Abstract
Objective: The aims of this study were to compare the clinical outcomes between ultrasound (US)-guided percutaneous microwave ablation (MWA) and surgical resection (SR) in patients with thoracoabdominal wall implants from hepatocellular carcinom (HCC) and to identify the prognostic factors associated with the two treatment methods.
Materials and methods: A total of 47 patients (mean age, 56.7 ± 15.9 years, range, 18–78 years; 34 men and 13 women) with 61 thoracoabdominal wall HCC seeding were included from April 2007 to May 2017. Twenty-five patients underwent US-guided MWA and 22 patients underwent SR. Survival, recurrence and liver function were compared between the two groups. Effect of changes in key parameters (i.e. overall survival (OS), disease-free survival (DFS) and local tumour reoccurrence-free (LTRF)) was statistically analysed with the log-rank test. Univariate and multivariate analyses were performed on several clinicopathological variables to identify factors affecting long-term outcome and recurrence.
Results: The OS, DFS and LTRF after MWA were comparable to those of SR (p =0.493, p = 0.578 and p =0.270, respectively). Estimated 5-year overall survival rates were 63% after MWA and 48.1% after SR; for disease-free survival, estimated 5-year rates were 67.5% after MWA and 48.8% after SR; estimated 24-month LTRF rates were 71.3% after MWA and 87.8% after SR. The MWA group had less surgical time (p = <0.001), estimated blood loss (p = <0.001) and post-operative hospitalisation (p = 0.032) and cost (p = 0.015). Multivariate analysis showed remnant intrahepatic tumour (p =0.007), Child Pugh grade (p = 0.009) and metastasis (p= <0.001), were predictors for survival rate.
Conclusions: Ultrasound-guided percutaneous MWA is a safe and effective treatment method for metastatic HCC on the thoracoabdominal wall with similar outcomes to SR. Residual intrahepatic HCC, Child Pugh grade and distant metastasis are predictors for survival.
Introduction
Hepatocellular carcinoma (HCC) is one of the most common malignancies and the incidence was 28.71/100 000 in China [Citation1], which is closely related with viral hepatitis B and C infections. HCC seeding on the thoracoabdominal wall is considered as a highly advanced disease stage [Citation2], resulting from poorly controlled primary disease, which occasionally emerged after the percutaneous procedures or surgery [Citation3–5]. Multiple treatment options are currently available for seeding, including traditional surgical resection and newer approaches such as percutaneous thermal ablative techniques and 125I brachytherapy. Among these, surgical resection (SR) has been reported as the most popular treatment modality [Citation6]. In addition, ultrasound (US)-guided microwave ablation (MWA) of abdominal wall metastatic tumours has been reported [Citation7], but more detailed clinical outcomes are lacking.
In recent years, various thermal ablative modalities have been applied to treat thoracoabdominal wall metastatic tumours, including high intensity focussed ultrasound (HIFU) and radiofrequency ablation (RFA). As a newer modality, MWA has been frequently reported in terms of its therapeutic effectiveness for solid malignancies [Citation8–10]. Moreover, compared with RFA [Citation11,Citation12], MWA was associated with many theoretical advantages such as higher intratumoural temperature, larger ablation volume, less operation time, less dependence on the electrical conductivities of tissues and less limited energy delivery brought about by the exponential rise in electrical impedance of tumour tissues. Although previous studies have reported encouraging short-term therapeutic response and survival data in treatment of HCC [Citation13,Citation14], the role of percutaneous MWA is still controversial. Finally, the available comparison data related to seeding between MWA and traditional SR are rare.
Therefore, we investigated the US-guided percutaneous MWA and SR for thoracoabdominal wall implants from HCC in terms of technical effectiveness, oncologic outcomes and complications. The purpose of this study was to review the intermediate-term effectiveness of MWA vs. SR in the management of HCC seeding on the thoracoabdominal wall and to identify prognostic factors associated with these two techniques.
