Abstract
Purpose: To evaluate the efficacy and safety of ultrasound-guided percutaneous microwave ablation (MWA) for large hepatic cavernous haemangiomas (HCHs) with ablation of the feeding artery and aspiration of blood from haemangioma simultaneously.
Materials and methods: From January 2011 to December 2016, 40 patients (29 females, 11male; average age 43.25 ± 7.65 years) with 42 giant hepatic haemangiomas (mean diameter 7.02 ± 1.55 cm, range 4.1–10.8 cm) were treated with image-guided percutaneous MWA with ablation of the feeding artery and aspiration of blood from haemangioma simultaneously. After MWA, therapeutic efficacy was assessed by contrast-enhanced imaging during follow-up. Median follow-up time was 24 months (range 6–60 months).
Results: Technical effectiveness rate was 100% with a mean ablation time of 1175 ± 516 (range 480–2340) seconds. The mean number of antenna insertions was 3.82 ± 1.23 for each lesion. Clinical effectiveness rate was 95% (38/40). There was a significant decrease of the tumour volume within three days after ablation compared with before ablation (p < 0.001). The mean tumour volume shrinkage rate was 59.67 ± 16.73% (range 28.20–89.72%) within three days after ablation. Minor complications mainly included fever, transient elevation of transaminase, pleura effusion, and haemoglobinuria. One patient developed a major complication with acute kidney injury (AKI) shortly after MWA, whose renal function gradually recovered after haemodialysis. No other severe complications occurred in perioperative and follow-up periods.
Conclusions: Ultrasound-guided percutaneous MWA for large HCHs with ablation of the feeding artery and aspiration of blood from haemangioma simultaneously is safe and effective.
Introduction
Hepatic cavernous haemangiomas (HCHs) are the most common benign neoplasms in liver, with the prevalence in the general population as many as 20% in autopsy studies [Citation1]. Haemangiomas are referred to as “giant” if greater than 4 cm in diameter [Citation2]. Although most haemangiomas are asymptomatic and can be managed safely with observation alone, larger lesions may produce a variety of symptoms and signs, including abdominal pain, dyspepsia, jaundice, thrombocytopenia and even spontaneous rupture [Citation3,Citation4]. The primary treatment is surgical resection, transarterial embolization, radiation therapy, thermal ablation, and the use of a vascular endothelial growth factor (VEGF) inhibitor have also been reported [Citation5–8]. Unfortunately, surgical resection is associated with morbidity up to 27% and low risk of mortality [Citation9,Citation10]. Transarterial embolization treatment is not considered as curative because recurrence is common due to vascular recanalization, and may result in severe complications including ectopic embolizations and destructive biliary damage [Citation6,Citation11,Citation12]. Radiation therapy provides partial reduction in haemangioma size and relief of symptoms, but has certain risks including radiation hepatitis, veno-occlusive disease, and the development of hepatomas [Citation7].
The most extensively studied percutaneous thermal ablation modality for treatment of hepatic haemangiomas is radiofrequency (RF) ablation. While limited power and small ablation zones can result in long ablation times and incomplete tumour devascularisation requiring repeated ablation, presumably the result of perfusion-mediated tissue cooling (heat-sink) and limited power deposition [Citation13–16]. Compared with RF ablation, the benefits of microwave ablation (MWA) are less limitation by heat sink effect and tissue charring and the ability to achieve larger ablation volume in shorter time [Citation17], so MWA would be a better way in ablating symptomatic HCHs. Ziemlewicz et al. [Citation18] reported seven patients with symptomatic HCHs performed percutaneous MWA, and results showed that percutaneous MWA was safe, well-tolerated, and effective in markedly shrinking large HCHs and improving symptoms in most patients. Because haemangiomas are sinusoids and the main component inside is the blood, conventional ablation by inserting the ablation needle into the tumour can lead to massive red cell destruction, intravascular haemolysis, and even acute kidney injury (AKI), which was related with the size of the ablation zone and length of the procedure [Citation19]. When treating large HCHs with thermal ablation, the number of ablations and the duration of the ablation sequences should be reduced to the absolute minimum. The blood supply of hepatic haemangioma is predominantly derived from the hepatic artery [Citation20], so if blocking the feeding artery of haemangioma firstly by ablation and aspirating the blood from haemangioma simultaneously may reduce the volume of haemangioma and shorten the ablation time, thus reducing the risk of ablation. Therefore, the purpose of the study is to explore the clinical value of US-guided percutaneous MWA for HCHs with ablation of the feeding artery and aspiration of blood from haemangioma simultaneously. To our knowledge, this is the first report about US-guided percutaneous MWA for HCHs with ablation of the feeding artery and aspiration of blood from haemangioma simultaneously.
