Abstract
Background: Retroperitoneal metastases are common, and most present with symptoms; however, treatments for this condition are limited. This retrospective study verified the efficacy and safety of microwave ablation (MWA) in retroperitoneal metastases patients.
Methods: Patients with pathologically confirmed malignant carcinoma and imaging showing retroperitoneal metastases were enrolled and underwent MWA. The end-points included objective response rate, time to local progression (TTLP), overall survival, visual analogue scale (VAS) score, dose of morphine pre- and post-ablation and complications.
Results: Twenty-three patients were enrolled. The mean tumour diameter was 3.6 cm. Altogether, 29 tumour sites in 23 patients were ablated during 23 procedures; technical success was achieved in all 23 patients. The objective response and disease control rates were 95.7% and 100.0%, respectively. The mean TTLP and median OS were 22.8 months (95% CI: 16.1–29.6 months) and 10.6 months (95% CI: 7.4–13.8 months), respectively. In 13 patients with symptoms, the VAS values before ablation and 48 h, 1 month, 2 months, 3 months and 6 months after ablation were 5.38, 2.77 (p = 0.015), 2.15 (p = 0.001), 2.17 (p = 0.001), 1.40 (p = 0.000) and 1.71 (p = 0.006), respectively. The corresponding morphine doses were 76.9 mg, 70.7 mg (p = 0.584), 50.7 mg (p = 0.031), 55.0 mg (p = 0.097), 46.0 mg (p = 0.057) and 40.0 mg (p = 0.363), respectively. No ablation-associated mortality was observed. Major complications, minor complications and adverse events were observed in eight (34.8%), five (21.7%) and four (17.4%) patients, respectively.
Conclusion: MWA for the treatment of retroperitoneal metastases was effective and the complications were common.
Introduction
Retroperitoneal metastases commonly occur in multiple solid tumours and especially in digestive system, genitourinary, gynaecological and testicular carcinomas [Citation1–10]. Patients with retroperitoneal metastases have a poor prognosis, with symptoms of abdominal pain, abdominal distention and back pain [Citation1–10]. Treatment strategies include radical surgery, irradiation and chemotherapy. Most patients with retroperitoneal metastases are in advanced stages with metastases to other tumour sites as well, making surgery difficult. Only those with oligometastases in addition to primary tumour sites can benefit from operations for both primary and retroperitoneal metastases. Irradiation is restricted because of the interval of 3–4 weeks and the fixed position during the treatment procedure. The relatively low response rate of chemotherapy indicates that it cannot play an important role in the management of local tumour control [Citation11–16].
Image-guided thermal ablation including radiofrequency ablation (RFA), microwave ablation (MWA) and laser ablation developed considerably during the past decade [Citation17–24]. Thermal ablation, especially RFA and MWA, has been widely applied in the treatments of primary hepatic carcinoma, primary or secondary pulmonary carcinoma and primary renal cancer [Citation17–27]. Their efficacy and safety were verified in those tumours. Several small sample studies showed that RFA in the management of retroperitoneal metastases was effective and safe [Citation1,Citation2,Citation6,Citation8,Citation10].
Compared with RFA, MWA had several advantages as follows: larger ablation zones, shorter ablation times, less impact of the heat sink effect, lack of interference between two or more antennae and lack of effect on cardiac pacemakers [Citation28,Citation29]. However, to date, there has been no study focussed on MWA in the treatment of retroperitoneal metastases. We conducted this retrospective study to verify the efficacy and safety of MWA in the treatment of retroperitoneal metastases.
Materials and methods
Patients
Twenty-three patients with the following characteristics were enrolled in the retrospective study: (1) pathologically confirmed presence of malignant tumours in the primary tumour sites, (2) contrast-enhanced computed tomography or magnetic resonance imaging (MRI)-confirmed existence of retroperitoneal metastases, (3) Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–1 and (4) adequate hepatic, renal and bone marrow function that could afford MWA: Alanine transaminase and aspartate aminotransferase ≤1.5 upper limit of normal (ULN); bilirubin ≤1.5 ULN; creatinine and urea nitrogen within the normal range; prothrombin time (PT) and activation of partial thrombin time (APTT) within the normal range; white blood cells ≥3.0 × 109/L, neutrophils ≥1.5 × 109/L, platelets ≥50 × 109/L and haemoglobin ≥95 g/L. The study was approved by the ethics committee of Shandong Provincial Hospital affiliated with Shandong University, and informed consents were obtained from all enrolled patients.
