Abstract
Introduction: In clinical practice and experimental settings, cutaneous premalignant and malignant lesions are commonly diagnosed by histopathological biopsy. However, this technique is invasive and results in functional or cosmetic defects. Dynamic thermal imaging is a non-invasive technique that quantifies the infra-red (IR) radiation emitted by a subject after the introduction of external thermal stimuli (such as heat or cold).
Methods: Forty hairless albino (Crl:SKH1-hr) mice were randomised to the control group or the experimental group. The experimental group was regularly irradiated with artificial ultraviolet. Clinical photographs, immunohistochemical staining and dynamic thermal imaging results of both groups were obtained.
Results: As photocarcinogenesis proceeded, faster thermal recovery to basal temperature after heat stimuli was significant on dynamic thermal imaging. With histopathological correlations, it was possible to differentiate normal, premalignant and malignant cutaneous lesions according to thermal imaging results. CD 31 staining analysis showed that increased vasculature was the key change responsible for different thermal imaging results among photocarcinogenesis steps.
Conclusions: Dynamic thermal imaging is useful to differentiate normal, premalignant and malignant cutaneous lesions. Increased vasculature is the key change responsible for different thermal imaging results.
Introduction
Skin cancer is common in humans, and squamous cell carcinoma (SCC) is the second most common form of skin cancer [Citation1,Citation2]. Actinic keratosis (AK) is benign tumour of dysplastic keratinocytes considered a precancerous lesion to SCC [Citation3–5]. Cumulative chronic sun exposure is the most important risk factor for AK and SCC [Citation3,Citation6]. Sun light, especially ultraviolet (UV) B, causes mutation of the p53 tumour suppressor gene, which is an important step in cutaneous oncogenesis [Citation7,Citation8]. Chronic UV exposure causes clusters of clonal keratinocytes with elevated mutated p53 expressions, which are commonly referred as mutant p53 foci or patches [Citation6,Citation9,Citation10]. Selected numbers of these mutant p53 foci progress to AK and ultimately to SCC [Citation4,Citation6].
Repetitive artificial UV exposure of hairless albino (Crl:SKH1-h) mouse results in cutaneous lesions similar to AK or SCC [Citation6,Citation11]. This is a well-established animal model for photocarcinogenesis study [Citation4,Citation10]. Although UVB is the important UV type that induces p53 mutation, the combination of UVB and UVA produces an additive effect [Citation4]. Different doses and frequencies of UV application were used in previous experiments, which all resulted in cutaneous lesions similar to AK or SCC [Citation6,Citation10,Citation12,Citation13].
Suspected AK or SCC lesions are usually diagnosed by biopsy in experimental settings and clinical practice. However, this technique is invasive that can possibly result in scars or defects. In addition, there is reluctance to repetitive biopsies to verify therapeutic effect and detect tumour recurrence. Therefore, there is a need for a non-invasive diagnostic technique.
Thermal imaging is a non-invasive technique that quantifies the infra-red (IR) radiation emitted by surface of a subject [Citation1]. Various studies attempted to detect cancerous cells or tumours by measuring IR emitted from the surface of animal models or humans [Citation5,Citation7,Citation14–18]. This technique has two subtype methods. The passive (static) method detects IR radiation emitted from subjects without any manipulation. The dynamic (active) method quantifies IR radiation after introducing external thermal stimulus (heating or cooling) to the subject in order to enhance relevant thermal contrast [Citation1,Citation19].
Malignant tissue is known to exhibit abnormal cell proliferation, excess metabolism and increased blood flow. Thus, it was hypothesised that malignant tissue has excess heat generation compared to normal tissue [Citation1,Citation14,Citation15,Citation18]. With this hypothesis, passive thermal imaging was first used in diagnosis of cutaneous melanoma [Citation20]. However, the passive method has a high rate of false negatives [Citation1]. The dynamic method was introduced to overcome this shortcoming with promising results [Citation1,Citation16,Citation17].