Materials and methods
Patient and tumours characteristics
This study was conducted in accordance with the principles of the Declaration of Helsinki, and the study protocol was approved by the ethics committee of Chinese PLA General Hospital. Because of the retrospective nature of the study, patient consent for inclusion was waived. The medical records of all patients with thoracoabdominal wall HCC seeding between April 2007 and May 2017 were reviewed. The inclusion criteria for this study were as follows: (1) clear visualisation of the entire tumour on imaging; (2) maximum diameter of the tumour ≤5 cm; (3) tumour number was not more than two; (4) tumour location on the thoracoabdominal wall was unilateral; (5) the HCC seeding was treated after the intrahepatic HCC treatment; (6) the patients with Barcelona Clinic Liver Cancer (BCLC)stage B disease were adopted. The exclusion criteria were as follows: (1) thoracoabdominal wall primary tumour; (2) severe cardiopulmonary dysfunction; (3) severe coagulation abnormalities; (4) HCC was located proximity to the skin surface (<5 mm). The patients were divided according to the mode of treatment received for the thoracoabdominal wall HCC seeding (US-guided MWA vs. SR).
During the study period, 172 consecutive patients underwent percutaneous MWA (n = 87) and SR (n = 85). Among them, 47 patients (mean age, 56.7 years +15.9, range, 18–78 years; 34 men and 13 women) met the inclusion criteria and were included in our study. In addition, each patient received a Karnofsky Performance Status (KPS) score to show his physical condition before treatment. The KPS score describes a patient’s functional status as a comprehensive 11-point scale correlating to percentage values ranging from 100% (no evidence of disease, no symptoms) to 0% (death). In all, 25 patients (53.2%; 25 of 47 patients) with 32 lesions underwent MWA (MWA group) and 22 patients (46.8%; 22 of 47 patients) with 29 lesions underwent SR (SR group). In previous study [Citation7], we reported preliminary study of MWA for abdominal wall metastatic cancer in 11 patients bearing 23 tumours including five patients with HCC, with tumour sizes not more than 5 cm in diameter. In this study, we added 20 new patients to the five patients with HCC in previously reported. Therefore, a portion of the patient cohort in the present study was from a different, previously published study.
The following demographic and clinicopathologic parameters was obtained from each patient: age, gender, comorbidities, Child–Pugh grade, maximum diameter of the lesions, number of lesions, pathologic differentiation, perioperative primary disease control, metastasis, seeding side, seeding location and presumed cause of HCC implantation. The remnant intrahepatic tumour was analysed separately. The procedural or treatment variables collected were post-operative hospital stay, operation time, estimated blood loss, complications, cost, date and site of recurrence or metastasis and date of and status on the last follow-up. We also recorded the reasons for death.
Indications for MWA in this study were as follows: advanced age or poor surgical candidates for major comorbidities in 14 patients, poor liver function tests in 4 patients, poor renal function in 2 patients and patient preference in 5 patients. SR was offered to patients who were relatively healthy and young enough to endure the surgery.
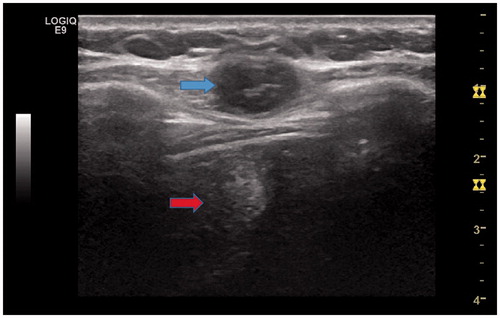
The two groups were similar in terms of the results of pre-operative diagnostic examinations, including chest radiography, contrast-enhanced abdominal US, dynamic computed tomography (CT) and magnetic resonance imaging (MRI). The US image before operation show in . The diagnosis of HCC was confirmed by pathologic findings in all patients from the SR group. For all patients in the MWA group, the metastatic tumours were diagnosed based on pathologic findings of needle biopsy samples before ablation.
Figure 1. A 46-year-old female patient with abdominal wall implants from hepatocellular carcinoma (HCC) after US-MWA treatment. US image shows one low level echo mass (3.1 × 2.5 × 2.2 cm) located on the abdominal wall (blue arrow). There is a high level echo field located behind the mass, and it is MWA field (red arrow).
