Abstract
Background: The peritoneal surface is the second most common site of disease recurrence, after the liver, following definitive surgery for colorectal cancer. Adjuvant intraperitoneal (IP) chemotherapy delivered at time of surgical resection has the potential to delay or prevent future spread to the peritoneal surface and improve clinical outcome. The exact role of adjuvant IP chemotherapy in colorectal cancer, including its associated morbidity and mortality, is not well defined.
Study design: Systematic review and pooled random effect analysis of comparative trials examining the addition of adjuvant IP chemotherapy compared to surgery alone in colorectal cancer. The primary outcome was overall survival, and the secondary outcomes were of post-operative morbidity and mortality.
Results: In nine colorectal cancer studies identified, seven were two-arm trials comparing adjuvant IP chemotherapy to surgery alone. Of these, four trials had outcome reporting and met criteria that allowed inclusion into a random effects model. Heterogeneity was measured by Cochran’s Q-test (Q = 13.9; p = 0.01) and random effect models were utilised. Pooling eligible trials together revealed a 0.55 odds ratio of death associated with the administration of IP chemotherapy compared to surgery alone (CI = 0.31, 0.98; p = 0.04). Trials selecting patients at elevated risk for the development of peritoneal carcinomatosis by clinicopathological biomarkers for administration of adjuvant IP chemotherapy reported more favourable overall outcomes. There was no increase in mortalities or IP chemotherapy-related abdominal complication rates among patients undergoing IP chemotherapy (OR = 1.4; CI = 0.52, 3.8; p = 0.5).
Conclusions: This systematic review supports the use of adjuvant IP chemotherapy in resectable colorectal cancer at risk for peritoneal spread. Future trials should seek to standardise inclusion criteria and IP chemotherapy modalities to better define the role of this treatment in patients with resectable colorectal cancer.
Introduction
Colorectal malignancies are the most common gastrointestinal cancers in the United States. In 2016, approximately 135 000 new cases of colorectal cancers were diagnosed and nearly 50 000 of those were fatal [Citation1]. Colorectal cancer is expected to remain the third most common cause of cancer-related death in the United States since 2016 [Citation1]. Due to improved primary prevention and screening, in up to 40% of cases, colorectal cancer presents while still amenable to surgical removal [Citation2]. While stages I and II colon cancer are associated with 5-year survival rates of 90% and 78%, mortality increases sharply thereafter with stages III and IV having 50%, and less than 5% of 5-year survivals [Citation3,Citation4]. Approximately 25% of colon cancer patients presents with distant disease, and the third of those not presenting with metastases eventually develops them [Citation5,Citation6]. This rate of recurrence persists in spite of adjuvant therapy chemotherapy, which is aimed at reducing recurrence and improving clinical outcome in stages II and III patients [Citation4,Citation7,Citation8].
Therapies targeted to organs at high risk of recurrence are a current area of research focus, as part of an effort to improve patient selection for adjuvant treatment. The second most common site of recurrence in colorectal cancer is the peritoneum, which is involved in 25–35% relapses [Citation9–13]. Patients who do develop peritoneal spread have a particularly dismal prognosis, with a shorter expected median survival compared to patients with non-peritoneal metastatic colon cancer [Citation14]. Additionally, there is a disproportionally higher impact on quality of life associated with peritoneal recurrence due to the difficulties in managing GI failure with frequent intestinal obstructions. Thus, there appears to be rationale for the development of locoregional adjuvant therapy for the prevention of spread to the peritoneum, in particular in patients who are at high risk.
Cytoreductive surgery (CRS) in combination with intraperitoneal (IP) chemotherapy has become a clinically proven treatment in the management of overt peritoneal carcinomatosis from colorectal cancer. This trend follows the established management of peritoneal involvement of appendiceal cancer, ovarian cancer and other primary peritoneal surface malignancies [Citation15–19]. There is also a growing body of evidence supporting (IP) chemotherapy as a preventative strategy in gastrointestinal (GI) malignancies, including gastric cancer [Citation20,Citation21]. Preclinically, this strategy is supported by finding that cells undergo epithelial-mesenchymal transition during the peritoneal metastatic process. Recent studies have shown that this cell population acquires an increased ability to migrate and invade, and becomes exquisitely resistant to systemic chemotherapy [Citation22,Citation23]. Additionally, following implantation at distant sites, these metastatic deposits frequently have already created a microenvironment favourable for further growth and spread. They have been shown to attract tumour-promoting cell populations such as M2-tumour associated macrophages, T-regulatory cells, stellate cells and cancer-associated fibroblasts [Citation24]. These factors are thought to mediate additional resistance to chemotherapy which may in part explain the failure of systemic intravenous chemotherapy to treat peritoneal carcinomatosis [Citation25].
Furthermore, early administration of locoregional cytotoxic therapy with known pharmacokinetic advantages over IV chemotherapy may reach tumour cells prior to metastatic implantation. Reaching tumours prior to the development of cellular resistance-mediating pathways is known to yield improved efficacy [Citation26–28]. Clinically, this strategy has been demonstrated to yield improved survival with the addition of IP chemotherapy in optimally debulked stage-III ovarian cancers [Citation29]. Additionally, there is evidence from multiple randomised phase-II studies in gastric cancer supporting IP chemotherapy at the time of surgery to prevent peritoneal dissemination [Citation20]. Currently, it is not known whether adjuvant IP chemotherapy at the time of definitive surgery might play a useful role in colorectal cancer [Citation10,Citation20,Citation21,Citation30,Citation31]. A previous review on the topic identified seven comparative studies, while attesting to the heterogeneity of evidence, suggested an improved outcome with IP treatment. On the other hand, the largest study on the subject, which comprised 753 patients, was excluded from this review. Further, this review did not include a formal analysis quantifying the impact of IP chemotherapy.
