Abstract
Objectives: To prospectively evaluate the effectiveness of intra-articular application of pulsed radiofrequency (PRF) combined with viscosupplementation in patients with knee osteoarthritis suffering from chronic pain refractory to conservative therapies.
Methods: During a 30-month period, PRF combined with viscosupplementation was performed on 53 cases of knee osteoarthritis (45 patients, 8/45 with bilateral knee osteoarthritis). Pre-operational imaging included standard knee X-rays on anterior–posterior and lateral views used to evaluate patients according to the Kellgren–Lawrence classification. Pain, prior, one week/one, 6 and 12 months post were compared by means of a numeric visual scale (NVS) questionnaire.
Results: Mean pain score prior to PRF was 8.19 ± 1.4 NVS units. This score was reduced to a mean value of 2.47 ± 2.5 NVS units at 1 week after, 2.55 ± 2.6 at 1 month, 3.1 ± 2.8 at 6 months and 5.02 ± 3.09 at 12 months of follow-up (p < 0.01). Overall mobility improved in 47/53 (88.6%) patients. No complication was observed.
Conclusions: Combining PRF with viscous supplementation is an effective and safe technique for palliative management of chronic pain in patients with knee osteoarthritis. Results seem to be reproducible and long lasting. There seems to be a need of repeating the session at 1 year.
Introduction
Knee osteoarthritis is a degenerative type of arthritis, the prevalence of which increases with age [Citation1]. Knee osteoarthritis ranks as the 11th leading cause of years lived with disability and as the third greatest contributor to loss of health-related quality of life [Citation2,Citation3]. Risk factors include aging, obesity and mechanical stress [Citation4]. Osteoarthritis in the knee can be defined either on the basis of radiographic findings using the Kellgren–Lawrence scale or can be clinically defined according to the proposal of the American College of Rheumatology (ACR) [Citation5,Citation6]. Symptomatic patients report pain, stiffness, swelling, joint instability, reduced mobility and muscle weakness [Citation7]. Current treatment options include physical and occupational therapy, weight loss, stretching exercises, analgesics and anti-inflammatory drugs, intra-articular injections of corticosteroids, concentrated platelet injections or hyaluronic acid into the knee, extra-articular neurolysis of genicular nerves, intra-articular application of pulsed radiofrequency (PRF) and surgery [Citation8–11].
In accordance with the 2012 guidelines of the ACR it is recommended to use intraarticular hyaluronic acid injection for the treatment of knee osteoarthritis since these products are locally administered (minimising thus the risk of systemic side effects) and additionally they seem to delay the need for total knee arthoplasty [Citation12–14]. During the last 40 years, RF energy (in the continuous mode application) has been used for chronic pain therapy in cases unresponsive to conservative treatment; nowadays, technological advances include the use of continuous RF energy in multiple or quadrapolar systems as well as the PRF mode [Citation15–18]. During PRF application the generator produces RF pulses each of which is followed by a silent phase keeping thus the temperature below 42 °C [Citation19]. Both continuous and PRF modes have been applied for pain reduction in symptomatic patients with knee osteoarthritis [Citation20,Citation21].
Purpose of this study is to prospectively evaluate the effectiveness of intra-articular application of PRF combined with viscosupplementation in patients with knee osteoarthritis suffering from chronic pain refractory to conservative therapies.
Material and methods
Institutional review board approval was obtained. All patients were informed about the technique itself as well as possible benefits and complications and they signed a written consent form to the procedure and the study. Authors have no conflict of interest to declare. No industry support was received for this study. All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. No funding was received for this paper.
Patient selection and evaluation
This is a prospective study upon safety and efficacy of intra-articular application of PRF combined with viscosupplementation in symptomatic patients with knee osteoarthritis. During a 30-month period (January 2014–July 2016), PRF and viscosupplementation was performed on 53 cases of knee osteoarthritis (45 patients, 8/45 with bilateral knee osteoarthritis cases). Patient demographics are reported in and .
Table 1. Patient demographics.