US-guided percutaneous MWA
Before treatment, all patients were scanned using contrast-enhanced computed tomography (CT)/magnetic resonance imaging (MRI) and ultrasound, and an appropriate puncture route was chosen by ultrasound. With the patient under moderate sedation and local anaesthesia, MWA was performed by three interventional radiologists, namely, P. L., X. L. Y. and Z. G. C., who had 20, 20 and 10 years of experience on MWA, respectively. The microwave unit (KY-2000; Kangyou Medical, Nanjing, China) was capable of producing 100 W of power at 2450 MHz. The cool tip needle antenna had a diameter of 1.9 mm (15 gauge) and a length of 18 cm. After application of local anaesthesia with 1% lidocaine (Yi you, Beijing, China), US-guided biopsy was performed in 2–3 separate punctures using an automatic biopsy gun with an 18-gauge cutting needle. Subsequently, the microwave antenna was percutaneously inserted into the tumour and placed on the desired location under US guidance. A power output of 50 W for 10 min was routinely used during MWA. After all the punctures, intravenous anaesthesia with a combination of propofol (Diprivan; Zeneca Pharmaceuticals, Wilmington, Del) and ketamine (Shuang he Pharmaceuticals, Beijing, China) was administered via the peripheral vein. If the heat-generated hyperechoic water vapour did not completely encompass the entire tumour, prolonged microwave emission was applied until the desired temperature (60 °C) was reached. If the tumour was adjacent to bowel, gallbladder or other important tissues, a 21 gauge thermocouple was inserted close to these tissues for real-time temperature monitoring during MW ablation. In addition, microwave needle tract was cauterised during the needle withdrawal. For tumours with subcutaneous invasion, an ice bag was placed on the skin to avoid scalding during MW ablation.
Surgical resection
The patients were placed on either a supine or lateral position and were under general anaesthesia or continuous epidural anaesthesia. In all cases, a urinary catheter was placed during the operation. After dissecting and dividing the thoracic or abdominal subcutaneous tissue and muscle, the tissues surrounding the metastatic tumour were dissected meticulously, followed by complete resection of the tumour. The operation was carried out by two surgeons who had 15 and 10 years of experience on general surgery. The entire procedure was completed with secure haemostasis. Stitches were removed on post-operative day 7 or 8.
Follow-up
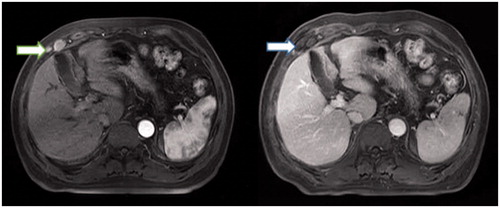
At 1 and 3 months after treatment and at 6-month intervals thereafter, the follow-up visit covered several evaluations, including routine physical examination; laboratory tests such as total bilirubin, serum albumin, prothrombin time and tumour marker levels and parameter related to liver function; and three-phase contrast-enhanced US, CT or MRI. Technical success was defined as the absence of contrast-enhancement on imaging in any area of the mass after one month. All patients who underwent pre- and post-operative contrast-enhanced MRI were shown in . Local tumour recurrence was defined based on imaging findings of irregular nodular, scattered or eccentric pattern of peripheral enhancement around the ablation zone in the MWA group and around the resected margin in the SR groups. Intervals for CT/MRI were shortened when levels of tumour marker were increased after the treatment. If no viable lesion was detected on CT/MRI (with enhancement in the arterial phase), the intrahepatic lesions were regarded as well controlled. If viable lesions were decreased or unchanged in size and number, the disease was considered as stable, whereas the presence of new lesions or an increase of at least 20% in the sum of the diameters of remnant intrahepatic viable lesions indicated poor control. Major complications were defined in line with the HCC ablation reporting standards [Citation15]. The definition of major complication is an event that leads to substantial morbidity and disability that increases the level of care or results in hospital admission. Follow-up was closed until the last visit or on the time of death and seeding recurrence.
Figure 2. Sixty-four year old male with abdominal wall implants from hepatocellular carcinoma (HCC). (A) Two nodules were found in the right abdominal wall in the arterial phase in the axial enhanced MRI (arrow) before US-guided MWA, and one was measured in 3.3 × 3.2 × 2.1 cm, another one was in 1.3 × 1 × 0.8 cm. (B) Two nodules were treated after 1 month undergoing US-guided MWA. The thermal ablation field was found in the right abdominal wall in the delay phase in the axial enhanced MRI (arrow).