The current study explored the use of dynamic thermal imaging on a well-established photocarcinogenesis mouse model. This animal model enabled the acquisition of sequential results during photocarcinogenesis from normal skin to AK and ultimately to SCC. This study evaluates the usefulness of dynamic thermal imaging analysis in AK lesions as well as SCC lesions. In addition, it aims to provide possible explanation for different thermal imaging results between normal and malignant cutaneous tissue.
Methods
SKH1/hr mouse model
Forty 8-week-old outbred male hairless albino (Crl:SKH1-h) mice were obtained from Orient-Bio Inc (Seoul, Korea). They were randomised to the control group or the experimental group. The experimental group (20 mice) was irradiated with UV (500 J/m2) five times per week for 16 weeks. The dose corresponds approximately to one minimal erythema dose (MED) for the mouse model [Citation9,Citation10]. Three TL-12/40 W fluorescent tubes (Phillip, Amsterdam, Netherlands) were the artificial UV source. These lamps emit 54% output of UVB (280–315 nm) and 46% of UVA (315–400 nm). The lamps were placed in parallel approximately 23–26 cm above the cage during UV irradiation. Before UV irradiation, the power of the light source was measured with optical power metre (S120VC power sensor with PM100D power metre, Thorlabs, Newton, NJ) at the same position where the mouse was irradiated. The measured power was 120 μW/cm2. Therefore, it took approximately 417 s per each session to give 500 J/m2.
Clinical photographs of all mice in both groups were taken every week. Additionally, dynamic thermal imaging of both groups were taken every other week under inhalational anaesthesia. The mouse was initially anaesthetised with 4% isoflurane (JW Pharmaceuticals, Korea) mixed in 30% O2 and 70% N2O for 4 min in a ventilated anaesthesia induction chamber (Harvard apparatus, Holliston, MA). The anaesthesia was maintained by gas inhalation with inhalational mask, which was continuously supplied with 2% isoflurane in 30% O2 and 70% N2O. Finally, punch biopsy (4 mm diameter) of a representative lesion was obtained from a selected mouse in the experimental group every week.
Institutional Animal Care and Use Committee (IACUC) of Korea University approved this study.
Immunohistochemistry and imaging analysis
Skin biopsy samples were immediately fixed with formaldehyde solution and embedded in paraffin. Sections were stained with H&E. Ki67 (Abcam, Cambridge, UK) immunostaining was done to detect cellular proliferation. CD31 (Abcam, Cambridge, UK) immunostaining was performed to detect the degree of angiogenesis.
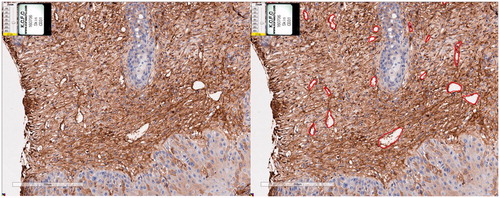
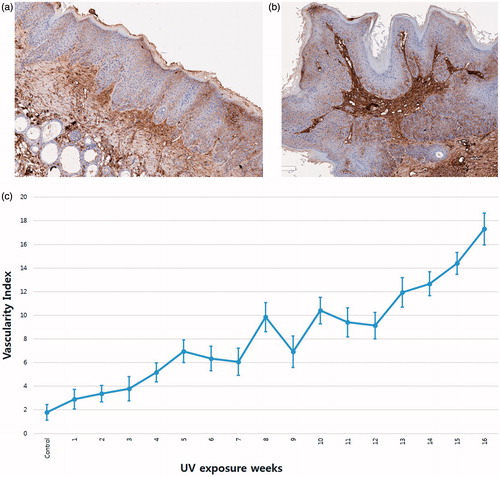
All histopathological slides were scanned using an Aperio AT2 (Leica Biosystems, Vista, CA). All images were analysed using an Aperio imagescope (Leica Biosystems). Especially with CD31 immunostaining results, we manually marked the lumens of all stained vessels. () The total lumen area of sectioned vessels was calculated and was divided by the total area of epidermis and dermis of the corresponding specimen. We defined this calculated value as the vascularity index which was adapted from previous studies [Citation21,Citation22]. Vascularity indexes of each specimen were obtained to compare vasculature development as UV exposure proceeded.
