterial haemorrhage [Citation32]. However, thermal ablation has been reported for the successful treatment of trauma-caused bleeding in solid organs [Citation33]. Compared with TAE and surgery, ablation for needle tract bleeding right after bleeding detection may greatly reduce the time, cost and substantial complications. Three cases with rapid bleeding were successfully terminated after CEUS-guided ablation at needle tract, indicating that the combination of CEUS guidance and thermal ablation may provide a convenient and minimally invasive method for the treatment of needle tract bleeding.
In this study, one case of bleeding was found 4 h after ablation, which received ablation together with splenectomy. CEUS failed to detect the bleeding at the suture of the spleen fossa until the laparotomy was conducted. Haemostatic materials were used to maintain haemostasis at the spleen fossa suture site during the splenectomy. We considered that the block of haemostatic materials may be the reason why CEUS failed to discover the bleeding at the suture of the spleen fossa.
After complementary ablation guided by CEUS at needle tract, the major haemorrhage rate related to thermal ablation was 0% in this study. One bleeding after ablation was related to splenectomy. No other major complications or ablation-related death occurred. This rate was lower than those of other studies in which major complication rates of 2.0%-3.4% were reported [Citation13,Citation14,Citation18,Citation29,Citation34]. In addition, our study showed that the 1-year OS rate was 84.8%, which was lower than those of previous studies [Citation13,Citation14,Citation18,Citation29,Citation34,Citation35], and 5 cases (62.5%) died within 1 year after ablation. However, the 3-year OS rate was 82.7%, which was similar to that of previous studies [Citation13,Citation14,Citation18,Citation29,Citation34,Citation35], showing that with gastroscopy before ablation, platelet concentrate or fresh frozen plasma transfusion during ablation and CEUS-guided ablation to cease needle tract bleeding, HCC patients with abnormal coagulation function might also achieve comparable OS rate.
There were some limitations in this study. First, a better randomised controlled trial should be designed, because our results were compared to previous studies. Thermal ablation should be compared with other treatments to further evaluate the value of thermal ablation in HCC with abnormal coagulation. Second, larger sample sizes and longer follow-ups are needed to observe the long-term safety. Third, all the cases in this study were of haemoperitoneum. However, when the bleeding occurs in intercostal vessels, the diaphragm, pleura, and biliary tract, the safety of CEUS-guided thermal ablation for haemothorax and haemobilia should be further investigated.
In conclusion, with gastroscopy before ablation, platelet concentrate or fresh frozen plasma transfusion during ablation and CEUS-guided ablation to cease needle tract bleeding, thermal ablation achieved a low rate of major complications and satisfied OS rate for HCC in patients with lowest platelet count of 16 × 109/L or highest INR of 2.14, indicating it to be a safe treatment method.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
Additional information
Funding
References
- Torre LA, Bray F, Siegel RL, et al. (2015). Global cancer statistics, 2012. CA Cancer J Clin 65:87–108.
- Bruix J, Sherman M. (2011). Management of hepatocellular carcinoma: an update. Hepatology 53:1020–2.
- Bruix J, Gores GJ, Mazzaferro V. (2014). Hepatocellular carcinoma: clinical frontiers and perspectives. Gut 63:844–55.
- De Pietri L, Bianchini M, Montalti R, et al. (2016). Thrombelastography-guided blood product use before invasive procedures in cirrhosis with severe coagulopathy: a randomized, controlled trial. Hepatology 63:566–73.
- Tejima K, Masuzaki R, Ikeda H, et al. (2010). Thrombocytopenia is more severe in patients with advanced chronic hepatitis C than B with the same grade of liver stiffness and splenomegaly. J Gastroenterol 45:876–84.
- Andriulli A, Tripodi A, Angeli P, et al. (2016). Hemostatic balance in patients with liver cirrhosis: report of a consensus conference. Dig Liver Dis 48:455–67.
- Ghadimi K, Levy JH, Welsby IJ. (2016). Perioperative management of the bleeding patient. Br J Anaesth 117:iii18–30.
- Lee MW, Raman SS, Asvadi NH, et al. (2017). Radiofrequency ablation of hepatocellular carcinoma as bridge therapy to liver transplantation: a ten year intention-to-treat analysis. Hepatology 65:1979–90.
- Qi X, Wang D, Su C, et al. (2015). Hepatic resection versus transarterial chemoembolization for the initial treatment of hepatocellular carcinoma: a systematic review and meta-analysis. Oncotarget 6:18715–33.
- Taner T, Nagorney DM, Tefferi A, et al. (2012). Splenectomy for massive splenomegaly: long-term results and risks for mortality. Ann Surg 258:1034–9.
- Ahuja C, Farsad K, Chadha M. (2015). An overview of splenic embolization. AJR Am J Roentgenol 205:720–5.
- Han J-B, Kong F-W, Ding H, et al. (2017). Hepatectomy combined with microwave ablation of the spleen for treatment of hepatocellular carcinoma complicated with splenomegaly: a retrospective study. Mol Clin Oncol 6:204–8.