Table 2. Demographics per gender.
Inclusion criteria were as follows: adult patients who were capable of providing consent with symptomatic, advanced knee osteoarthritis diagnosed with X-rays and classified as grade II–IV according to the Kellgren–Lawrence classification (16 knees were classified as grade 2, 18 knees as grade 3 and 19 knees as grade 4 according to the Kellgren–Lawrence classification); all patients reported a pain score ≥4NVS units with the pain located at the level of the knee joint with pain duration lasting longer than 1 month [Citation5]. The diagnosis was made by an interventional radiologist with 15 years of experience or the referring orthopaedic surgeon who identified the potential participants and verified their eligibility. All enrolled patients had undergone different conservative therapies in the past six months without success. In our study, pre-enrolment conservative therapy was not pre-specified in the protocol but potentially included the following: analgesics and non-steroidal anti-inflammatory drugs as well as physiotherapy. Before undergoing the procedure none of the patients had received viscosupplementation as conservative measure. At the time of treatment all patients had discontinued all drug therapy for at least two weeks. Before each session each patient underwent clinical examination, whilst in correlation with his/her medical record, evaluation of all imaging and laboratory studies was performed. Pre-operational imaging included standard knee X-rays on anterior–posterior and lateral views used to evaluate patients according to the Kellgren–Lawrence classification. Exclusion criteria for the procedure included untreatable coagulopathy, active, systemic or local infections and patient unwilling to consent to the procedure and the study.
Technique
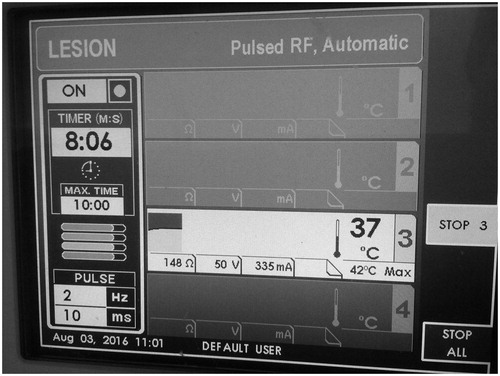
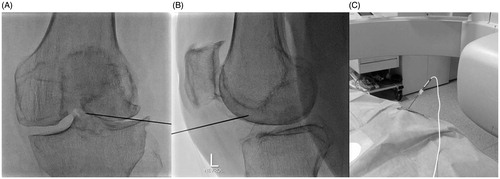
Under local sterility and fluoroscopic guidance, selection of the entrance skin point was performed and local anaesthesia (3–5 ml of lidocaine hydrochloric 2%) was applied. No preoperative antibiotics were intravenously administered. A 20 gauge/10 cm RF trocar (Diros OWL® RF Probe, Diros Technology Inc, Ontario, Canada) was percutaneously inserted from the antero-lateral region of the knee joint. The final position of the RF trocar inside the joint (midline and in an equidistant level between tibial and femoral bones) was fluoroscopically verified in anteroposterior and lateral projections (). Coaxially, a RF electrode with a 10-mm “active tip” (Diros OWL® RF Probe, Diros Technology Inc, Ontario, Canada) was introduced and a 10 min neurolysis session was performed with PRF (1200 pulses at 50 V with 10-ms duration followed by a 480-ms silent phase) (). Subsequently, viscosupplementation was performed through the same trocar by intra-articular injection of 60 mg/6 ml of sodium hyaluronate (Suplasyn 1-shot treatment, Mylan Institutional, Zurich, Switzerland). Each patient remained in the hospital for 30–45 min (only for observation) and was then discharged with suggestions of 1 day rest and then being free to engage in normal activities.
Figure 1. (A) Face fluoroscopy view of the left knee illustrating the final position of the trocar at the level of the tibial crest. (B) Lateral fluoroscopy of the left knee illustrating the final position of the trocar anteriorly to the tibial crest. (C) The RF electrode is coaxially inserted in the knee joint through the trocar and connected to the generator.