Dynamic thermal imaging
The dynamic thermal imaging system consists of two parts. The first part is the heating system. A metal ceramic heater (HT19R, Thorlabs, Newton, CA) and a resistance temperature detector (TH100PT, Thorlabs) was the heating source. A heating controller (TC200-EC, Thorlabs) was used to ensure that stabilised heat was delivered to the subject. The second part is the data analysis system. Sequential real-time recording of IR emitted from the mouse dorsal skin was detected by an IR thermal camera (C2, FLIR system, Croissy-Beaubourg, France). This camera contains 80 × 60 pixels resolution (IR sensor) and has thermal sensitivity of 0.1 °C.
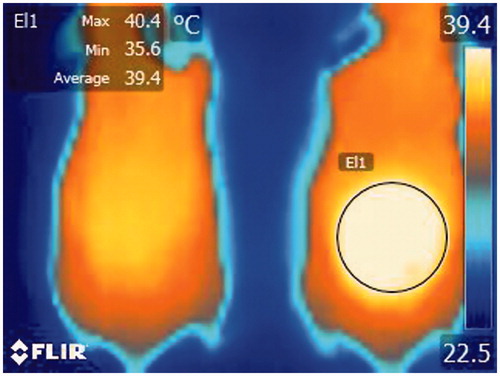
A mouse was placed in an ambient environment and under anaesthesia as described above. The dorsal skin of the mouse was heated with the heating system. As soon as the target temperature (40 °C) was reached, the heating source was removed. All thermal images of the dorsal skin were sequentially recorded during the procedure. This procedure was done every other week. Acquired images were analysed by the thermal imaging processing software (Altair, FLIR system, Croissy-Beaubourg, France) to select a target site (normal skin, or suspicious AK or SCC sites) and analyse the thermal change of the selected region of interest. Suspicious lesions were selected by a single experienced dermatologist, and the selected sites were approximately in the same area ().
Statistical analysis
Data from thermal imaging was analysed using the Excel programme (Microsoft, Redmond, WA). Temperature changes of selected region of interest were drawn as a graph, which corresponded to a thermal response graph. Exponential curves were fitted to thermal response graph according to the following equation.
We converted the above equation to logarithmic form as below.
The gradient component (value “b”) was compared between different UV exposure weeks with ANOVA. Statistical analysis was carried out by using SPSS 20.0 (IBM, Armonk).
Results
Tumor development
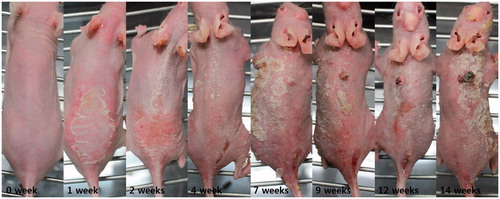
The mice developed erythema and desquamation after 1 week of UV exposure. After 2 weeks, some mice showed hyperkeratosis on the dorsal skin. By 7 weeks, erythematous protruding nodule begins to appear on some mice. The size and numbers of nodules, and the degree of hyperkeratosis increased as UV exposure proceeded. There were no significant changes in the control group ().
Figure 3. Clinical photographs of hairless albino (Crl:SKH1-h) mice from control (week 1) to week 14. The mice developed erythema and desquamation after 1 week of UV exposure. By 7 weeks, erythematous protruding nodule begins to appear on some mice. The size and numbers of nodules, and the degree of hyperkeratosis increased as UV exposure proceeded.
Immunohistochemistry and imaging results
Weekly haematoxylin and eosin (H&E) staining revealed that dyskeratotic keratinocytes constituted one-third to two-thirds of the epidermis at 6–8 weeks. Dyskeratotic cells occupied the entire epidermis during 9–13 weeks. These stages are similar to AK lesions in humans. After 14 weeks, the dyskeratotic cells began to invade the epidermal–dermal junction (i.e. dermal invasion). This status corresponds to SCC in humans ().