MWA procedure
A General Electric (GE)-light speed 64 V spiral CT machine was used for the scanning of all patients. The MWA instrument used was an MTC-3C (YZB 1408–2003, No: SFDA (III) 20073251059: Nanjing Qiya Medical Equipment Co., Jiangsu, China). The microwave emission frequency was 2.450 ± 50 MHz, and the adjustable output level of the continuous wave ranged between 0 and 100 W. The microwave antennae had an effective length of 100–180 mm and an outside diameter of 14–20 G with a long, tapered end; a water circulation cooling system was used to reduce the surface temperature of the antennae. MWA with an output of 60–80 W has an ablative zone of nearly 3.5 × 3 cm2. For tumours that were larger than 3.5 cm, two ablation antennae were applied. Local anaesthesia (lidocaine and bupivacaine) and pre-emptive analgesia (morphine) were used [Citation14]. Preoperative localisation was confirmed by observation of CT images and patient movement in different positions. After the achievement of satisfactory anaesthesia (when the patient had no reaction to a local stimulus), the MWA procedure was performed by cutting the skin at the punctured point and puncturing the ablation microwave antennae through the deeper layers of tissue to the nodular lesion; this procedure was performed according to the preoperative-planned channel, with the puncture depth as the preoperative-planned “target skin distance”. MWA could be performed after the cold circulating pipes and pumps had been connected to the MWA antennae and machine with cables. After the procedure, the microwave ablation antennae were extracted, local disinfection was performed and a bandage was used to seal the wound. CT scans without IV contrast were obtained immediately after MWA to observe the size and shape in relation to nearby organs, as well as to determine if there were any signs of bleeding or other problems ( and ).
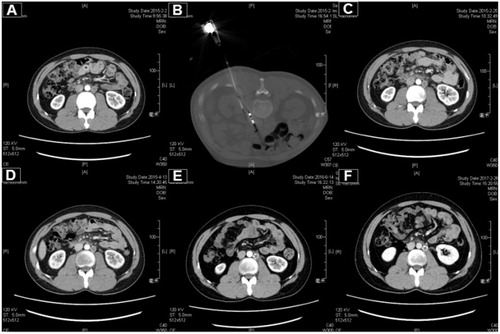
Figure 2. A 27-year-old male patient with testicular embryonal carcinoma and peritoneal metastasis was treated with MWA. (A) The enhanced CT pre-ablation. (B) The procedure of ablation. (C) One month post-ablation. (D) Three months post-ablation. (E) Sixteen months post-ablation. (F) Twenty-four months post-ablation.
Follow-up
The response to MWA
According to the follow-up protocol, contrast-enhanced abdominal CT was conducted each month during the first three months and then every three months thereafter for up to 2 years. The efficacy of MWA was assessed according to the modified response evaluation criteria in solid tumours (mRECIST) criteria. Both objective response rate (ORR) and disease control rate (DCR) were considered [Citation30].
Change in pain levels and dose of morphine
An 11-point score was used for the visual analogue scale (VAS) evaluation [Citation31]. VAS scores were assessed by physicians before (baseline) and at 48 h and 1, 2, 3 and 6 months after the procedure. The dose of daily morphine administration was also recorded at the same intervals.
Complications
Complications were assessed according to the standards of the International Working Group on Image-Guided Tumour Ablation [Citation32]. The SIR classification system for complications by outcome was as follows: Minor complications included the following: (A) No therapy, no consequence and (B) Nominal therapy, no consequence, including overnight admission for observation only. Major complications included the following: (C) Requires therapy, minor hospitalisation (<48 h), (D) Requires major therapy, unplanned increase in level of care, prolonged hospitalisation (>48 h), (E) Permanent adverse squeal and (F) Death. Adverse events referred to pain, post-ablation syndrome and asymptomatic minor bleeding or fluid accumulation as detected by CT. Post-ablation syndrome is induced by the absorption of necrotic material and the release of inflammatory factors. This syndrome is a transient, self-limiting symptom/sign complex of low-grade fever, nausea, vomiting and general malaise [Citation33].
Statistical analyses
Continuous variables were expressed as the mean ± standard deviation. Paired Student’s t test was applied to evaluate the VAS score and the dose of morphine before and after the procedure.