To better assess the existing data supporting this approach, we systematically searched the literature and quantified the impact of adjuvant IP chemotherapy on clinical outcome with random effects models. We additionally sought to assess and quantify any potential morbidity associated with adjuvant IP chemotherapy.
Materials and methods
Search strategy
A systematic search of published literature was conducted using PubMed and Cochrane databases in August 2015. Combinations of the following terms were used to identify relevant studies: peritoneal or IP chemotherapy, carcinomatosis, colorectal cancer, cholangiocarcinoma, bile duct cancer, pancreas cancer, hepatocellular cancer and gastric cancer. Studies originally not published in English or not categorised as clinical trials were excluded. Additional review articles were screened for clinical studies not identified by the original search.
Selection criteria
Titles and abstracts were screened for eligibility by two independent researchers (PLF and MMK). All studies examining adjuvant IP chemotherapy for GI malignancies were included, studies in patients with established carcinomatosis were excluded and studies focussed on other malignancies including appendiceal and ovarian tumours were excluded as well. Other exclusion criteria included duplicates, studies published as conference abstracts only, phase I or preclinical studies, studies that did not report patient outcome data, studies that included more than 50% of patients with established peritoneal carcinomatosis, studies in which ≥50% of subjects received another form of regional therapy like intra-portal chemotherapy infusions, or studies that did not separate outcomes for those without macroscopic peritoneal carcinomatosis. Studies that used a non-chemotherapeutic IP therapy such as an immune or radiation therapy, either alone or in combination with IP chemotherapy, were also excluded. Studies that gave neoadjuvant systemic chemotherapy were excluded as well. In the case of disagreement about inclusion, the full text was examined to render a final decision.
Data analysis
Trials were sorted by histology and design. For each article, the following data were collected: author, year of publication, total number of patients including number of patients enrolled into IP chemotherapy vs. standard treatment arm in case of a randomised study, stage of disease, details on type of IP chemotherapy (including dose, administered volume and heat and timing), outcome data, toxicity, median follow-up, disease-free survival, peritoneal recurrence-free survival and overall survival. Reported toxicities were summarised and subjectively described.
Statistical analysis
Random effect models were used to correlate outcomes reported in treatment arms. First, as part of a comparative analysis, a “bubble chart” visualisation was generated. Each trial arm for both randomised and case control studies was plotted according to sample size and main reported clinical outcome. Studies that were randomised or well-matched case control designs were selected and compared using pooled odds ratio in random-effect models. Statistical heterogeneity was quantified by the I2 inconsistency test and the tau2 test, all summary statistics and analysis were performed within the R statistical and programming environment [Citation32]. Graphical displays were generated through the “ggplot2” software package [Citation33].
Results
Study population
The database search resulted in a total of 444 studies, of which 386 studies were excluded based on title and abstract. The most common reason for exclusion was that the study examined IP chemotherapy as a treatment for established peritoneal spread. A flow chart describing the literature search according to the preferred reporting items for systematic reviews and meta-analysis (PRISMA) statement is shown in [Citation34]. Full texts of the remaining 57 studies were screened for eligibility and 30 studies were excluded for reasons outlined in . There were nine studies in colorectal cancer identified for detailed analysis. The remainder consisted of 17 studies in gastric cancer and one in pancreatic cancer. There were no studies identified in biliary tract malignancies.
Figure 1. Article identification and screening according to PRISMA reporting guidelines [Citation34].
![Figure 1. Article identification and screening according to PRISMA reporting guidelines [Citation34].](/cms/asset/65009c34-f9d3-4492-83f8-11134db63937/ihyt_a_1401742_f0001_b.jpg)
Among the nine colorectal cancer trials, 853 patients were treated with 595 receiving adjuvant IP therapy and surgery and 359 undergoing surgical resection alone. A total of 681 patients were enrolled in phase II randomised studies with 393 patients receiving IP chemotherapy and 288 undergoing surgery alone. Prospective single arm and comparative case control studies grouped together included 494 patients in intervention arms and 359 patients in standard surgery arms. The earliest article on adjuvant IP therapy in colorectal cancer was published in 1985 and the most recent in 2013 (). All RCTs had pre-specified inclusion criteria.
Table 1. Characteristics and outcomes of studies examining surgery plus adjuvant IP chemotherapy versus surgical resection alone in colorectal cancer.
The studies in show heterogeneity in terms of inclusion criteria (tumor node metastasis (TNM) stage), number of individuals enrolled, IP administration modalities and IP chemotherapy selection. Stage-IV patients comprised the minority of enrolled patients, and only Noura et al. reported more than 10% of patients with stage-IV disease (31%). Distant disease cannot be completely excluded with preoperative workup alone, and a small number of stage-IV patients are to be expected in patients undergoing operations with curative intent. The smallest trial was that of Virzi et al. with only 12 patients in a prospective single arm study, while the largest was that of Nordlinger et al. with 753 patients in total enrolled. Administered IP chemotherapy regimens included 5-FU, platinum and mitomycin-C-based regimens. Among the 5 RCTs identified, all utilised some form of early post-operative IV chemotherapy, with only Tentes et al., also having an intra-operative IV chemotherapy group. Taken together, these differences in trial characteristics significantly limit the impact of any conclusions from pooled statistical analysis.