Figure 4. (a) Hematoxylin and eosin (H&E) stain of control group. (b) H&E stain of week 6 showing dyskeratotic keratinocytes constituting lower one-third to two-thirds of epidermis. (c) H&E stain of week 14 showing dermal invasion of dyskeratotic keratinocytes. (d) Ki stain of week 8 showing mitosis limited to lower one-third of epidermis (e) Ki stain of week 14 showing mitosis present in dermis as well as epidermis.
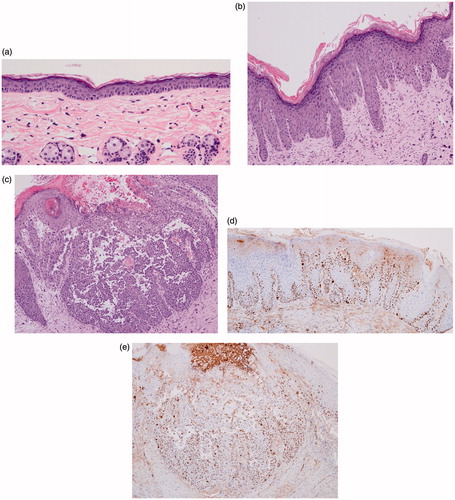
Ki 67 staining revealed that cellular mitotic activity was limited to the basal layer in the control group. However, with more UV exposure, cellular proliferations became apparent in upper parts of epidermis and the basal layer. Ki 67 staining of SCC stage showed that mitosis is also visible in the dermis ().
CD 31 staining results of the experimental group revealed increasing numbers of vessels with increasing UV exposure. This was especially evident in a vascularity index graph with UV exposure. The vascularity index ranged from 1.7858 in control group to 17.3131 in week 16. Although the vascularity index fluctuated slightly, it generally tends to increase as UV exposure increases ().
Dynamic thermal imaging
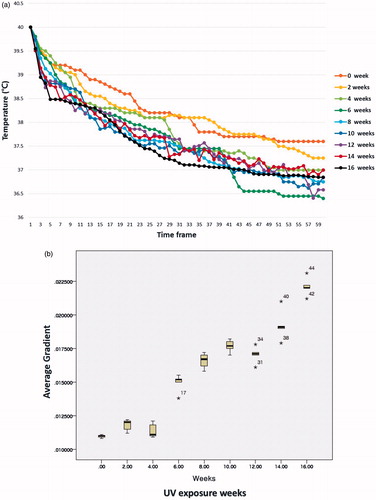
It took about 40–60 frames (approximately 12–18 s) for the temperature of the selected region of interest (normal skin or a site suspicious for AK or SCC) to recover to basal temperature after reaching the target temperature (40 °C). Comparison of the early thermal response graphs of mice at different lengths of UV exposure revealed faster thermal recovery to basal level as UV exposure increased. (, Supplementary Table 1) After fitting exponential curve (Supplementary Graph), logarithmic equations for each UV exposure weeks were calculated. When comparing the gradient value of each equation, it was possible to significantly group the UV exposure weeks into five groups: 0 to 4, 6, 8–12, 14 and 16 weeks. (, Supplementary Table 2) With histopathological correlation, the first group corresponded to the normal stage, the second and third groups to the AK stage, and the last two groups to the SCC stage.
Figure 6. (a) Comparison of the early thermal response graphs of mice at different lengths of UV exposure revealed faster thermal recovery to basal level as UV exposure increased. (b) Tukey or Duncan analysis of average gradient significantly grouped the UV exposure weeks into five groups; 0 to 4, 6, 8 to 12, 14 and 16 weeks.