The time to local progression (TTLP) was defined from the date of ablation to the date of local progression. The overall survival post-ablation was calculated from the time of ablation to the date of death. Kaplan–Meier univariate analyses and Cox regression multivariate analyses were used for survival analyses. SPSS 17.0 (SPSS Inc., Chicago, IL) was used for the analysis. Tests were two-sided, and a p values of less than 0.05 was considered statistically significant.
Results
Baseline characteristics
From 1 October 2012, to 31 August 2016, 23 patients with retroperitoneal metastases were recruited (). The mean age was 61 years, with a range from 27 to 82 years. Among them, 19 patients (82.6%) were male, eight (34.8%) were 65 years of age or older, and 22 (95.7%) had an ECOG PS status of 1. For the primary tumour sites, the digestive system (15 patients, 65.2%) and urogenital system (four patients, 17.4%) were the most common sites. Adenocarcinoma and squamous cell carcinoma were the common histological types, accounting for 34.8% and 30.4%, respectively. Poor and moderate differentiation were the most common differentiation types, accounting for 17.4% and 21.7%, respectively. The details of the 23 enrolled patients are recorded in .
Table 1. Baseline characteristics of 23 enrolled patients.
Local effects
The mean tumour diameter in the enrolled patients was 3.6 cm, ranging from 1.0 to 11.5 cm. Among them, 18 patients had tumours sized 3.0 cm or larger and 13 patients had tumours sized 3.5 cm or larger. Five and nine patients were treated with irradiation and chemotherapy before the MWA, respectively. Eleven patients were treated with two antennas. Three (13.0%), 13 (56.5%), 6 (26.1%) and 1 (4.3%) patients were treated with a power of 50, 60, 70 and 80 W, respectively. The mean ablation times were 7.1 min, with a range of 3.5–16.0 min. A total of 29 tumour sites in 23 patients were ablated during 23 procedures ( and ). Technical success was achieved in all 23 patients (100%). CR, PR and SD were observed in 9, 13 and 1 patient, respectively. The ORR and DCR were 95.7% and 100.0%, respectively. Two patients who recurred were treated with MWA again and achieved CR.
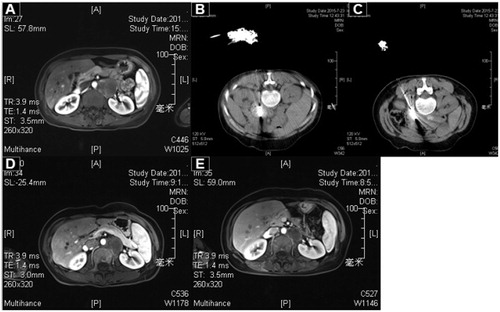
Figure 3. A 45-year-old female patient with poorly differentiated cervical squamous cell carcinoma and peritoneal metastasis was treated with MWA. (A) The enhanced CT pre-ablation. (B, C). The procedure of ablation (two antennae were applied). (D) Two months post-ablation. (E) Four months post-ablation.
Time to local progression and overall survival
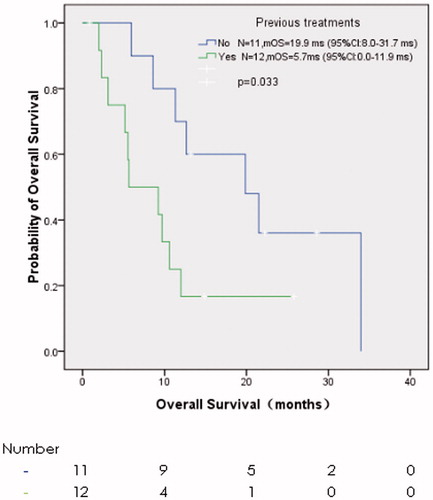
The last follow-up was on 5 March 2017, with a median follow-up of 10.6 months. Six patients (26.1%) progressed in ablative sites, and 17 patients (73.9%) died. The mean TTLP and median OS were 22.8 months (95% confidence interval, CI: 16.1–29.6 months) () and 10.6 months (95% CI: 7.4–13.8 months) (), respectively. TTLP had no correlation with the baseline characteristics and treatments including gender, age, ECOG PS, previous treatments, tumour size, tumour number and the response to MWA in the univariate analysis (). OS was associated with previous treatments only. The median OS of those without previous treatments and with previous treatments was 19.9 months (95% CI: 8.0–31.7 months) and 5.7 months (95% CI: 0.0–11.9 months), respectively (p = 0.033) (, ). The sample was too small, and thus it was not fit for univariate analyses for both TTLP and OS.