Overall mortality in patients treated with surgery vs. surgery plus IP chemotherapy
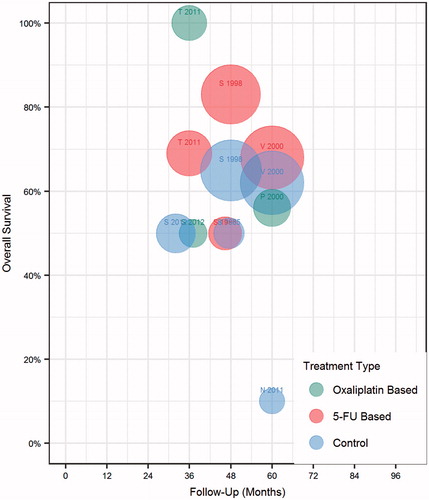
Overall survival was compared for patients undergoing surgery and IP chemotherapy vs. surgery alone (). Displaying all trial arms in a bubble chart, there was a subjective trend toward improved survival among arms administering IP chemotherapy (red circles positioned higher on the y-axis) vs. surgery only (blue circles). Attesting to the known heterogeneity in outcome reporting, overall survival rates ranged from 50% to 75% at reported intervals between 36 and 60 months [Citation10]. We next sought to identify whether study outcomes could be separated based on the chemotherapy agent utilised. As displayed in , there was a trend toward improved overall survival with 5-FU-based IP regimens, compared to oxaliplatin regimens, or just surgery alone. The trend is skewed by the strikingly positive results of Tentes et al., who compared intraoperative administration of IP MMC and oxaliplatin vs. post-operative IV 5FU and found a dramatic survival difference in favour of IP chemotherapy [Citation35].
Figure 2. Colorectal cancer trial treatment arms by overall survival and reported clinical outcome. Each bubble represents one arm of a study. Size represents number of patients. First initial of first author and study year is displayed as a label above each corresponding bubble. Red represents surgery plus adjuvant IP treatment arm, blue surgery arm alone.
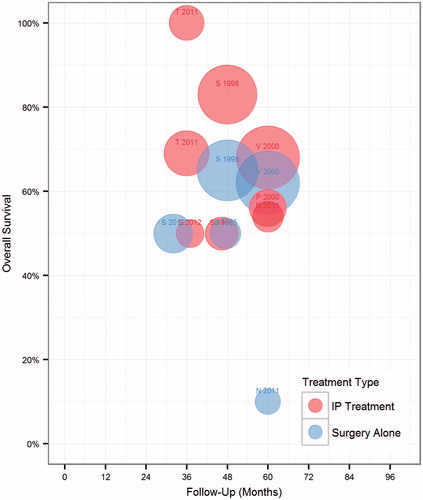
Figure 3. Colorectal cancer trial treatment arms by IP chemotherapy agent. Each bubble represents one arm of a study. Size represents number of patients. First initial of first author and study year is displayed as a label above each corresponding bubble. Colors denote IP chemotherapy agent utilised.
Next, we selected randomised controlled trials that compared IP chemotherapy plus surgery to surgery alone and also reported overall survival, for initial random effects modelling. This group of studies included Vaillant, Scheithauer, Nordlinger and Noura et al. The choice whether to specify fixed or random effects in the meta-analysis was guided by the degree of effect heterogeneity in the included trials. Under Cochran's Q-test (13.9, p = 0.01), the null hypothesis of a homogeneous effect is rejected at the 5% level. The I2 statistic of 78% (CI 42%, 92%) also reveals considerable “unexplained” heterogeneity in the studies [Citation44]. Under such circumstances, random effects explicitly accounting for intra-trial variance should provide more conservative estimates of the pooled effects than modelled fixed effects will. This study, therefore, reports estimates from meta-analysis with random effects. When the studies were pooled in this manner, IP chemotherapy was associated with a statistically significant decreased risk of death of 0.55 (, 95% CI = 0.3053, 0.9849; p = 0.0443).
Figure 4. Random effect comparison overall mortality odds ratios in colorectal cancer Mantel Haenszel random effects pooled analysis of comparative colorectal cancer trials reporting overall survival. Size of boxes is relative to weight of each study. 95% of confidence intervals represented by whiskers, arrow indicating interval clipping. Asterisk (*) denotes IP group included all “regional” chemotherapy, which included intraportal and intraperitoneal administration.
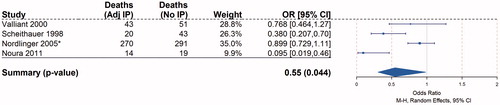
The effect would be stronger without the inclusion of Nordlinger et al., which, in the largest study on the subject, randomised 753 stage-II and -III colorectal cancer patients to IP regional chemotherapy followed by systemic chemotherapy, or to resection plus systemic chemotherapy alone. The IP regional chemotherapy group in the Nordlinger study included patients who received chemotherapy washing of the peritoneal cavity and others that received intraportal regional liver chemotherapy perfusion. Unfortunately, outcomes for the groups receiving intraportal therapy vs. IP therapy were not separated, and together they were reported to achieve a 5-year survival rate exceeding 70% with no significant difference from surgery alone [Citation36]. It is possible that the inferior outcomes among patients who received liver perfusion as their adjuvant treatment might have negated the greater impact seen in the IP chemotherapy group. Of note, despite the study of Nordlinger et al., having significantly more patients than the other studies, included its weight in the random effect model was calculated as 31% of reflecting the significant differences among study size, an advantage compared to fixed-effect analysis which weights studies relatively more equally [Citation45].