Discussion
Although AK or cutaneous SCC itself is not usually fatal, it constitutes a substantial public health burden due to its relatively high prevalence [Citation2,Citation23]. There are other risk factors such as burns and immunosuppression. Still, cumulative UV exposure is the main aetiological factor responsible for AK or cutaneous SCC development [Citation2,Citation24]. UV is involved in all three steps of UV carcinogenesis: initiation, promotion and progression [Citation24]. DNA bases can directly absorb UV radiation, which can result in C to T or CC to TT transitions. These transitions of DNA bases are called UV signature mutations [Citation10,Citation24]. UV signature mutations of p53 genes are present in AK and SCC of humans as well as in UV-induced skin tumours of experimental mouse models [Citation3,Citation4,Citation11]. The p53 gene mutation plays a pivotal role in photocarcinogenesis, because the normal p53 protein is involved in multiple regulatory pathways in cell cycle, cell apoptosis and DNA damage repair [Citation4,Citation10]. Chronic UV exposure results in clusters of clonal keratinocytes that overexpress mutant p53 [Citation6,Citation9,Citation10]. These p53 mutant foci are histologically detectable, and some progress to AK [Citation4,Citation6,Citation8,Citation9,Citation10]. Only a small percentage of AK further transforms to SCC with estimated rate ranging from 0 to 20% per lesion-year [Citation2,Citation25,Citation26].
Similar photocarcinogenesis steps were also proven in a hairless albino mouse model [Citation6,Citation11]. Various doses, times and wavelengths of UV were applied; all of them yielded skin lesions similar to AK or SCC [Citation6,Citation10,Citation12,Citation13]. The mice in the present study also produced cutaneous lesions histologically similar to AK after 6 weeks of UV exposure. After 14 weeks, microscopically proven SCC lesions appeared on some mice.
It is important as well as cost-effective to prevent these AK or SCC lesions at the population level. The main method of prevention of AK or SCC is skin protection from undue sunlight [Citation2]. This includes lifestyle modifications, such as appropriate and regular use of sunscreens, public education and health policies [Citation2,Citation24]. A great example of health policies to prevent public from UV exposure is regulation of indoor artificial tanning that some countries has introduced [Citation2]. Besides primary protection, early diagnosis of premalignant AK and SCC is crucial for better outcome, minimising risk of morbidity, recurrence and mortality [Citation2]. Unfortunately, there is no definite screening tool for these lesions, and clinical diagnosis often results in false-positive or false-negative findings. For instance, even well-trained dermatologists clinically count significantly different numbers of AKs in the same patient [Citation27]. Therefore, definite diagnosis of AK or SCC is usually performed by histopathological biopsy. However, skin biopsy may cause cosmetic or functional defects. Therefore, other non-invasive methods, such as dermoscopy and confocal scanning laser microscopy, are applied as an additional diagnostic procedure [Citation28,Citation29]. IR imaging is one of promising non-invasive auxiliary diagnostic tool in skin cancer diagnosis [Citation5].
Brasfield et al. first introduced IR imaging in skin cancer field when they reported that melanoma lesions are hyperthermic compared to surrounding tissue [Citation20]. Unfortunately, early studies showed poor findings with a high rate of false-negatives [Citation30]. There are several explanations. First, at that time, there was no IR imaging device sensitive enough to detect slight temperature differences between normal and malignant tissue. More importantly, the concept of dynamic thermal imaging was not applied during the early studies [Citation1,Citation15,Citation31]. Dynamic IR imaging involves applying external thermal stimulus (heating or cooling) to the subject for a defined period [Citation1,Citation15,Citation19,Citation31]. This method enhances relevant thermal contrast of the subject of interest [Citation1]. In addition, by choosing IR images from a specific time after thermal stimulus, it is possible to discard unnecessary temperature variations of the subject [Citation1,Citation31]. Di Carlo first attempted to use dynamic IR imaging method to detect cutaneous melanoma [Citation7]. Çetingül and Herman did more in-depth research on actual patients as well as using a mathematical model to evaluate use of dynamic IR imaging in melanoma diagnosis [Citation1,Citation32]. Di Carlo also used dynamic video thermography in AK patients [Citation5].
To our knowledge, there was no experiment of dynamic IR imaging involving the hairless albino mouse model. Using this model enables the analysis of sequential IR imaging results along the photocarcinogenesis continuum from normal skin to AK, and ultimately to SCC. Similar studies cannot ethically be performed in humans. In our results, the early thermal response was faster with longer UV exposure. It was possible to statistically classify the UV exposure weeks into five groups using exponential curve results. With histological correlation, it was possible to match these groups to photocarcinogenesis stages (normal skin, AK and SCC). Therefore, dynamic thermal imaging results can be used as an additional diagnostic criterion when diagnosing AK-similar or SCC lesions in the mouse model. With further research, similar methods may be developed or applied in humans.