Outcome measures
Patients were followed by clinical visits at one week/one, 6 and 12 months. Questions asked during the follow-up period concerned the pain reduction and mobility improvement and whether the procedure had decreased or totally relieved the symptoms they were treated for. Pain, prior, one week/one, 6 and 12 months post were compared by means of a numeric visual scale (NVS) questionnaire [Citation22]. Improvement was defined as any pain decrease of more than 4 NVS Units after the treatment. Recurrence during the follow-up was defined as any pain increase lower than the score before treatment, despite initial improvement. Mobility evaluation was performed using four criteria: normal mobility, moderate mobility (painful walking without English canes), limited mobility (mobility with English canes), and very limited mobility (impossible). Complications were graded according to international reporting standards [Citation23].
Statistical analysis
Repeated Measures ANOVA was used to compare means of pain before the procedure and during follow-up. Results were studied after the corrections of Greenhouse-Geisser, Huynh-Feldt and lower-bound had been applied. Furthermore, paired-samples Student t-test was used to compare mean pain score at each follow-up point with baseline. A p value <0.05 was considered statistically significant. Pain score values were given as mean ± SD. All statistical analysis was performed using SPSS v.22.0 (IBM Corp., Armonk, NY).
Results
Technical success (i.e. positioning of the electrode on the mid-line with an equal distance from the tibia and the femur) was obtained in 100% cases; 8/45 patients received a bilateral procedure in the same interventional session. Mean pain score prior to PRF and viscosupplementation was 8.19 ± 1.4 NVS units. This score was reduced to a mean value of 2.47 ± 2.5 NVS units at 1 week after, 2.55 ± 2.6 at 1 month, 3.1 ± 2.8 at 6 months and 5.02 ± 3.09 at 12 months of follow-up (p < 0.01) (, ). Initial improvement (pain decrease of more than 4 NVS Units during the first month) was noticed in 46/53 knees (86.8%). Improvement was noticed in 15/16 (93.8%) cases of grade 2 Kellgren–Lawrence osteoarthritis, vs. 16/18 (88.9%) cases of with grade 3 and 15/19 (78.9%) cases of grade 4 osteoarthritis. Pain recurrence during the follow-up period was noticed in 18 (39.1%) joints, who had initially (first month) responded well to treatment. Pain recurrence was noticed in nine knees with grade 4 Kellgren–Lawrence osteoarthritis, six knees with grade 3 and three knees with grade 2 osteoarthritis.
Figure 3. Charts illustrating mean pain scores and score deviation prior and during the follow-up period.
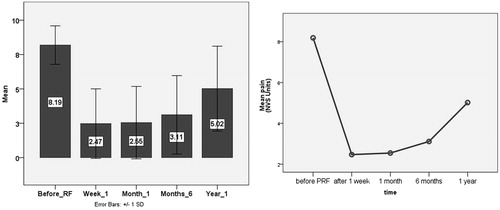
Table 3. Descriptive statistics.
Overall mobility improved in 47/53 (88.6%) patients (19 patients reported moderate mobility before treatment and normal mobility after 1 year, 14 patients limited mobility before and moderate mobility after 1 year, nine patients limited mobility before and normal mobility after 1 year, three patients very limited mobility before and limited after 1 year and two patients very limited mobility before and moderate mobility after 1 year). No clinically significant complications were noted in our study population. During the early or late follow-up period no patients developed haemorrhage/haemarthrosis, infection thermal injury or loss of motor and sensory control in the corresponding area.
Discussion
When compared to continuous RF, pulsed mode has much less (if any) neuro-destructive characteristics; the long silent phases (480 ms) between the short bursts of energy application (10–20 ms) maintain tissue temperature under 42 °C which is below the irreversible tissue damage threshold [Citation24,Citation25]. In addition, up until now there is no reported evidence of long-lasting structural effects by pulsed RF application [Citation17]. Histological studies upon the biological effects of pulsed RF report architectural impairment of the axonal myelin sheath bundles, interstitial oedema (which is temporary and persists for a few weeks post the session) as well as ultra-structural changes of the C and A delta nociceptive fibres [Citation21,Citation26,Citation27]. Intra-articular application of pulsed RF suppresses the excitatory C fibre response and the synaptic transmission resulting in immediate pain relief; in addition, it causes an immune response interrupting production of pro-inflammatory cytokines, such as interleukin-1 b and interleukin-6 [Citation21].