Heterogeneity in patient selection and study design
A stronger single in favour of IP chemotherapy may have been further obscured by the heterogeneity in tumour stage and risk for peritoneal recurrence across the studies analysed. The majority of studies attempted to select patients at high risk for peritoneal carcinomatosis via the TNM system (patients with T stage(s) ≥3 in Tentes et al., Vaillant et al., Scheithauer et al.) assuming a higher risk of peritoneal dissemination with increased T and lymph node stages [Citation35,Citation37,Citation38]. Only the studies of Noura et al. and Sammartino et al. included additional criteria such as positive peritoneal lavage (Noura et al.) or presence of mucinous or signet ring tumour histology (Sammartino et al.) [Citation40,Citation41]. Interestingly, these studies showed the highest differences in measured clinical outcome with the addition of IP chemotherapy to definitive surgery. Noura reported a 5-year cancer specific OS of 54.3% vs. 9.5% for those receiving IP chemotherapy vs. surgery alone. Furthermore, Sammartino et al., which reported only PFS and was therefore excluded from the random effects model, did show an increase in disease free survival of 36.8 months for the IP chemotherapy group for 21.9 months for surgery alone.
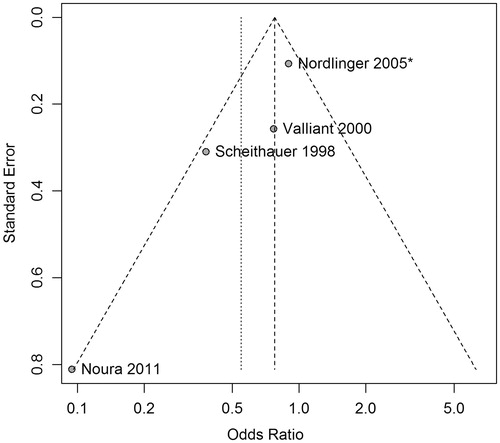
An additional concern from this pooled analysis is the potential effect of the under-reporting of negative studies. Statistical tests of symmetry and visual inspection of a funnel plot (i.e. a scatterplot of the odds ratios against standard errors for studies in the meta-analysis) can help to detect publication bias. Inspection of reveals an acceptable distribution of the four analysed studies, each lying within the meta-analytic 99% of confidence interval. Four tests of symmetry each fail to reject the null hypothesis of symmetry at the 5% level: Begg’s test (p = 0.09), Egger’s test (p = 0.07), Peters’ test (p = 0.07) and Harbord’s test (p = 0.08) [Citation46–49]. The conclusions that can be drawn from these tests are limited by the small number of trials in the analysis, but evidence of publication bias does not emerge.
IP chemotherapy and associated operative morbidity
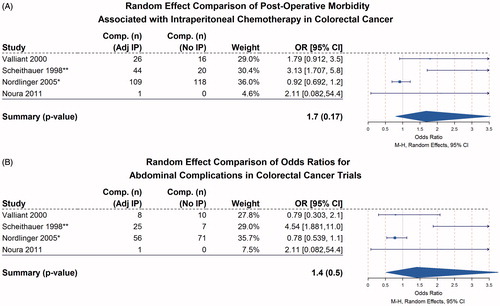
IP chemotherapy-related complications due to the cytotoxic agent were infrequent and relatively minor. In-hospital mortality was rare, and the most commonly reported complications were those who are known to be associated with systemic chemotherapy. The most common abdominal complications reported were anastomotic failure, peritonitis and small bowel obstruction (). A random effect analysis of the four eligible trials was used to quantify the association between morbidity and IP chemotherapy.
Table 2. Peri-operative morbidity and mortality of studies examining surgery plus adjuvant IP chemotherapy vs. surgical resection alone in colorectal cancer.
IP chemotherapy was not associated with a statistically significant increase in morbidity of general post-operative complications (, OR = 1.7, p = 0.17). Reported complications were broadly grouped into two categories, chemotherapy-related issues such as agranulocytosis, febrile neutropenia and other known haematologic and general issues, or IP chemotherapy specific abdominal complications such as anastomotic leak, diarrhoea, abdominal pain, obstruction/ileus or ostomy/wound breakdown (). When these IP chemotherapy-specific complications were entered into random effect models, there was no associated increase in morbidity associated with IP chemotherapy (, OR = 1.4, p = 0.5). While not statistically significant, the trend toward increased complications with IP chemotherapy was in large part driven by Scheithauer et al., which performed the largest series examining delayed post-operative IP chemotherapy (as late as 35 d after definitive surgery) in using peritoneal dialysis catheters vs. surgery alone, and reported a significantly higher severe treatment-associated side effect rate (13% vs. 3%; p = 0.01) in the IP arm [Citation38]. Among studies included in the random effects model, only four mortalities were reported, two in the IP arm of Vaillant et al., and one in the regional and one in the systemic arm of Nordlinger et al., resulting in no significant associations.