It is important to understand the pathophysiological changes of malignant or pre-malignant tissue that cause faster thermal response shown in dynamic IR imaging. Previous reports suggested several changes in malignant tissue as a possible cause. Abnormal cell proliferation, excess metabolism and increased blood flow are well known examples [Citation1,Citation14,Citation15,Citation18]. Most previous dynamic IR imaging studies used cold stimulus and suggested that the aforementioned changes are responsible for the rapid thermal recovery. In contrast, the present study applied a heat stimulus. Pre-malignant and malignant cutaneous lesions had faster thermal recovery after heating compared to normal skin. This has a significant meaning because it proved that increased blood flow, rather than increased metabolism or cell proliferation, is the most important pathophysiologic finding responsible for rapid thermal recovery. Theoretically, if increased metabolism or cell proliferation were to have more significant effect on temperature change than increased blood flow, the temperature would slowly decrease after heat stimulus. Therefore, the increased vascularity is the key pathophysiologic change accountable for rapid thermal response in dynamic IR imaging. This is consistent with a mathematical model study that showed that surface temperature is most sensitive to blood perfusion change [Citation32]. We suspect that applied heat energy on premalignant or malignant tissue is dissipated at a higher rate due to increased vascularity and hyperpermeability of tumour vessels. This increase rate of heat dissipation is evident as a faster thermal recovery as measured by IR imaging. The imaging analysis of serial CD31 staining results in the present study revealed the actual vascular change during photocarcinogenesis. The total lumen area of tumour vessels increased as photocarcinogenesis proceeded.
Conclusion
Dynamic thermal imaging is useful to differentiate normal, premalignant and malignant cutaneous lesions using a photocarcinogenesis mouse model. Increased vasculature is the key change responsible for different thermal imaging results.
IHYT_1408858_Supplemental_File.zip
Download Zip (221.4 KB)Disclosure statement
No potential conflict of interest was reported by the authors.
Additional information
Funding
References
- Çetingül MP, Herman C. (2011). Quantification of the thermal signature of a melanoma lesion. Int J Thermal Sci 50:421–31.
- Green AC, Olsen CM. (2017). Cutaneous squamous cell carcinoma: an epidemiological review. Br J Dermatol 177:373–81.
- Ratushny V, Gober MD, Hick R, et al. (2012). From keratinocyte to cancer: the pathogenesis and modeling of cutaneous squamous cell carcinoma. J Clin Invest 122:464–72.
- Ortonne JP. (2002). From actinic keratosis to squamous cell carcinoma. Br J Dermatol 146 Suppl 61:20–3.
- Di Carlo A, Elia F, Desiderio F, et al. (2014). Can video thermography improve differential diagnosis and therapy between basal cell carcinoma and actinic keratosis? Dermatol Ther 27:290–7.
- Cozzi SJ, Ogbourne SM, James C, et al. (2012). Ingenol mebutate field-directed treatment of UVB-damaged skin reduces lesion formation and removes mutant p53 patches. J Invest Dermatol 132:1263–71.
- Di Carlo A. (1995). Thermography and the possibilities for its applications in clinical and experimental dermatology. Clin Dermatol 13:329–36.
- Rebel H, Kram N, Westerman A, et al. (2005). Relationship between UV-induced mutant p53 patches and skin tumours, analysed by mutation spectra and by induction kinetics in various DNA-repair-deficient mice. Carcinogenesis 26:2123–30.
- Rebel HG, Bodmann CA, van de Glind GC, et al. (2012). UV-induced ablation of the epidermal basal layer including p53-mutant clones resets UV carcinogenesis showing squamous cell carcinomas to originate from interfollicular epidermis. Carcinogenesis 33:714–20.
- Rebel H, Mosnier LO, Berg RJ, et al. (2001). Early p53-positive foci as indicators of tumor risk in ultraviolet-exposed hairless mice: kinetics of induction, effects of DNA repair deficiency, and p53 heterozygosity. Cancer Res 61:977–83.