Figure 4. Kaplan–Meier survival curve of time to local progression (the mean TTLP was 22.8 months, with the 95% CI from 16.1 months to 29.6 months).
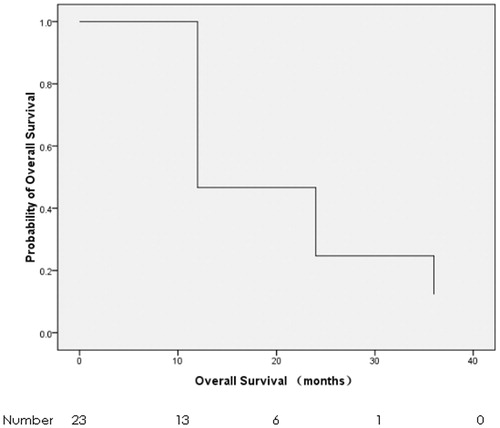
Figure 5. Kaplan–Meier survival curve of overall survival (the median overall survival was 10.6 months, with the 95% CI from 10.4 months to 20.6 months).
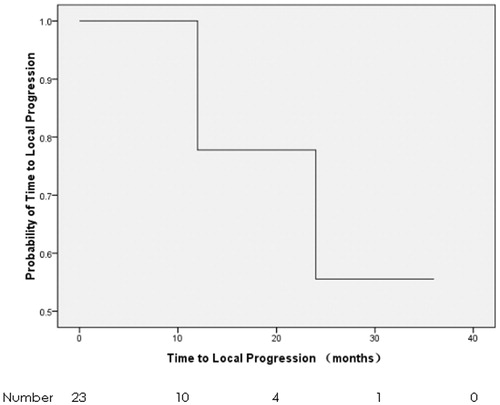
Table 2. Univariant analyses of time to local progression and overall survival.
VAS score and morphine dose
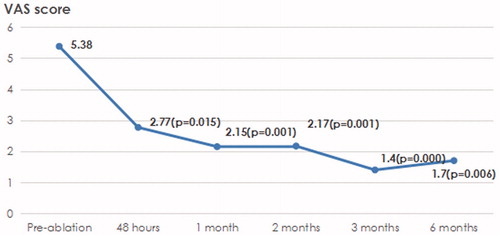
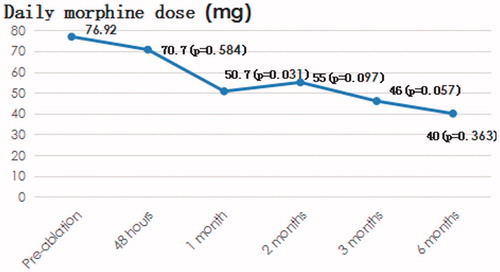
Thirteen patients presented with pain, and 10 patients used morphine to relieve the pain. In the 13 patients with pain, the VAS scores before ablation and 48 h, 1 month, 2 months, 3 months and 6 months after ablation were 5.38, 2.77, 2.15, 2.17, 1.40 and 1.71, respectively (), with p values of 0.015, 0.001, 0.001, 0.000 and 0.006, respectively. The corresponding morphine doses were 76.92, 70.70, 50.70, 55.00, 46.00 and 40.00, respectively (), and the p values were 0.584, 0.031, 0.097, 0.057 and 0.363, respectively.
Complications
Eighteen patients had complications, including nine during the MWA procedure, eight during the post-ablation period and one during both the ablation procedure and in the post-ablation period. No ablation-associated mortality was observed during the perioperative period. Major complications, minor complications and adverse events were observed in eight (34.8%), five (21.7%) and five (21.7%) patients, respectively. The major complications included haemorrhage, nerve injury, pneumothorax requiring chest tube insertion, acute pancreatitis, splenic infarction and infection. For patients with bleeding, the haemostasis drugs and red blood cells transfusion were needed. For those with acute pancreatitis, diet, nutrition support therapy and somatostatin were applied. For those with Pheumothorax, chest tube insertion was required. For patients with splenic infarction or infection, antibiotics were applied. The average days of staying in the hospital was 7 days (range 3–21 days). Most complications, except the splenic infarction, were relieved during the follow-up period (). The minor complications included sinus bradycardia, hypertension and pain.