Statistical analysis
Data between the MWA and SR groups were compared with the Student’s t-test. Continuous variables were analysed by the Wilcoxon signed-rank test and categorical variables were analysed using either the Pearson’s chi-squared test or the Fisher’s exact test. Overall survival, disease-free survival and LTRF were assessed by the Kaplan–Meier method with log-rank test. A Cox proportional hazards model was used to identify the significant effects of multiple factors on survival rate. Statistical analyses were performed using SPSS 19.0 (SPSS Inc., Chicago, IL). For all tests, a p values of less than 0.05 was considered to be statistically significant.
Results
Patient and tumours characteristics
The characteristics of the patients and tumours are summarised in . The mean age and sex were comparable between the two groups (p = 0.892 and p = 0.956, respectively). The mean maximum diameter and number of the metastatic nodules were comparable between the two groups (p = 0.426 and p = 0.325, respectively). The side and location of implanted nodules were comparable between the two groups (p = 0.133 and p = 0.234, respectively). Patients who underwent MWA had lower KPS score than those who received surgery (p = 0.032). Patients who underwent MWA had more comorbid diseases than those who received surgery (p = 0.014). The presumed causes of tumour implantation were including percutaneous treatment, hepatic resection and spontaneous rupture, which were based on image examination and patient history. The pre-operative treatment of intrahepatic HCC as follows: hepatic resection, percutaneous MWA, TACE and 125I brachytherapy and the presumed causes of tumour implantation and pre-operative treatment of intrahepatic HCC were comparable between the two groups (p = 0.765 and p = 0.588, respectively). The perioperative primary disease control was comparable between the two groups (p = 0.989), which was based on CT/MRI image analysis. The pre-operative liver function in two groups are summarised in .
Table 1. Baseline characteristics of patients undergoing MWA and SR.
Table 2. The pre-operative liver function in two groups.
Treatment parameters
The treatment parameters are summarised in . Twenty-five patients with 32 tumours received a total of 34 treatments sessions in MWA group. Thirty tumours were successfully treated in one MWA session and two tumours were treated in two sessions. The surgical time and hospitalisation in the SR group were significantly longer than in the MWA group (p = < 0.001 and p = 0.032). There was more estimated blood loss and cost in the SR group (p ≤ 0.001 and p = 0.015). One patient in the SR group needed blood transfusion treatment of 200–400 ml. Transfusion was not necessary in the MWA group.
Midterm oncologic outcome and recurrence
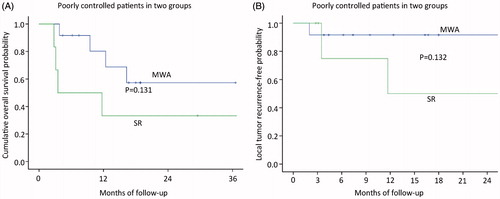
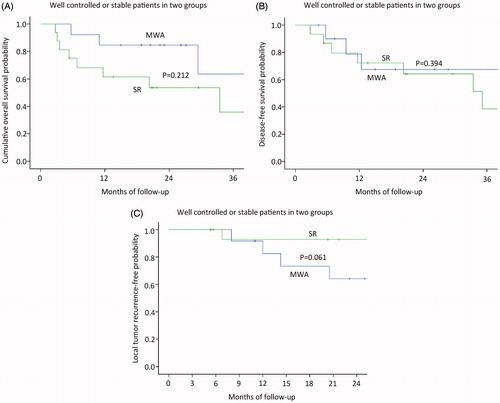