Materials and methods
Patients
From January 2011 to December 2016, 40 patients (29 females, 11 males; average age 43.25 ± 7.65 years) with 42 giant hepatic haemangiomas (mean diameter 7.02 ± 1.55 cm, range 4.1–10.8 cm) were treated with image-guided percutaneous microwave (MW) ablation. Inclusion criteria for performing MWA were (1) definite diagnosis of a giant cavernous haemangioma >4 cm based on the typical enhancement pattern on contrast-enhanced multiphase computed tomography (CT) or magnetic resonance imaging (MRI); (2) clinical symptoms typically caused by the giant haemangioma present for at least one year, including abdominal pain, nausea, vomiting, abdominal fullness. And other aetiologies for these symptoms were excluded. The diagnoses of HCHs were proven pathologically in all patients using US-guided core needle biopsy during the same session as ablation. This clinical application was approved by our institutional human research review committee. Written informed consent was obtained from all patients.
Microwave equipment and ablation technique
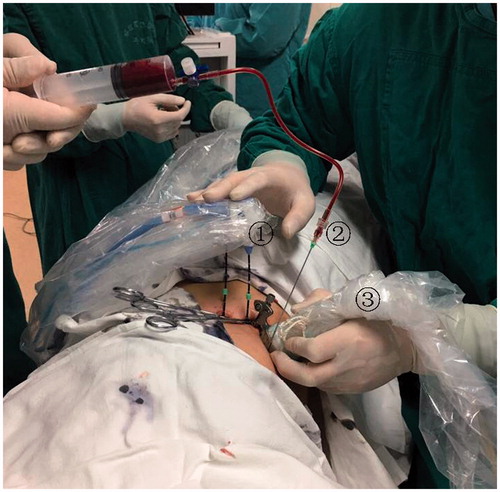
All treatments were performed in our institution and were carried out under US guidance with the patients under unconscious intravenous anaesthesia (Propofol, 6–12 mg/kg/h; Ketamine, 1–2 mg/kg) in the operating room. Additionally, when the tumours were not well visualised with conventional B-mode US, a contrast-enhanced ultrasound (CEUS)-guided ablation with SonoVue (Bracco, Milan, Italy) was performed. The 2450 MHz or 915 MHz MW system was used. The 2450 MHz MW system (KY-2000; Kangyou Medical, China) consists of three independent MW generators, three flexible coaxial cables and three water-pumping machines, which can drive three 15-gauge cooled-shaft antennae (1.1 cm antenna tip) simultaneously. The 915 MHz MW system (KY-2001;, Kangyou Medical, China) consists of two independent MW generators, two flexible coaxial cables and two water-pumping machines, which can drive two 15-gauge cooled-shaft antennae (2.2 cm antenna tip) simultaneously. The two MW system generators are capable of producing 1–100 W of power output. The antenna was 15 gauges in diameter with cool-shaft. All therapy was performed by two experienced radiologists according to the preoperative planning. Colour Doppler US or CEUS was performed to determine the location of the tumour-feeding artery of haemangioma. During the ablation, the feeding artery of haemangioma was ablated firstly, and a 18 G percutaneous transhepatic cholangiography (PTC) needle (Hakko, Japan) was inserted into the haemangioma and was used to aspirate the blood from the haemangioma simultaneously (). After ablation the feeding artery of haemangioma, if the amount of aspiration blood was less than the tumour volume and the blood could not be extracted, the ablation of the feeding artery was considered to be a success. Conversely, it was considered a failure. We monitored the hyperechoic area of ablation using grey-scale sonography and thermal monitoring to decide the endpoint of treatment. When withdrawing the antennae, the needle tracks were routinely cauterised to avoid bleeding. Hydrodissection technique and thermal monitoring technique were applied for haemangiomas abutting vital structures to avoid thermal damage. Within three days after ablation, every patient received CEUS and contrast-enhance CT or MRI to evaluate ablation area.