Figure 6. Random effect comparison of post-operative morbidity associated with intraperitoneal chemotherapy in colorectal cancer Mantel Haenszel random effects pooled analysis of comparative colorectal cancer trials reporting post-operative morbidity. Size of boxes is relative to weight of each study. 95% of confidence intervals represented by whiskers, arrow indicating interval clipping. (A) All reported complications included. (B) Abdominal only complications, including, GI obstruction, ileus, anastomotic leak, diarrhoea, abdominal pain, ostomy and wound complications. Asterisk (*) denotes IP group included all “regional” chemotherapy, which included intraportal and intraperitoneal administration. (**) denotes Scheithauer et al. reported grade III and above complications only.
Several studies were excluded from the pooled analysis, but still provided valuable insights. Sammartino et al. enrolled patients at high risk for peritoneal involvement, including those with T3/T4 tumours, signet cell or mucinous histology and reported improved median disease-free survival with no difference on OS [Citation40]. This study, in addition to the small initial report of Sugarbaker et al., which did not find a difference in favour of IP chemotherapy, was both excluded from the random effects analysis as they did not explicitly report survival odds ratios. Of note, the study of Tentes et al. was also excluded from the random effects model as it did not include a surgery alone control group. As mentioned previously, this study is notable for its dramatic reported survival advantage for the immediate IP chemotherapy group [Citation50].
Discussion
Adjuvant systemic chemotherapy for unselected stages II and III colorectal cancer patients is known to improve outcome, but only in a minority of patients. Recent efforts, therefore, have shifted focus on improving patient selection for adjuvant therapy. Studies have demonstrated promise in areas such as using molecular markers like high microsatellite instability, BRAF mutant tumours or new genetic predictors (Oncotype DX) [Citation51,Citation52]. Clinical predictors such as lymphovascular invasion, T4 tumours with signs of perforation or presence of intestinal obstruction in stage-II cancers are currently under investigation.
The use of adjuvant IP chemotherapy, in this regard, remains a relatively unexploited area, despite its potential to focus prevention at the site of likely recurrence, afford a more personalised use of treatment and reduce toxicity. This concept, to a certain degree, is an extension of hepatic artery chemotherapy infusion pump results reported by Kemeny et al. [Citation53]. Using a similar site-directed approach, the rationale of adjuvant hepatic artery perfusion is to target the organ at highest risk of recurrence with 5-FUDR, seeking to increase the dose delivered, target the expected pattern of recurrence and reduce toxicity compared to systemic treatment. For similar reasons, we focussed on the peritoneal cavity, the second-most likely site of distant recurrence in colorectal cancer.
Our main finding is an association between adjuvant IP chemotherapy and improved overall survival following definitive surgery for early stage colorectal cancer. This finding shares similarities with previous analyses on adjuvant IP chemotherapy in gastric cancer, where several reviews have identified improved outcomes in patients undergoing surgery plus IP chemotherapy compared to surgery alone [Citation20,Citation21,Citation54,Citation55]. Specifically, our group has previously identified associations between survival and IP chemotherapy that was delivered intra-operatively vs. post-operatively, or between the use of mitomycin C and cisplatin [Citation20]. These reported conclusions, however, are limited by the paucity of randomised studies, as well as heterogeneity in eligibility criteria, IP chemotherapy agents used and outcome reporting. With the more limited dataset of adjuvant IP chemotherapy in colorectal cancer, similar comparisons were not possible. Furthermore, previous random effects analysis on the use of adjuvant IP chemotherapy in gastric cancer derived its signals from trials which delivered immediate, intra-operative IP chemotherapy, while all trials included in presented random effects model utilised early post-operative IP chemotherapy. The later postoperative rather than immediate intraoperative administration of IP chemotherapy loses the advantage of hyperthermia, which has been shown to have an anti-neoplastic advantage in several clinical and preclinical studies [Citation20,Citation56,Citation57].
Our study seeks to quantify the impact of IP chemotherapy delivered at the time of definitive surgery in patients with colorectal cancer. Despite the sentinel study of Verwaal et al. showing that CRS and IP chemotherapy has merit in the management of established peritoneal carcinomatosis due to colorectal cancer, our report, as well as others, shows that there is a lack of high-quality randomised trials focussed on this clinical question. This support for IP chemotherapy in metastatic disease is distinctly different from the case of gastric cancer, where adjuvant IP chemotherapy is supported by a number of randomised studies, but evidence for IP chemotherapy in within the setting of peritoneal carcinomatosis is limited [Citation58,Citation59]. Therefore, future development of IP chemotherapy in colorectal cancer might benefit from early identification of patients most likely to benefit. It is noteworthy that the two most recent studies identified here used histopathological and cytological criteria in addition to clinical criteria to identify patients at high risk for peritoneal recurrence. This approach yielded improved clinical outcomes [Citation40,Citation41]. Mucinous histology and the presence of signet ring cells, used by Sammartino et al., are known to be associated with increased peritoneal spread [Citation40]. The same is true of positive peritoneal cytology, which was used by Noura et al. [Citation41]. In line with these findings, the recently begun multi-institutional Adjuvant HIPEC in High Risk Colon Cancer (COLOPEC) trial, designed to randomise to either surgery plus IP chemotherapy or systemic chemotherapy, is limited to T4 tumour stage or intra-abdominally perforated colon cancer [Citation31]. The selection criteria utilised by Sammartino et al. and Noura et al. were in contrast to that of Nordlinger et al. [Citation31,Citation40,Citation41]. This phase-III multi-institutional trial failed to disclose which patients in the regional chemotherapy arm received intraportal therapy vs. IP therapy. Additionally, Nordlinger et al. enrolled a large number of stage-II or -III patients with N0 disease. Both of these factors might have contributed to the study’s negative result [Citation36].