The median follow-up period was 27.8 months (range, 3.8–108.9 months) in the MWA group and 38.3 months (range, 2.8–103.8 months) in the SR group. Mortality rate was 32% (eight of 25 patients) in the MWA group; Causes of death for all patients were primary liver cancer progression. Four of the patients had comorbid diseases, including hypertension in two patients and diabetes in two patients. Eleven patients (50%; eleven of 22 patients) died in the SR group, and the cause of death in both was HCC local or systemic progression. Based on the follow-up imaging, 100% technique success rate was achieved in both groups (MWA: 27 of 27 treatments; SR: 23 of 23 treatments). Six LTP lesions (24%; six of 25 patients) were discovered after MWA treatment and four LTP lesions (18.1%; four of 22 patients) were discovered after SR treatment. Nine patients after MWA (36%; nine of 25 patients) and eight patients after SR (36.4%; eight of 22 patients) had distant metastasis. The 1-, 3- and 5-year overall survival rates in the MWA group and SR group were 82.6, 73.5 and 63% and 72.2, 56.1 and 48.8%, respectively (), showing no significant statistical difference (p = 0.493). Among patients without HCC, The 1-, 3- and 5-year disease-free survival rates in the MWA group and SR group were 78.8, 67.5 and 67.5% and 72.2, 48.8 and 48.8%, respectively (), showing no significant statistical difference (p = 0.578). The median period of the local tumour reoccurrence was 7.9 months (range, 2.8–25.9 months). The 3-, 6-, 12-, 18- and 24-month local tumour reoccurrence-free (LTRF) rates in the MWA group and SR group were 96, 96, 85.3, 79.2 and 71.3% and 100, 100, 94.1, 94.1 and 87.8%, respectively (), showing no significant statistical difference (p = 0.270).The patients of intrahepatic HCC well controlled or stable in two groups, the 1-, 2- and 3-year overall survival rates in the MWA group and SR group were 84.6 84.6 and 63.5% and 61.4, 53.7 and 35.8%, respectively (), showing no significant statistical difference (p = 0.212). The 1-, 2- and 3-year disease-free survival rates in the MWA group and SR group were 80.2, 57.3 and 57.3% and 72.2, 64.2 and 51.4%, respectively (), showing no significant statistical difference (p = 0.394). The 3-, 6-, 12-, 18- and 24-month local tumour reoccurrence-free (LTRF) rates in the MWA group and SR group were 100, 91.7, 82.5, 64.2 and 71.3% and 100, 92.9, 92.9, 92.9 and 92.9%, respectively (), showing no significant statistical difference (p = 0.061). The patients of intrahepatic HCC poorly controlled in two groups, the 1-, 2- and 3-year overall survival rates in the MWA group and SR group were 82.6, 73.5 and 63 and 33.3, 33.3 and 0% (), showing no significant statistical difference (p = 0.131), respectively. The 3-, 6-, 12-, 18- and 24-month local tumour reoccurrence-free (LTRF) rates in the MWA group and SR group were 91.7,91.7,91.7, 91.7 and 91.7% and 100, 75, 75, 50 and 25%, respectively (), showing no significant statistical difference (p = 0.132).
Figure 3. (A) Graph shows the 1-, 3- and 5-year overall survival rates in the MWA group and SR group; (B), Graph shows the 1-, 3- and 5-year disease-free survival rates in the MWA group and SR group; (C) Graph shows the 3-, 6-, 12-, 18- and 24-month local tumour reoccurrence-free (LTRF) rates in the MWA group and SR group.
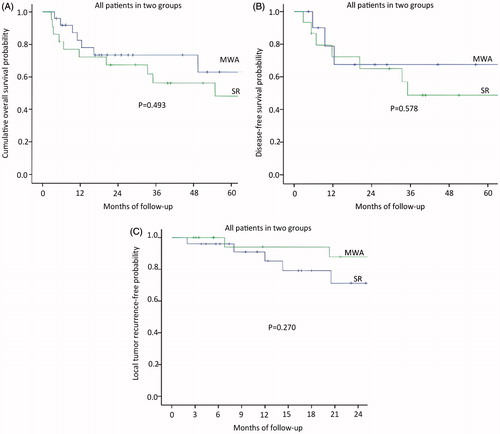
Figure 4. The patients of intrahepatic HCC well controlled or stable in two groups. (A) Graph shows the 1-, 2- and 3-year overall survival rates in the MWA group and SR group; (B) Graph shows the 1-, 2- and 3-year disease-free survival rates in the MWA group and SR group; (C) Graph shows the 3-, 6-, 12-, 18- and 24-month local tumour reoccurrence-free (LTRF) rates in the MWA group and SR group.