Hydrodissection technique
After administering a local anaesthetic comprising 1% lidocaine, a 16-gauge intravenous catheter (BD Angiocath; Sandy, UT, USA) was punctured into the peritoneal cavity between the edge of the liver and the abutting gastrointestinal tract (GIT) under US guidance. A sufficient amount of normal saline was delivered until a separation of more than 0.5 cm between the target lesion and the adjacent GIT was achieved. Drip infusion was continued during the MWA procedure to maintain a distance of more than 0.5 cm.
Thermal monitoring procedure
A thermal monitoring system attached to the microwave unit was used during treatment for haemangiomas abutting vital structures to avoid thermal damage. With US guidance, one or two 21 G thermal monitoring needles (Kangyou Medical, Nanjing, China) were placed into marginal tissue of tumour or liver proximal to the surrounding tissues or organs for real-time temperature monitoring during the ablation to protect the surrounding tissues or organs from thermal mediated injury. Based on our experimental evidence and clinical experience, the temperature cut off of ablation therapy was set at 60 °C in the patients. If the measured temperature reached 60 °C, emission of microwave antenna was stopped immediately and was activated again after the temperature decreased to 50 °C.
Therapeutic efficacy assessment and follow-up
Therapeutic efficacy assessment and follow-up was assessed by contrast enhanced imaging after the treatment. Technical effectiveness was defined by treatment of 90–100% of the volume of the haemangioma based on contrast enhanced imaging within three days after ablation, as the goal was to debulk the tumour while minimising the risk of complications. Clinical effectiveness was defined as improvement of symptoms notated during follow-up. Complications were classified according to the Society of Interventional Radiology Classification system for Complications by Outcome [Citation21]. The number of antenna insertions was defined as the total number of antenna placements for each tumour during ablation. The follow-up period was calculated starting from the beginning of MWA in all patients. Contrast-enhanced US and contrast-enhanced CT or MRI were repeated at one month and at three months intervals within one year and then at six-month intervals after MWA.
Statistical analysis
Data analysis was performed using SPSS17.0 for windows (SPSS Inc., Chicago, IL, USA) and the continuous data were expressed as mean ± standard deviations (SD). Paired t-test was used to compare the means between two groups. The level of statistical significance was set at p values less than 0.05.
Results
All patients underwent MWA successfully. Technical effectiveness rate was 100% with a mean ablation time of 1175 ± 516 (range 480–2340) seconds. The mean number of antenna insertions was 3.82 ± 1.23 for each lesion. Within three days after ablation, 38 lesions were completely necrotic, and four lesions were more than 90% necrotic with marginal residual tissue remaining in the periphery of the tumour adjacent to at risk structures based on contrast enhanced imaging within three days after ablation. The mean tumour volume was 122.50 ± 70.70 ml before ablation. The mean tumour volume was 63.24 ± 47.89 ml within three days after ablation. There was a significant decrease of the tumour volume after ablation compare with before ablation (p < 0.001). The mean tumour volume shrinkage rate was 59.67 ± 16.73% (range 28.20–89.72%) within three days after ablation (). During the follow-up, the ablation zone gradually decreased. Median follow-up time was 24 months (range 6–60 months).
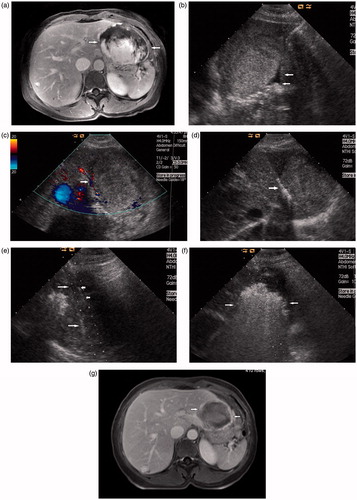
Figure 2. Images in a 50-year-old-woman who underwent MWA for hepatic cavernous haemangioma 8.8 cm in diameter. (a) Preoperative contrast-enhanced MRI showed the haemangioma was hyperenhanced in portal vein phase in left lobe and abutting the stomach (arrow). (b) The injection of normal saline (arrow) to separate the tumour and the stomach. (c) Colour Doppler ultrasonography depicts feeding arteries entering the tumour on the right side (arrow). (d) The antenna was inserted to ablate the feeding arteries (arrow). (e) US image showed the second antenna (long arrow) and the PTC needle (short arrow) were placed in the tumour. (f) US image showed the hyperechoic area of ablation covered the tumour (arrow). (g) Postoperative contrast-enhanced MRI showed complete necrosis of the tumour and the maximum diameter was reduced to 5.8 cm 3 days after MWA (arrow).