Table 3. The complications of MWA in retroperitoneal metastases.
Discussion
In the study, we verified that MWA for the treatment of patients with retroperitoneal metastases was effective and safe. After MWA, the VAS score and the dose of morphine decreased dramatically.
We first explored the efficacy and safety of MWA in retroperitoneal metastases. In the study, ORRs post-MWA were achieved in 95.7% patients, which was similar to the rates identified in previous studies on thermal ablation. Arellano et al. [Citation8] showed that all patients treated with RFA achieved complete ablation, which was verified by positron emission tomography/computer tomography (PET/CT) during the post-ablation follow-up period. Fan et al. [Citation34] reported that the ORR of cryoablation ranged from 72.8% to 82.7%. Mou et al. [Citation35] showed that in patients treated with ultrasound-guided laser ablation, ORR reached 62.5%. Jiang et al. [Citation36] summarised a total of 18 studies, which included 398 patients with 491 retroperitoneal tumours that were treated with thermal ablation, and showed that the ORR ranged from 74.5% to 100.0% [Citation1–10,Citation37–42].
The mean TTLP was 22.8 months, and the median OS from the start of MWA to death was 10.6 months. Previous thermal ablation, such as high-intensity focussed ultrasound (HIFU), FRA and laser ablation, showed PFS rates that ranged from 5.5 to 37.0 months, and the OS rates ranged from 9.0 to 43.0 months [Citation36]. The TTLP was similar to that in previous studies, and the OS rates were shorter than those of previous studies [Citation36]. The differences in OS between our study and previous studies are mainly because in our study, most patients had large tumours. Eighteen patients (78.3%) had tumours >3.0 cm in size, and 13 patients (56.5%) had tumours >3.5 cm in size. Fan et al. [Citation34] showed that tumour size was an independent prognostic factor of survival. Furthermore, most patients in our study presented with other metastases besides the retroperitoneal metastases.
Thermal ablation plays an important role in the treatment of pain relief. In this study, 13 patients with abdominal pain and back pain were enrolled. Another 10 patients without pain who had only one to two retroperitoneal metastases that could be cured were also enrolled. It is estimated that VAS scores and the doses of morphine decreased in most patients with pain, even for those who achieved incomplete ablation and for these not ablated at all tumour sites. In our study, the VAS scores decreased for at least 6 months, but the use of morphine was maintained for only 3 months. Machi et al. [Citation1] showed that all four patients with symptoms in their study were controlled with RFA. MWA could relieve pain due to several reasons, as follows: (1) direct damage of pain-associated nerves and (2) influence on the release of inflammation factors, which induced pain signals [Citation43,Citation44].
Major complications and minor complications occurred in 34.8% and 21.7%, respectively. The major complications included haemorrhage, nerve injury, pneumothorax needing chest tube insertion, acute pancreatitis, splenic infarction and infection. Most were relieved during the follow-up. Jiang et al. [Citation36] reported the complications of thermal ablation of retroperitoneal metastases ranged from 6.5% (2/31) to 63.9% (46/72). Pain was the most common complication of thermal ablation, followed by fever, haematoma, elevated amylase, skin burns, fistula and portal vein thrombosis [Citation36]. The rate of complications in this study was higher than those in previous studies mainly due to the difference in enrolled patients. In our study, eight patients (34.9%) had three tumours or more, while in other studies, patients with no more than two tumours were recruited. In order to reduce the complications, patients should be restricted to those with one or two tumour sites and the diameter of tumours less than 3.0 cm.
There were two major limitations in the study: one was that the samples were small, and the other was that only 13 patients in the study had abdominal and/or back pain.
In conclusion, MWA for the treatment of retroperitoneal metastases was effective and the complications were common.
Disclosure statement
The authors report no conflicts of interest. The authors alone are responsible for the content and writing of the paper.
References
- Machi J, Oishi AJ, Furumoto NL, Oishi RH. (2003). Sonographically guided radio-frequency thermal ablation for unresectable recurrent tumors in the retroperitoneum and the pelvis. J Ultrasound Med 22:507–13.
- Keil S, Bruners P, Brehmer B, Mahnken AH. (2008). Percutaneous radiofrequency ablation for treatment of recurrent retroperitoneal liposarcoma. Cardiovasc Intervent Radiol 31:S213–S6.