Findings from this study will lend support for choosing well-defined criteria to select patients at increased risk for peritoneal involvement following surgical resection. Such selection criteria will need to extend beyond the traditional clinicopathological criteria of TNM system, grade and histopathology (signet ring cell, mucinous histpathology), and should involve a direct measure like positive IP cytology or peritoneal micrometastasis detected intraoperatively via use of near-infrared imaging and indocyanine green (ClinicalTrials.gov identifiers NCT02032485 and NCT01982227, respectively). A study employing positive cytology as a selection criterion for IP chemotherapy administration at time of gastrectomy has been recently opened at the National Cancer Institute for Gastric Cancer (ClinicalTrials.gov NCT03092518).
Overall, the prognostic value of mutational derangements across tumours is likely to surpass stage and histology as the basis for surgical selection of patients. Indeed, genetic predictors specific for peritoneal surface involvement have already been shown to have value for differentiating between low- and high-risk appendiceal and colorectal peritoneal carcinomatosis [Citation60]. Although metastatic deposits contain significant mutational heterogeneity, recent work has identified certain genomic signatures in primary tumours that are predictive of future metastatic sites [Citation61,Citation62]. Thus, correlative tissue profiling could help to identify patients at high risk for peritoneal spread and likely to benefit from IP chemotherapy.
Given recent changes in surgical care, the adjuvant IP chemotherapy approach will need to be adjusted to fit better overall trends in cancer care. For colon cancer in particular, recent evidence points to the non-inferiority of minimally invasive resections that also reduce length of stays. Taking this into account, a one-time administration of IP chemotherapy at the time of resection is likely to be more easily accepted compared to multi-day post-surgical EPIC treatment(s). This approach is also supported by the direct comparison of the two modalities in Tentes et al., reporting a clinical outcome advantage in patients who received adjuvant IP chemotherapy immediately intra-op vs. delayed post-operatively. Furthermore, the recently opened multi-institutional COLOPEC study in Europe also uses a one-time IP chemo infusion during surgery [Citation31].
While the quantitative analysis presented here supports the need for additional study of adjuvant IP chemotherapy in high-risk colorectal cancer, our study has significant limitations. These include the small number of randomised trials, which was further narrowed in the random effects analysis due to heterogeneous outcome reporting. Additionally, significant heterogeneity remained in the clinicopathological characteristics of patients included in the model, in terms of risk for recurrence, administration of additional adjuvant treatment, as well as risk for peritoneal involvement.
Conclusion
This systematic review provides rationale in favour of adjuvant IP chemotherapy in resectable colorectal cancer patients at risk for peritoneal spread. Summary analyses do not support the traditional assumption that IP chemotherapy is associated with adverse post-operative outcomes such as intra-abdominal abscess formation and anastomotic failure. Future clinical trials should seek to enrich patients at high risk for peritoneal carcinomatosis as well as formalise standard inclusion criteria and IP chemotherapy treatment agent use to better define the role of this therapy in colorectal cancer.
Disclosure statement
No potential conflict of interest was reported by the authors.
References
- Siegel RL, Miller KD, Jemal A. (2016). Cancer statistics, 2016. CA Cancer J Clin 66:7–30.
- Siegel R, Desantis C, Jemal A. (2014). Colorectal cancer statistics, 2014. CA Cancer J Clin 64:104–17.
- Sabiston DC, Townsend CM. (2012). Sabiston textbook of surgery: the biological basis of modern surgical practice. Philadelphia (PA): Elsevier Saunders.
- Edwards BK, Ward E, Kohler BA, et al. (2010). Annual report to the nation on the status of cancer, 1975–2006, featuring colorectal cancer trends and impact of interventions (risk factors, screening, and treatment) to reduce future rates. Cancer 116:544–73.
- van der Pool AEM, Damhuis RA, Ijzermans JNM, et al. (2012). Trends in incidence, treatment and survival of patients with stage IV colorectal cancer: a population-based series. Colorectal Dis 14:56–61.
- Brenner H, Kloor M, Pox CP. (2014). Colorectal cancer. Lancet 383:1490–502.
- Elferink MAG, de Jong KP, Klaase JM, et al. (2015). Metachronous metastases from colorectal cancer: a population-based study in North-East Netherlands. Int J Colorectal Dis 30:205–12.
- Yothers G, O'Connell MJ, Allegra CJ, et al. (2011). Oxaliplatin as adjuvant therapy for colon cancer: updated results of NSABP C-07 trial, including survival and subset analyses. JCO 29:3768–74.
- Brodsky JT, Cohen AM. (1991). Peritoneal seeding following potentially curative resection of colonic carcinoma: implications for adjuvant therapy. Dis Colon Rectum 34:723–7.
- Sloothaak DA, Mirck B, Punt CJ, et al. (2014). Intraperitoneal chemotherapy as adjuvant treatment to prevent peritoneal carcinomatosis of colorectal cancer origin: a systematic review. Br J Cancer 111:1112–21.
- Koppe MJ, Boerman OC, Oyen WJ, Bleichrodt RP. (2006). Peritoneal carcinomatosis of colorectal origin: incidence and current treatment strategies. Ann Surg 243:212–22.