Complication
No death in the two groups was directly related to any treatment. One major complication (4%; one of 25 patients) occurred in the MWA group after 32 MWA sessions, including 1 patient who had skin burn and this patient had bigger seeding (4.8 cm in diameter) and diabetes. One major complication (4.5%, one of 22 patients) occurred in cases of SR group after 23 surgical procedures, including one incision ruptures. This patient had hypertension and diabetes. SR complication was successfully treated by suturing the incision a second time. The overall complication rates for the two groups were comparable (p = 0.593) were comparable.
Univariate and multivariate analyses
The univariate analysis () showed statistically significant differences between groups in terms of survival rates, depending on the tumour diameter (χ2 = 36.074; p = <0.001), control of the primary tumour (χ2 = 6.021; p = 0.013), distant metastasis (χ2 = 5.121; p = 0.024) and major complications (χ2 = 25.359; p ≤0.001). The multivariate analysis () showed that the factors that significantly affected the survival rate were remnant intrahepatic tumour (p = 0.007), Child–Pugh grade (p = 0.009) and metastasis (p ≤0.001). Six of these patients had Child–Pugh B, 18 of these patients had poor control of the primary tumour and 17 of these patients had distant metastasis.
Table 3. Univariable analysis of factors affecting overall survival.
Table 4. Multivariate analysis of prognostic factors with cox proportional hazards model.
Discussion
The thoracoabdominal wall seeding is usually transferred from the primary sites through needle tracks or incisions. Among these, metastasis from HCC is the most common, although its incidence is very low [Citation16]. Takemur et al. [Citation17] reported that SR of thoracoabdominal wall HCC metastasis can provide acceptable long-term survival as long as the intrahepatic lesions are controlled. In their study, the cumulative 1-, 3- and 5-year overall survival rates were 71, 44 and 39%, respectively. Likewise, Yeh et al. [Citation18] reported median survival times of 16 and 12.5 months in 30 patients with HCC implants on the thoracoabdominal wall. These data were similar to the results in this study. Minimally invasive interventional therapy has received much attention as an alternative method in patients with poor physical condition and who are inoperable [Citation19]. Indeed, both physical condition and immunologic status were the factors affecting prognosis in the patients [Citation20]. However, the therapeutic equivalence between minimally invasive procedures and surgical resection is still controversial.
In general, the thoracoabdominal wall seeding are manifestations of the terminal stage of cancer, which is difficult to control and treat. In such cases, surgical resection could impose difficulties during operation and risks for complications, including hernia [Citation21]. For metastatic nodules at an ideal site or those with smaller diameter, percutaneous therapy has achieved good therapeutic effects. At present, RFA, MWA, transcatheter arterial embolisation (TACE), HIFU and 125I brachytherapy have emerged as the percutaneous treatment modalities. Such interventional therapies are more ideal for patients with poor physical condition. In this study, we showed that MWA, compared with RFA, may allow a larger ablation zone and higher intratumoural temperature [Citation10,Citation12,Citation16,Citation22].
Compared with HIFU, MWA was reported to be less affected by the perfusion-mediated heat-sink effect, which may help in treating common malignant tumours [Citation23]. Compared with 125I brachytherapy, MWA has fewer side effects [Citation24].
Comparison of the clinical efficacy between MWA and traditional SR for the treatment of HCC seeding on the thoracoabdominal wall was performed in the present study. To ensure that the two sets of data are balanced, we chose HCC patients in stage B based on BCLC. And, under the condition of approximately same liver function [Citation25], compared with SR, MWA had comparable survival outcomes in the patients with HCC seeding and similar local recurrence rate. In addition, we compared with survival outcomes and local tumour recurrence rate in patients with well controlled primary tumours between two groups, showing no significant statistical difference. And the same for the patients with poorly controlled primary tumours. Data show that the patients between SR group and MWA group had same comparable survival outcomes and local tumour recurrence rate in the same situation of primary site (well-controlled or uncontrolled). Finally, reconstruction is troublesome [Citation26], and a significant number of patients require abdominoplasty for larger abdominal wall tumours. However, MWA was associated with the advantages of being minimally invasive, less expensive and having a shorter hospital stay [Citation27]. Therefore, it could be an effective method for the treatment of HCC seeding on the thoracoabdominal wall.