RF treatments for pain reduction in symptomatic patients with knee osteoarthritis have been proposed with two ways: extra-articular application of continuous RF at the level of genicular nerves or intra-articular application of pulsed RF [Citation20,Citation24]. The latter aims at pericapsular nerve endings responsible for pain [121]. Choi et al. [Citation20] compared continuous RF at the genicular nerves with a placebo therapy reporting significantly lower pain scores in the RF group during follow-up. Karaman et al. [Citation24] applied with a blind technique pulsed RF intra-articularly reporting significant pain decrease (>50%) over a 6 months follow-up. Masala et al. [Citation21] report significant pain decrease and improved autonomy in daily life post intra-articular application of pulsed RF over 12 months follow-up period.
Similarly, in our study significant pain reduction is reported from the first week throughout the 12 months follow-up period whilst overall mobility improved in the vast majority of the patients included in the study (47/53 – 88.6%). What is common in our study and to the one performed by Masala et al. [Citation21] is that towards the end of the 12-month period there is a tendency for pain increase although the self-reported pain scores are significantly lower than those of the baseline. At this time point a new therapeutic session could provide further symptom improvement; however, this potential evaluation of repeated PRF success upon pain reduction was not evaluated in the present study.
In all three studies (including the present one), for intra-articular application of pulsed RF, there is a different neuromodulation protocol applied. Karaman et al. [Citation24] applied with a blind technique pulsed RF for 15 min (1200 pulses with 20-ms duration) while Masala et al. [Citation21] performed a session of similar technical characteristics under fluoroscopic guidance (verifying thus trocar’s position in the middle of the joint) but without any mention of the duration. In the present study a 10 min neuromodulation session was applied intra-articularly with pulsed RF (1200 pulses at 50 V with 10-ms duration) while the trocar was placed in the centre of the joint under fluoroscopic guidance. No doubt different vendors and operator’s preference play a major role in these variations; however, a consensus upon a standard protocol is a near future necessity. The common technical parameter in all these systems is that the generator stops the pulses if temperature exceeds 42 °C until the temperature decreases to this value.
In the present study PRF was combined to viscosupplementation; this combination seems to further increase the duration of pain reduction as reported in the study by Karaman et al. [Citation24]. Intra-articular sodium hyaluronate injections in knee osteoarthritis are performed aiming to viscoelastic supplementation of the joint. There is a multifactorial mechanism of action including both mechanical (protective effect on the superficial layer of cartilage) and non-mechanical pathways such as reaggregation of proteglycan molecules, inhibition of articular nociceptive receptors (analgesic effect), prostaglandin-E2 synthesis blockade and inhibition of arachidonic acid release (anti-inflammatory action) [Citation28–30]. Due to the fact that PRF does not cause osteophytes to regress or cartilage and meniscus to regenerate in patients with substantial bone and cartilage damage adding hyaluronate to these patients seems to be a necessity. It must be noted, however, that from the data available in this paper it is not possible to distinguish whether PRF or viscosupplementation contribute more to the pain management process.
Limitations of our study include that this is a single centre study lacking control groups receiving sham procedure, neither RFA nor viscosupplementation, only viscosupplementation without RFA or RFA alone, respectively. Furthermore, there was no direct comparison of intra-articular application of pulsed RF to the extra-articular neurolysis of the genicular nerves by means of continuous RF.
Percutaneous, intra-articular application of PRF combined with viscosupplementation is an effective and safe technique for palliative management of chronic pain in patients with knee osteoarthritis. Results seem to be reproducible and long lasting. There seems to be a need of repeating the session at 1 year. Further evaluation of the technique against sham trial is warranted.