- Segelman J, Granath F, Holm T, et al. (2012). Incidence, prevalence and risk factors for peritoneal carcinomatosis from colorectal cancer. Br J Surg 99:699–705.
- Bouvier AM, Launoy G, Bouvier V, et al. (2015). Incidence and patterns of late recurrences in colon cancer patients. Int J Cancer 137:2133–8.
- Franko J, Shi Q, Goldman CD, et al. (2012). Treatment of colorectal peritoneal carcinomatosis with systemic chemotherapy: a pooled analysis of north central cancer treatment group phase III trials N9741 and N9841. J Clin Oncol 30:263–7.
- Sugarbaker PH. (2001). Cytoreductive surgery and peri-operative intraperitoneal chemotherapy as a curative approach to pseudomyxoma peritonei syndrome. Eur J Surg Oncol 27:239–43.
- El Halabi H, Gushchin V, Francis J, et al. (2012). The role of cytoreductive surgery and heated intraperitoneal chemotherapy (CRS/HIPEC) in patients with high-grade appendiceal carcinoma and extensive peritoneal carcinomatosis. Ann Surg Oncol 19:110–14.
- Bijelic L, Jonson A, Sugarbaker PH. (2007). Systematic review of cytoreductive surgery and heated intraoperative intraperitoneal chemotherapy for treatment of peritoneal carcinomatosis in primary and recurrent ovarian cancer. Ann Oncol 18:1943–50.
- Ji ZH, Peng KW, Yu Y, et al. (2017). Current status and future prospects of clinical trials on CRS + HIPEC for gastric cancer peritoneal metastases. Int J Hyperthermia 33:562–70.
- Polom K, Marano L, Roviello G, et al. (2016). Evolution and emerging future of cytoreducxtive surgery and hyperthermic intraperitoneal chemoperfusion in gastric cancer: from treating the incurable to preventing recurrence. Int J Hyperthermia 32:173–9.
- Feingold PL, Kwong ML, Davis JL, Rudloff U. (2017). Adjuvant intraperitoneal chemotherapy for the treatment of gastric cancer at risk for peritoneal carcinomatosis: a systematic review. J Surg Oncol 115:192–201.
- Yan TD, Black D, Sugarbaker PH, et al. (2007). A systematic review and meta-analysis of the randomized controlled trials on adjuvant intraperitoneal chemotherapy for resectable gastric cancer. Ann Surg Oncol 14:2702–13.
- Zheng X, Carstens JL, Kim J, et al. (2015). Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature 527:525–30.
- Fischer KR, Durrans A, Lee S, et al. (2015). Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature 527:472–6.
- Psaila B, Lyden D. (2009). The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9:285–93.
- Fidler IJ. (2003). The pathogenesis of cancer metastasis: the ‘seed and soil’ hypothesis revisited. Nat Rev Cancer 3:453–8.
- Fushida KO, Kinoshita J, Tsukada T, et al. (2013). Intraperitoneal chemotherapy as a multimodal treatment for gastric cancer patients with peritoneal metastasis. J Cancer Ther 4:6–15.
- Lu Z, Wang J, Wientjes MG, Au JL. (2010). Intraperitoneal therapy for peritoneal cancer. Future Oncol 6:1625–41.
- Goodman MD, McPartland S, Detelich D, Saif MW. (2016). Chemotherapy for intraperitoneal use: a review of hyperthermic intraperitoneal chemotherapy and early post-operative intraperitoneal chemotherapy. J Gastrointest Oncol 7:45–57.
- Armstrong DK, Bundy B, Wenzel L, et al. (2006). Intraperitoneal cisplatin and paclitaxel in ovarian cancer. New Engl J Med 354:34–43.
- Coccolini F, Cotte E, Glehen O, et al. (2014). Intraperitoneal chemotherapy in advanced gastric cancer: meta-analysis of randomized trials. Eur J Surg Oncol 40:12–26.
- Klaver CE, Musters GD, Bemelman WA, et al. (2015). Adjuvant hyperthermic intraperitoneal chemotherapy (HIPEC) in patients with colon cancer at high risk of peritoneal carcinomatosis; the COLOPEC randomized multicentre trial. BMC Cancer 15:428.
- R Foundation for Statistical Computing. (2014). R: a language and environment for statistical computing. Vienna, Austria: R Foundation for Statistical Computing.
- Wickham H. (2009). ggplot2: elegant graphics for data analysis. New York: Springer-Verlag.
- Moher D, Liberati A, Tetzlaff J, Altman DG. (2009). Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement the PRISMA statement. Ann Internal Med 151:264–9.
- Tentes AA, Spiliotis ID, Korakianitis OS, et al. (2011). Adjuvant perioperative intraperitoneal chemotherapy in locally advanced colorectal carcinoma: preliminary results. ISRN Surg 2011:529876.
- Nordlinger B, Rougier P, Arnaud JP, et al. (2005). Adjuvant regional chemotherapy and systemic chemotherapy versus systemic chemotherapy alone in patients with stage II-III colorectal cancer: a multicentre randomised controlled phase III trial. Lancet Oncol 6:459–68.
- Vaillant JC, Nordlinger B, Deuffic S, et al. (2000). Adjuvant intraperitoneal 5-fluorouracil in high-risk colon cancer: a multicenter phase III trial. Ann Surg 231:449–56.
- Scheithauer W, Kornek GV, Marczell A, et al. (1998). Combined intravenous and intraperitoneal chemotherapy with fluorouracil + leucovorin vs fluorouracil + levamisole for adjuvant therapy of resected colon carcinoma. Br J Cancer 77:1349–54.