- Handoko HY, Ferguson B, Walker GJ. (2015). Mouse models for actinic keratosis and squamous cell carcinoma. Curr Probl Dermatol 46:42–8.
- Cozzi SJ, Le TT, Ogbourne SM, et al. (2013). Effective treatment of squamous cell carcinomas with ingenol mebutate gel in immunologically intact SKH1 mice. Arch Dermatol Res 305:79–83.
- Burns EM, Tober KL, Riggenbach JA, et al. (2013). Preventative topical diclofenac treatment differentially decreases tumor burden in male and female Skh-1 mice in a model of UVB-induced cutaneous squamous cell carcinoma. Carcinogenesis 34:370–7.
- Moustafa AMN, Muhammed HH, Hassan M. (2013). Skin cancer detection using temperature variation analysis. Engineering 5:18–21.
- Bonmarin M, Le Gal FA. (2014). Lock-in thermal imaging for the early-stage detection of cutaneous melanoma: a feasibility study. Comput Biol Med 47:36–43.
- Herman C, Cetingul MP. (2011). Quantitative visualization and detection of skin cancer using dynamic thermal imaging. J Vis Exp 51:e2679.
- Santa Cruz GA, Bertotti J, Marin J, et al. (2009). Dynamic infrared imaging of cutaneous melanoma and normal skin in patients treated with BNCT. Appl Radiat Isot 67:S54–S8.
- Song C, Appleyard V, Murray K, et al. (2007). Thermographic assessment of tumor growth in mouse xenografts. Int J Cancer 121:1055–8.
- Kim JY, Chang KS, Kook MH, et al. (2013). Measurement of thermal properties of magnetic nanoparticles using infrared thermal microscopy. Infrared Phys Technol 57:76–80.
- Brasfield RD, Laughlin JS, Sherman RS. (1964). Thermography in the Management of Cancer. Ann N Y Acad Sci 121:235–47.
- Reyes-Aldasoro CC, Williams LJ, Akerman S, et al. (2011). An automatic algorithm for the segmentation and morphological analysis of microvessels in immunostained histological tumour sections. J Microsc 242:262–78.
- Rossi A, Sozio F, Sestini P, et al. (2010). Lymphatic and blood vessels in scleroderma skin, a morphometric analysis. Hum Pathol 41:366–74.
- Siegel JA, Korgavkar K, Weinstock MA. (2017). Current perspective on actinic keratosis: a review. Br J Dermatol 177:350–8.
- Erlendsson AM, Thaysen-Petersen D, Bay C, et al. (2016). Repeated treatments with ingenol mebutate for prophylaxis of UV-induced squamous cell carcinoma in hairless mice. J Photochem Photobiol B 163:144–9.
- Quaedvlieg PJ, Tirsi E, Thissen MR, et al. (2006). Actinic keratosis: how to differentiate the good from the bad ones?. Eur J Dermatol 16:335–9.
- Werner RN, Sammain A, Erdmann R, et al. (2013). The natural history of actinic keratosis: a systematic review. Br J Dermatol 169:502–18.
- Lee KC, Lew R, Weinstock MA. (2014). Improvement in precision of counting actinic keratoses. Br J Dermatol 170:188–91.
- Rishpon A, Kim N, Scope A, et al. (2009). Reflectance confocal microscopy criteria for squamous cell carcinomas and actinic keratoses. Arch Dermatol 145:766–72.
- Zalaudek I, Giacomel J, Schmid K, et al. (2012). Dermatoscopy of facial actinic keratosis, intraepidermal carcinoma, and invasive squamous cell carcinoma: a progression model. J Am Acad Dermatol 66:589–97.
- Cristofolini M, Perani B, Piscioli F, et al. (1981). Uselessness of thermography for diagnosis and follow-up of cutaneous malignant melanoma. Tumori 67:141–3.
- Bonmarin M, Le Gal FA. (2015). A lock-in thermal imaging setup for dermatological applications. Skin Res Technol 21:284–90.
- Cetingul MP, Herman C. (2010). A heat transfer model of skin tissue for the detection of lesions: sensitivity analysis. Phys Med Biol 55:5933–51.