- Wang C, Wang HH, Yang W, et al. (2015). Multicenter randomized controlled trial of percutaneous cryoablation versus radiofrequency ablation in hepatocellular carcinoma. Hepatology 61:1579–90.
- Lee DH, Lee JM, Lee JY, et al. (2014). Radiofrequency ablation of hepatocellular carcinoma as first-line treatment: long-term results and prognostic factors in 162 patients with cirrhosis. Radiology 270:900–9.
- Fonseca AZ, Santin S, Gomes LGL, et al. (2014). Complications of radiofrequency ablation of hepatic tumors: frequency and risk factors. World J Hepatol 6:107–13.
- Bertot LC, Sato M, Tateishi R, et al. (2011). Mortality and complication rates of percutaneous ablative techniques for the treatment of liver tumors: a systematic review. Eur Radiol 21:2584–96.
- Goto E, Tateishi R, Shiina S, et al. (2010). Hemorrhagic complications of percutaneous radiofrequency ablation for liver tumors. J Clin Gastroenterol 44:374–80.
- Kim Y, Lim HK, Rhim H, et al. (2013). Ten-year outcomes of percutaneous radiofrequency ablation as first-line therapy of early hepatocellular carcinoma: analysis of prognostic factors. J Hepatol 58:89–97.
- Basili S, Raparelli V, Violi F. (2010). The coagulopathy of chronic liver disease: is there a causal relationship with bleeding? Yes. Eur J Intern Med 21:62–4.
- Chinese Society of Liver Cancer, Chinese Anti-Cancer Association; Chinese Society of Clinical Oncology, Chinese Anti-Cancer Association; Liver Cancer Study Group, Chinese Society of Hepatology, Chinese Medical Association (2011). Expert consensus on the norms of local ablation therapy for hepatocellular carcinoma. Chinese J Hepatol 19:257–9.
- Rossi S, Ravetta V, Rosa L, et al. (2011). Repeated radiofrequency ablation for management of patients with cirrhosis with small hepatocellular carcinomas: a long-term cohort study. Hepatology 53:136–47.
- Livraghi T, Solbiati L, Meloni MF, et al. (2003). Treatment of focal liver tumors with percutaneous radio-frequency ablation: complications encountered in a multicenter study. Radiology 226:441–51.
- Rhim H, Chintapalli KN, Wood BJ, et al. (2004). Radiofrequency thermal ablation of abdominal tumors: lessons learned from complications. Radiographics 24:41.
- Rhim H, Lim HK, Kim YS, et al. (2008). Radiofrequency ablation of hepatic tumors: lessons learned from 3000 procedures. J Gastroenterol Hepatol 23:1492–500.
- Mulier DS, Mulier P, Ni Y, et al. (2002). Complications of radiofrequency coagulation of liver tumours. Br J Surg 89:1206–22.
- Garcia-tsao G, Bosch J. (2010). Management of varices and variceal hemorrhage in cirrhosis. N Engl J Med 362:823–32.
- LaBreque D, Dite P, Fried M, Gangl A, Kan AG, Esophageal varices. World Gastroenterology Organisation Global Guidelines; 2014, 2–3.
- Van Meer S, De Man RA, Coenraad MJ, et al. (2015). Surveillance for hepatocellular carcinoma is associated with increased survival: results from a large cohort in the Netherlands. J Hepatol 63:1156–63.
- Yu J, Yu X-L, Han Z-Y, et al. (2017). Percutaneous cooled-probe microwave versus radiofrequency ablation in early-stage hepatocellular carcinoma: a phase III randomised controlled trial. Gut, 2016,66(6):gutjnl-2016-312629.
- Zhou L, Kuang M, Xu Z, et al. (2015). Contrast-enhanced sonographically guided thermal ablation for treatment of solid-organ hemorrhage: preliminary clinical results. J Ultrasound Med 34:907–15.
- Miele V, Piccolo CL, Galluzzo M, et al. (2016). Contrast-enhanced ultrasound (CEUS) in blunt abdominal trauma. Br J Radiol 89:20150823
- Wu XY, Shi XL, Zhou JX, et al. (2012). Life-threatening hemorrhage after liver radiofrequency ablation successfully controlled by transarterial embolization. World J Hepatol 4:419–21.
- Maroulis I, Spyropoulos C, Kalogeropoulou C, Karavias D. (2013). Use of radiofrequency ablation for controlling liver hemorrhage in the emergency setting; report of two cases and review of the literature. Ulus Travma Ve Acil Cerrahi Derg 19:167–72.
- Xu Y, Shen Q, Liu P, et al. (2017). Microwave ablation for the treatment of hepatocellular carcinoma that met up-to-seven criteria: feasibility, local efficacy and long-term outcomes. Eur Radiol 27:3877–87.
- Peng Z-W, Lin X-J, Zhang Y-J, et al. (2012). Radiofrequency ablation versus hepatic resection for the treatment of hepatocellular carcinomas 2 cm or smaller: a retrospective comparative study. Radiology 262:1022–33.