The average amount of aspiration blood during ablation was 75.58 ± 73.19 ml (range 10–400 ml). The ablation of the feeding artery was considered to be a success for 39 patients. Only one patient was considered to be a failure, because when the volume of aspiration blood from the haemangioma during ablation was 400 ml, the blood could still be extracted. Hydrodissection with saline solution was performed successfully on 9 patients. The average amount of solution for hydrodissection was 1476.66 ± 586.28 ml (range 790–2600 ml). No patients had abnormal vital signs until the day after the procedure. The injected solution was completely absorbed in all patients at the one-month follow-up CT or MRI. Patient, tumour, and procedure details are outlined in .
Table 1. characteristic of patient, tumour and procedure details.
The procedure provided clinical effectiveness in 38 of 40 patients who had complete pain relief or improvement of pain and nausea, abdominal fullness following the procedure. Overall pain score on the visual analogue scale decreased from an average of 5.2 to 1.1(p < 0.05). Clinical effectiveness rate was 95% (38/40). All patients were able to return to their pre-procedure level of activity within a week of the procedure. Two patients whose pains were unchanged after MWA. Severe complications such as abscess, bile duct injury, perforation of gastrointestinal tracts and haemorrhage requiring embolization did not occur in peri-operation and follow-up periods. Minor complications included fever, transient elevation of transaminase, pleura effusion, and haemoglobinuria. Fever occurred in six cases. The highest body temperatures were between 37.2 and 38.5 Celsius degrees lasting 1–2 days with no need of further treatment. Pleura effusion occurred in two cases and did not require drainage. Laboratory examination of liver function showed a transient elevation of transaminase in most cases. Haemoglobinuria was found at the first urination after ablation in 15 cases with large HCHs. Subsequently, the colour of urine recovered normally gradually after basification of urine and hydration treatment in 14 cases. One case developed a major complication with AKI shortly after MWA, caused by massive heat-induced intravascular haemolysis. Lab results showed AKI (creatinine 227 micromol/l and urea 13.8 mmol/l in the second day after ablation, creatinine 353 micromol/l and urea 15.1 mmol/l 3 days later after ablation). Because of progressive dyspnoea and ongoing anuria haemodialysis through a femoral catheter was started. After 12 haemodialysis, 32 days later, the renal function gradually recovered and dialysis was stopped and the patient was discharged from the hospital 34 days after the procedure.
Discussion
For most patients with HCHs, open or laparoscopic surgical resection has been considered the preferred choice of treatment [Citation22]. However, it is important to keep in mind that many of the trade-offs encountered during the treatment of cancer are not applicable to HCHs due to their benign natural history. Thus, a highly effective but morbid treatment would be a poor choice for most patients. Various percutaneous treatment methods have been proposed for HCHs, and the largest experience to date is with RF ablation [Citation14,Citation23]. MWA has higher thermal effectiveness and less limitation by heat sink effect and tissue charring compared with RF ablation, MWA has the potential to replace RF ablation for benign and malignant tumours in the liver, kidney, and lung [Citation24–26]. Therefore, US-guided percutaneous MWA could be as a new alternative to treat HCHs.