- Narayanan G, Bhatia S, Echenique A, et al. (2014). Vessel patency post irreversible electroporation. Cardiovasc Intervent Radiol 37:1523–9.
- Gill IS, Novick AC, Meraney AM, et al. (2000). Laparoscopic renal cryoablation in 32 patients. Urology 56:748–53.
- Lee DI, McGinnis DE, Feld R, Strup SE. (2003). Retroperitoneal laparoscopic cryoablation of small renal tumors: intermediate results. Urology 61:83–8.
- Kariya S, Tanigawa N, Kojima H, et al. (2005). Radiofrequency ablation combined with CO2 injection for treatment of retroperitoneal tumor: protecting surrounding organs against thermal injury. Am J Roentgenol 185:890–3.
- Patel MN, Menon M, Rogers CG. (2008). Robot-assisted retroperitoneal renal cryoablation. J Robot Surg 2:257.
- Arellano RS, Flanders VL, Lee SI, et al. (2010). Imaging-guided percutaneous radiofrequency ablation of retroperitoneal metastatic disease in patients with gynecologic malignancies: clinical experience with eight patients. Am J Roentgenol 194:1635–8.
- Wan ZH, Bai QY. (2011). Focused ultrasound in patients with primary retroperitoneal sarcoma therapy. Chinese J Hernia Abdom Wall Surg 5:49–52.
- Gao F, Gu Y, Huang J, et al. (2012). Radiofrequency ablation of retroperitoneal metastatic lymph nodes from hepatocellular carcinoma. Acad Radiol 19:1035–40.
- Dusaud M, Malavaud B, Bayoud Y, et al. (2016). Post-chemotherapy retroperitoneal teratoma in nonseminomatous germ cell tumors: Do predictive factors exist? Results from a national multicenter study J Surg Oncol 114:992–6.
- MacNeill AJ, Gronchi A, Miceli R, et al. (2017). Postoperative morbidity after radical resection of primary retroperitoneal sarcoma: a report from the transatlantic RPS working group. Ann Surg. [Epub ahead of print]. doi:10.1097/SLA.0000000000002250.
- MacNeill AJ, Miceli R, Strauss DC, et al. (2017). Post-relapse outcomes after primary extended resection of retroperitonealsarcoma: a report from the trans-atlantic RPS working group. Cancer 123:1971–8.
- Gershman B, Takahashi N, Moreira DM, et al. (2016). Radiographic size of retroperitoneal lymph nodes predicts pathological nodal involvement for patients with renal cell carcinoma: development of a risk prediction model. BJU Int 118:742–9.
- Ju X, Li P, Shao P, et al. (2016). Retroperitoneal laparoscopic nephrectomy combined with bench surgery and autotransplantation for renal cell carcinoma in the solitary kidney or tumor involving bilateral kidneys: Experience at a single center and technical considerations. Urol Int 97:473–9.
- Rungruang BJ, Miller A, Krivak TC, et al. (2017). What is the role of retroperitoneal exploration in optimally debulked stage IIIC epithelial ovarian cancer? An NRG Oncology/Gynecologic Oncology Groupancillary data study. Cancer 123:985–93.
- Yang X, Ye X, Huang G, et al. (2017). Repeated percutaneous microwave ablation for local recurrence of inoperable Stage I nonsmall cell lung cancer. J Cancer Res Ther 13:683–8.
- Li W, Man W, Guo H, Yang P. (2016). Clinical study of transcatheter arterial chemoembolization combined with microwave ablation in the treatment of advanced hepatocellular carcinoma. J Cancer Res Ther 12(Suppl):C217–20.
- Kim C, Hoang CD, Kesarwala AH, et al. (2017). Role of local ablative therapy in patients with oligometastatic and oligoprogressive non-small cell lung cancer. J Thorac Oncol 12:179–93.
- de Baere T, Tselikas L, Catena V, et al. (2016). Percutaneous thermal ablation of primary lung cancer. Diagn Interv Imaging 97:1019–24.
- Howard J, Masterson L, Dwivedi RC, et al. (2016). Minimally invasive surgery versus radiotherapy/chemoradiotherapy for small-volume primary oropharyngeal carcinoma. Cochrane Database Syst Rev 12:CD010963.
- Facciorusso A, Serviddio G, Muscatiello N. (2016). Local ablative treatments for hepatocellular carcinoma: an updated review. World J Gastrointest Pharmacol Ther 7:477–89.