- Sugarbaker PH, Gianola FJ, Speyer JL, et al. (1985). Prospective randomized trial of intravenous v intraperitoneal 5-FU in patients with advanced primary colon or rectal cancer. Semin Oncol 12:101–11.
- Sammartino P, Sibio S, Biacchi D, et al. (2012). Prevention of peritoneal metastases from colon cancer in high-risk patients: preliminary results of surgery plus prophylactic HIPEC. Gastroenterol Res Pract 2012:141585.
- Noura S, Ohue M, Shingai T, et al. (2011). Effects of intraperitoneal chemotherapy with mitomycin C on the prevention of peritoneal recurrence in colorectal cancer patients with positive peritoneal lavage cytology findings. Ann Surg Oncol 18:396–404.
- Virzi S, Iusco D, Baratti D, et al. (2013). Pilot study of adjuvant hyperthermic intraperitoneal chemotherapy in patients with colorectal cancer at high risk for the development of peritoneal metastases. Tumori 99:589–95.
- Palermo JA, Richards F, Lohman KK, et al. (2000). Phase II trial of adjuvant radiation and intraperitoneal 5-fluorouracil for locally advanced colon cancer: results with 10-year follow-up. Int J Radiat Oncol Biol Phys 47:725–33.
- Higgins JP, Thompson SG. (2002). Quantifying heterogeneity in a meta-analysis. Stat Med 21:1539–58.
- Higgins J, Green SE. 2011. Cochrane handbook for systematic reviews of interventions version 5.1.0 [updated March 2011] - 10.4.4.1. Comparing fixed and random-effects estimates. The Cochrane Collaboration. Available from: www.handbook.cochrane.org [last accessed 11 Aug 2016].
- Begg CB, Mazumdar M. (1994). Operating characteristics of a rank correlation test for publication bias. Biometrics 50:1088–101.
- Egger M, Smith GD, Schneider M, Minder C. (1997). Bias in meta-analysis detected by a simple, graphical test. BMJ 315:629–34.
- Peters JL, Sutton AJ, Jones DR, et al. (2006). Comparison of two methods to detect publication bias in meta-analysis. JAMA 295:676–80.
- Harbord RM, Egger M, Sterne JA. (2006). A modified test for small-study effects in meta-analyses of controlled trials with binary endpoints. Stat Med 25:3443–57.
- Tentes AA, Kyziridis D, Kakolyris S, et al. (2012). Preliminary results of hyperthermic intraperitoneal intraoperative chemotherapy as an adjuvant in resectable pancreatic cancer. Gastroenterol Res Pract 2012:506571.
- Simon RM, Paik S, Hayes DF. (2009). Use of archived specimens in evaluation of prognostic and predictive biomarkers. J Nat Cancer Inst 101:1446–52.
- O'Connell MJ, Lavery I, Yothers G, et al. (2010). Relationship between tumor gene expression and recurrence in four independent studies of patients with stage II/III colon cancer treated with surgery alone or surgery plus adjuvant fluorouracil plus leucovorin. J Clin Oncol 28:3937–44.
- Kanat O, Gewirtz A, Kemeny N. (2012). What is the potential role of hepatic arterial infusion chemo-therapy in the current armamentorium against colorectal cancer. J Gastrointest Oncol 3:130–8.
- Coccolini F, Cotte E, Glehen O, et al. (2014). Intraperitoneal chemotherapy in advanced gastric cancer: meta-analysis of randomized trials. Eur J Surg Oncol 40:12–26.
- Sugarbaker PH. (2007). Laboratory and clinical basis for hyperthermia as a component of intracavitary chemotherapy. Int J Hyperthermia 23:431–42.
- Zhao C, Dai C, Chen X. (2012). Whole-body hyperthermia combined with hyperthermic intraperitoneal chemotherapy for the treatment of stage IV advanced gastric cancer. Int J Hyperthermia 28:735–41.
- Zhong H, Yang Y, Ma S, et al. (2011). Induction of a tumour-specific CTL response by exosomes isolated from heat-treated malignant ascites of gastric cancer patients. Int J Hyperthermia 27:604–11.
- Rudloff U, Langan RC, Mullinax JE, et al. (2014). Impact of maximal cytoreductive surgery plus regional heated intraperitoneal chemotherapy (HIPEC) on outcome of patients with peritoneal carcinomatosis of gastric origin: results of the GYMSSA trial. J Surg Oncol 110:275–84.
- Glehen O, Gilly FN, Arvieux C, et al. (2010). Peritoneal carcinomatosis from gastric cancer: a multi-institutional study of 159 patients treated by cytoreductive surgery combined with perioperative intraperitoneal chemotherapy. Ann Surg Oncol 17:2370–7.
- Levine EA, Blazer DG, 3rd, Kim MK, et al. (2012). Gene expression profiling of peritoneal metastases from appendiceal and colon cancer demonstrates unique biologic signatures and predicts patient outcomes. J Am College Surgeons 214:599–606. discussion -7.
- Yachida S, Jones S, Bozic I, et al. (2010). Distant metastasis occurs late during the genetic evolution of pancreatic cancer. Nature 467:1114–7.
- Nguyen DX, Bos PD, Massague J. (2009). Metastasis: from dissemination to organ-specific colonization. Nat Rev Cancer 9:274–84.