In our study, we used the method of ablation of the feeding artery and aspiration of blood from haemangioma simultaneously during US-guided percutaneous MWA for HCHs. The merits of the method were as follows: first, ablation the feeding artery of haemangioma firstly could lead to similar effect with TACE; second, aspirating the blood from haemangioma simultaneously may reduce the volume of haemangioma and shorten the ablation time. In addition, MWA is known to cause increased tissue shrinkage compared with RF ablation [Citation27], and this may be an advantage when treating symptoms caused by bulky tumours. Technical effectiveness rate was 100% in our study. In our study, the mean tumour volume shrinkage rate was 59.67 ± 16.73% (range 28.20–89.72%) within three days after MWA, which was similar with the previous report about MWA or RFA for haemangioma [Citation13–15,Citation18]. In our study, the mean ablation time was 1175 ± 516 (range 480–2340) seconds. Compared with RF ablation for giant hepatic haemangiomas in the previous reports, the ablation times of MWA for giant hepatic haemangiomas in our study was much shorter [Citation13–16,Citation28]. RF ablation for giant hepatic haemangiomas less than 10 cm in diameter, reported ablation time was 29.4–126.5 min [Citation13,Citation14,Citation28].
In our study, the complications of all patients belonged to minor ones such as fever, local pain with grade 1 and increment of liver enzymes test. The incidence rates were similar to the ones after the MWA of liver malignancies [Citation24]. About 14 out of 15 cases of haemoglobinuria after therapy tended to improve without additional remedy or management. The reason of occurrence of haemoglobinuria might be a mass of blood cells were ruined in an HCH ablation procedure. Blocking the feeding artery was failure in the case who developed AKI shortly after MWA, because when the volume of aspiration blood from the haemangioma during ablation was 400 ml, the blood could still be extracted. So we stopped aspirating. The failure to block the feeding artery may lead to more red blood cells undergoing budding and fragmentation, which presumably resulted in a more massive thermal haemolysis. Van Tilborg et al. [Citation19] reported two patients with very large symptomatic HCHs who developed AKI shortly after bipolar RF ablation, caused by massive heat-induced intravascular haemolysis, which was related with the size of the ablation zone and length of the procedure. The ablation time was 33 min for the patient in our study, the tumour maximum diameter was 10 cm. The failure to block the feeding artery and the long ablation time and the larger diameter may be risk factors for AKI. When treating large HCHs with thermal ablation, the number of ablations and the duration of the ablation sequences should be reduced to the absolute minimum, in order to prevent hemepigment-induced AKI.
This study had some limitations. First, the present study was a preliminary study, a further study, such as randomised controlled study and longer follow-up period may be needed. Second, this was only a single centre study; a multi-centre study would be more convincing.
Conclusions
In conclusion, ultrasound-guided percutaneous MWA for large HCHs with ablation of the feeding artery and aspiration of blood from haemangioma simultaneously can improve the clinical symptoms of most patients and reduce the volume of lesions, so it is safe and effective.
Disclosure statement
The authors alone are responsible for the correct and writing of the paper. No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Wanless IR. (1990). Micronodular transformation (nodular regenerative hyperplasia) of the liver: a report of 64 cases among 2500 autopsies and a new classification of benign hepatocellular nodules. Hepatology 11:787–97.
- Schnelldorfer T, Ware AL, Smoot R, et al. (2010). Management of giant hemangioma of the liver: resection versus observation. J Am Coll Surg 211:724–30.
- Corigliano N, Mercantini P, Amodio PM, et al. (2003). Hemoperitoneum from a spontaneous rupture of a giant hemangioma of the liver: report of a case. Surg Today 33:459–63.
- Watzke HH, Linkesch W, Hay U. (1989). Giant hemangioma of the liver (Kasabach-Merritt syndrome): successful suppression of intravascular coagulation permitting surgical removal. J Clin Gastroenterol 11:347–50.
- Singh RK, Kapoor S, Sahni P, Chattopadhyay TK. (2007). Giant haemangioma of the liver: is enucleation better than resection?. Ann R Coll Surg Engl 89:490–3.
- Giavroglou C, Economou H, Ioannidis I. (2003). Arterial embolization of giant hepatic hemangiomas. Cardiovasc Intervent Radiol 26:92–6.
- Gaspar L, Mascarenhas F, da Costa MS, et al. (1993). Radiation therapy in the unresectable cavernous hemangioma of the liver. Radiother Oncol 29:45–50.