- Lucchina N, Tsetis D, Ierardi AM, et al. (2016). Current role of microwave ablation in the treatment of small hepatocellular carcinomas. Ann Gastroenterol 29:460–5.
- Floridi C, De Bernardi I, Fontana F, et al. (2014). Microwave ablation of renal tumors: state of the art and development trends. Radiol Med 119:533–40.
- Sidoff L, Dupuy DE. (2017). Clinical experiences with microwave thermal ablation of lung malignancies. Int Hyperthermia 33:25–33.
- Zhou F, Yu X, Liang P, et al. (2016). Combined microwave ablation and systemic chemotherapy for liver metastases from oesophageal cancer: preliminary results and literature review. Int J Hyperthermia 32:524–30.
- Nour-Eldin NA, Exner S, Al-Subhi M, et al. (2017). Ablation therapy of non-colorectal cancer lung metastases: retrospectiveanalysis of tumour response post-laser-induced interstitial thermotherapy (LITT), radiofrequency ablation (RFA) and microwave ablation (MWA). Int J Hyperthermia 33:820–9.
- Singla N, Gahan J. (2016). New technologies in tumor ablation. Curr Opin Urol 26:248–53.
- Poulou LS, Botsa E, Thanou I, et al. (2015). Percutaneous microwave ablation vs radiofrequency ablation in the treatment of hepatocellular carcinoma. World J Hepatol 7:1054–63.
- Edeline J, Palmer D, Blanc JF, et al. (2017). mRECIST for systemic therapies: more evidence is required before recommendations can be made. J Hepatol S0168-8278:30135–6.
- Clarecon F, Jean B, Pham HP, et al. (2013). Value of percutaneous radiofrequency ablation with or without percutaneous vertebroplasty for pain relief and functional recovery in painful bone metastases. Skelet Radiol 42:25–36.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria – a 10-year update. Radiology 273:241–60.
- Ye X, Fan W, Chen JH, et al. (2015). Chinese expert consensus workshop report: guidelines for thermal ablation of primary and metastatic lung tumors. Thorac Cancer 6:112–21.
- Fan W, Niu L, Wang Y, et al. (2016). Percutaneous computed tomography-guided cryoablation for recurrent retroperitoneal soft tissue sarcoma: a study of safety and efcacy. Oncotarget 7:42639–49.
- Mou Y, Zhao Q, Zhong L, et al. (2016). Preliminary results of ultrasound-guided laser ablation for unresectablemetastases to retroperitoneal and hepatic portal lymph nodes. World J Surg Oncol 14:165.
- Jiang T, Deng Z, Tian G, et al. (2017). Percutaneous laser ablation: new contribution to unresectable high-risk metastatic retroperitoneal lesions. Oncotarget 8:2413–22.
- Zhao M, Li X, Wang J, et al. (2012). Retroperitoneal schwannoma treated with percutaneous computed tomography-guided radiofrequency ablation. J Neurosurg Spine 17:173–6.
- Littrup PJ, Bang HJ, Currier BP, et al. (2013). Soft-tissue cryoablation in difuse locations: feasibility and intermediate term outcomes. J Vasc Interv Radiol 24:1817–25.
- Araujo LH, Gouveia HR, Freitas Ede Q, et al. (2013). Hepatic transarterial chemoembolization and retroperitoneal lymph node radiofrequency ablation in the multidisciplinary approach of an overt metastatic leiomyosarcoma. Cancer Imaging 13:123–7.
- Molina R, Alvarez M, Capilla J, Paez A. (2014). Radiofrequency-treated recurrence of urothelial carcinoma of the upper urinary tract after nephroureterectomy. Korean J Urol 55:844–6.
- Monfardini L, Varano GM, Foa R, et al. (2015). Local recurrence of renal cancer after surgery: prime time for percutaneous thermal ablation. Cardiovasc Intervent Radiol 38:1542–7.
- Underhill CE, Walsh NJ, Bateson BP, et al. (2016). Feasibility and safety of irreversible electroporation in locally advanced pelvic and retroperitoneal tumors. Am Surgeon 82:263–5.
- Mannion RJ, Woolf CJ. (2000). Pain mechanisms and management: a central perspective. Clin J Pain 16:S144–S56.
- Honore P, Luger NM, Sabino MA, et al. (2000). Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med 6:521–8.