In this study, patients who underwent MWA had a lower KPS [Citation28] and more comorbid disease compared with the patients who underwent SR. These observations might have contributed to the fact that the patients with good physical condition and those who had less disease tended to choose SR over MWA. In fact, as a minimally invasive treatment method, MWA is more suitable the patients with poor condition compared with SR, which based on the advantage of high efficiency, minimal invasiveness and relatively short treatment durations. Technical effectiveness and primary tumour control seemed to be the two important factors that can help achieve MWA results similar to surgical outcomes. Remnant intrahepatic tumour, Child–Pugh grade and metastasis have a significant impact on prognosis. The above factors need to arouse our attention. Unfortunately, after MWA therapy, a patient skin was burned, which may be the reason that MWA energy range around the melting needle is difficult to control and the surrounding tissues such as the skin can be easily burned [Citation29]. To those patients with seeding adjoined to the skin surface, we should pay attention to control ablative range.
An important limitation of this study is its retrospective design with a relatively small patient series. Limited sample size might have reduced statistical power in comparative analysis so that some associations were not detected. The limited follow-up cannot provide long-term oncologic results for the two techniques. Also, the success of MWA was assessed by radiographic findings vs. pathologic margin-free status. Therefore, despite the intermediate follow-up reported, it might take longer to use radiographic techniques to detect MWA failures. In addition, the prognosis of patients of HCC seeding most likely relies upon the disease progress in the liver and distant metastasis, which is difficult to compare between MWA and SR. Finally, included in our population were patients with thoracoabdominal wall HCC seeding in stage B, which excluded HCC patients in other stages. A prospective trial that compares standard procedures with MWA is needed to validate the role of this developing modality in the clinical field.
In conclusion, compared with SR, MWA provided comparable results in terms of technical effectiveness, oncologic outcomes and complications. The present results were encouraging and suggested that MWA could be a safe and feasibly effective option in the management of HCC seeding on the thoracoabdominal wall.
Ethical approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. This article does not contain any studies with animals performed by any of the authors.
Disclosure statement
All authors declare that they have no conflict of interest.
References
- Zuo TT, Zheng RS, Zhang SW, et al. (2015). Incidence and mortality of liver cancer in China in 2011. Chin J Cancer 34:508–13.
- Tang A, Hallouch O, Chernyak V, et al (2017). Epidemiology of hepatocellular carcinoma: target population for surveillance and diagnosis. Abdom Radiol (NY) 4. doi: 10.1007/s00261-017-1209-1
- Zhong-Yi Z, Wei Y, Kun Y, et al. (2017). Needle track seeding after percutaneous radiofrequency ablation of hepatocellular carcinoma: 14-year experience at a single centre. Int J Hyperthermia 1. doi: 10.1080/02656736.2017.1278630
- Krengli M, Poletti A, Ferrara E, Fossati P. (2016). Tumour seeding in the surgical pathway after resection of skull base chordoma. Rep Pract Oncol Radiother 21:407–11.
- Yun JK, Kim MA, Choi CM, et al. (2015). Surgical outcomes after pulmonary resection for non-small cell lung cancer with localized pleural seeding first detected during surgery. Thorac Cardiovasc Surg 19(suppl 1). doi: 10.1055/s-0035-1564929
- Li A, Wu B, Cui L, Wu M. (2015). Successful en bloc resection of recurrent hepatocellular carcinoma directly invading the abdominal wall: a case report. J Med Case Rep 9:19.
- Qi C, Yu XL, Liang P, et al. (2012). Ultrasound-guided microwave ablation for abdominal wall metastatic tumors: a preliminary study. World J Gastroenterol 18:3008–14.
- Poggi G, Montagna B, DI Cesare P, et al. (2013). Microwave ablation of hepatocellular carcinoma using a new percutaneous device: preliminary results. Anticancer Res 33:1221–7.
- Gao Y, Liang P, Yu X, et al. (2016). Microwave treatment of renal cell carcinoma adjacent to renal sinus. Eur J Radiol 85:2083–9.