- Mahajan D, Miller C, Hirose K, et al. (2008). Incidental reduction in the size of liver hemangioma following use of VEGF inhibitor bevacizumab. J Hepatol 49:867–80.
- Clarke D, Currie E, Madhavan K, et al. (2004). Hepatic resection for benign noncystic liver lesions. HPB (Oxford) 6:115–19.
- Huang ZQ, Xu LN, Yang T, et al. (2009). Hepatic resection: an analysis of the impact of operative and perioperative factors on morbidity and mortality rates in 2008 consecutive hepatectomy cases. Chin Med J (Engl) 122:2268–77.
- Shrivastava DN, Gandhi D, Seith A, et al. (2001). Transcatheter arterial embolization in the treatment of symptomatic cavernous hemangiomas of the liver: a prospective study. Abdom Imaging 26:510–14.
- Huang XQ, Huang ZQ, Duan WD, et al. (2002). Severe biliary complications after hepatic artery embolization. World J Gastroenterol 8:119–23.
- Hinshaw JL, Laeseke PJ, Weber SM, Lee FT Jr. (2007). Multiple-electrode radiofrequency ablation of symptomatic hepatic cavernous hemangioma. AJR Am J Roentgenol 189:W146–9.
- Park SY, Tak WY, Jung MK, et al. (2011). Symptomatic-enlarging hepatic hemangiomas are effectively treated by percutaneous ultrasonography-guided radiofrequency ablation. J Hepatol 54:559–65.
- Gao J, Ke S, Ding XM, et al. (2013). Radiofrequency ablation for large hepatic hemangiomas: Initial experience and lessons. Surgery 153:78–85.
- Sharpe EE III, Dodd GD III. (2012). Percutaneous radiofrequency ablation of symptomatic giant hepatic cavernous hemangiomas: report of two cases and review of literature. J Vasc Interv Radiol 23:971–5.
- Wright AS, Sampson LA, Warner TF, et al. (2005). Radiofrequency versus microwave ablation in a hepatic porcine model. Radiology 236:132–9.
- Ziemlewicz TJ, Wells SA, Lubner MA, et al. (2014). Microwave ablation of giant hepatic cavernous hemangiomas. Cardiovasc Intervent Radiol 37:1299–305.
- van Tilborg AA, Dresselaars HF, Scheffer HJ, et al. (2016). RF ablation of giant hemangiomas inducing acute renal failure: a report of two cases. Cardiovasc Intervent Radiol 39:1644–8.
- Li GW, Chen QL, Jiang JT, Zhao ZR. (2003). The origin of blood supply for cavernous hemangioma of the liver. Hepatobiliary Pancreat Dis Int 2:367–70.
- Sacks D, McClenny TE, Cardella JF, Lewis CA. (2003). Society of interventional radiology clinical practice guidelines. J Vasc Interv Radiol 14:S199–S202.
- Miura JT, Amini A, Schmocker R, et al. (2014). Surgical management of hepatic hemangiomas: a multi-institutional experience. HPB (Oxford) 16:924–8.
- Ji J, Gao J, Zhao L, et al. (2016 Apr). Computed tomography-guided radiofrequency ablation following transcatheter arterial embolization in treatment of large hepatic hemangiomas. Medicine (Baltimore) 95:e3402.
- Liang P, Wang Y, Yu X, Dong B. (2009). Malignant liver tumors: treatment with percutaneous microwave ablation–complications among cohort of 1136 patients. Radiology 251:933–40.
- Yu J, Liang P, Yu XL, et al. (2012). US-guided percutaneous microwave ablation of renal cell carcinoma: intermediate-term results. Radiology 263:900–8.
- Vogl TJ, Naguib NN, Gruber-Rouh T, et al. (2011). Microwave ablation therapy: clinical utility in treatment of pulmonary metastases. Radiology 261:643–51.
- Brace CL, Diaz TA, Hinshaw JL, Lee FT Jr. (2010). Tissue contraction caused by radiofrequency and microwave ablation: a laboratory study in liver and lung. J Vasc Interv Radiol 21:1280–6.
- Cui Y, Zhou LY, Dong MK, et al. (2003). Ultrasonography guided percutaneous radiofrequency ablation for hepatic cavernous hemangioma. World J Gastroenterol 9:2132–4.