- Abdelsalam ME, Murthy R, Avritscher R, et al. (2016). Minimally invasive image-guided therapies for hepatocellular carcinoma. J Hepatocell Carcinoma 3:55–61.
- Vogl TJ, Farshid P, Naguib NN, et al. (2015). Ablation therapy of hepatocellular carcinoma: a comparative study between radiofrequency and microwave ablation. Abdom Imaging 40:1829–37.
- Abdelaziz A, Elbaz T, Shousha HI, et al. (2014). Efficacy and survival analysis of percutaneous radiofrequency versus microwave ablation for hepatocellular carcinoma: an Egyptian multidisciplinary clinic experience. Surg Endosc 28:3429–34.
- Han Y, Shao N, Xi X, Hao X. (2017). Use of microwave ablation in the treatment of patients with multiple primary malignant tumors. Thorac Cancer 8:365–71.
- Wang YM, Qian GJ, Xu Y, et al. (2017). [Efficacy of microwave ablation in treatment of hepatocellular carcinoma within the Milan criteria: a report of 696 cases]. Zhonghua Gan Zang Bing Za Zhi 25:344–8.
- Ahmed M, Solbiati L, Brace CL, et al. (2014). Image-guided tumor ablation: standardization of terminology and reporting criteria-a 10-year update. Radiology 273:241–60.
- Yu J, Liang P, Yu XL, et al. (2015). Local tumour progression after ultrasound-guided microwave ablation of liver malignancies: risk factors analysis of 2529 tumours. Eur Radiol 25:1119–26.
- Takemura N, Hasegawa K, Aoki T, et al. (2014). Surgical resection of peritoneal or thoracoabdominal wall implants from hepatocellular carcinoma. Br J Surg 101:1017–22.
- Yeh CN, Chen MF. (2004). Resection of peritoneal implantation of hepatocellular carcinoma after hepatic resection: risk factors and prognostic analysis. World J Surg 28:382–6.
- Wang QC, Cheng W, Zhang L, et al. (2015). Use of percutaneous sonographically guided microwave ablation therapy to treat inoperable malignant liver tumours. West Indian Med J 64:76–80.
- Goldfarb Y, Sorski L, Benish M, et al. (2011). Improving postoperative immune status and resistance to cancer metastasis: a combined perioperative approach of immunostimulation and prevention of excessive surgical stress responses. Ann Surg 253:798–810.
- Robertson JD, de la Torre JI, Gardner PM, et al. (2003). Abdominoplasty repair for abdominal wall hernias. Ann Plast Surg 51:10–16.
- Carrafiello G, Laganà D, Mangini M, et al. (2008). Microwave tumors ablation: principles, clinical applications and review of preliminary experiences. Int J Surg 6 Suppl 1:S65–S9.
- Wang Y, Wang W, Wang Y, Tang J. (2010). Ultrasound-guided high-intensity focused ultrasound treatment for needle-track seeding of hepatocellular carcinoma: preliminary results. Int J Hyperthermia 26:441–7.
- Yang Q, Peng S, Wu J, et al. (2015). Spectral CT with monochromatic imaging and metal artifacts reduction software for artifacts reduction of 125I radioactive seeds in liver brachytherapy. Jpn J Radiol 33:694–705.
- De Cobelli F, Marra P, Ratti F, et al. (2017). Microwave ablation of liver malignancies: comparison of effects and early outcomes of percutaneous and intraoperative approaches with different liver conditions: new advances in interventional oncology: state of the art. Med Oncol 34:49.
- Kovacević P, Velickov A, Stojiljković D, et al. (2014). Reconstruction of full thickness abdominal wall defect following tumor resection: a case report. Srp Arh Celok Lek 142:347–50.
- Yu J, Liang P. (2016). Status and advancement of microwave ablation in China. Int J Hyperthermia 33. doi: 10.1080/02656736.2016.1243261
- Terret C, Albrand G, Moncenix G, Droz JP. (2011). Karnofsky performance scale (KPS) or physical performance test (PPT)? That is the question. Crit Rev Oncol Hematol 77:142–7.
- Ding J, Zhou Y, Wang Y, et al. (2017). Percutaneous microwave ablation of exophytic tumours in hepatocellular carcinoma patients: safe or not. Liver Int 37:1365–72.