Disclosure statement
The authors report no declarations of interest.
References
- Kiadaliri AA, Lamm CJ, de Verdier MG, et al. (2016). Association of knee pain and different definitions of knee osteoarthritis with health-related quality of life: a population-based cohort study in southern Sweden. Health Qual Life Outcomes 14:121.
- Cross M, Smith E, Hoy D, et al. (2014). The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis 73:1323–30.
- Wu M, Brazier JE, Kearns B, et al. (2014). Examining the impact of 11 long-standing health conditions on health-related quality of life using the EQ-5D in a general population sample. Eur J Health Econ 16:141–51.
- Hunter DJ, Guermazi A. (2012). Imaging techniques in osteoarthritis. PMR 4:S68–S74.
- Kellgren JH, Lawrence JS. (1957). Radiological assessment of osteo-arthrosis. Ann Rheum Dis 16:494–502.
- Altman R, Asch E, Bloch D, et al. (1986). Development of criteria for the classification and reporting of osteoarthritis. Classification of osteoarthritis of the knee. Diagnostic and therapeutic criteria committee of the American rheumatism association. Arthritis Rheum 29:1039–49.
- Bijlsma JW, Berenbaum F, Lafeber FP. (2011). Osteoarthritis: an update with relevance for clinical practice. Lancet 377:2115–26.
- Gademan MG, Hofstede SN, Vliet Vlieland TP, et al. (2016). Indication criteria for total hip or knee arthroplasty in osteoarthritis: a state-of-the-science overview. BMC Musculoskelet Disord 17:463.
- Dai WL, Zhou AG, Zhang H, Zhang J. (2017). Efficacy of platelet-rich plasma in the treatment of knee osteoarthritis: a meta-analysis of randomized controlled trials. Arthroscopy 33:659–70.
- de Rooij M, van der Leeden M, Cheung J, et al. (2017). Efficacy of tailored exercise therapy on physical functioning in patients with knee osteoarthritis and comorbidity: a randomized controlled trial. Arthritis Care Res (Hoboken) 69:807–16.
- Bannuru RR, Brodie CR, Sullivan MC, McAlindon TE. (2016). Safety of repeated injections of sodium hyaluronate (SUPARTZ) for knee osteoarthritis: a systematic review and meta-analysis. Cartilage 7:322–32.
- Hochberg MC, Altman RD, April KT, et al. (2012). American college of rheumatology 2012 recommendations for the use of nonpharmacologic and pharmacologic therapies in osteoarthritis of the hand, hip, and knee. Arthritis Care Res 64:465–74.
- Neustadt DH. (2006). Intra-articular injections for osteoarthritis of the knee. Cleve Clin J Med 73:897–911.
- Migliore A, Bella A, Bisignani M, et al. (2012). Total hip replacement rate in a cohort of patients affected by symptomatic hip osteoarthritis following intra-articular sodium hyaluronate (MW 1,500–2,000 kDa) ORTOBRIX study. Clin Rheumatol 31:1187–96.
- Vallejo R, Benjamin RM, Aliaga L. (2010). Radiofrequency vs. pulse radiofrequency: the end of controversy. Tech Region Anesth Pain Manage 14:128–32.
- De Louw AJ, Vles HS, Freling G, et al. (2001). The morphological effects of a radio frequency lesion adjacent to the dorsal root ganglion (RF-DRG)-an experimental study in the goat. Eur J Pain 5:169–74.
- Kvarstein G. (2012). Pulsed radiofrequency – time for a clinical pause and more science. Scand J Pain 3:124–6.
- Organ LW, Burbham RS, Avila A, et al. Radiofrequency denervation of the sacroiliac joint. DIROS/OWL RF Monographs SIJ v6.0: 34–7. Available from: http://dirostech.com/techniques-procedures/
- Cahara A, van Zundert J, Macrea L, et al. (2006). Pulsed radiofrequency: current clinical and biological literature available. Pain Med 7:411–23.
- Choi WJ, Hwang SJ, Song JG, et al. (2011). Radiofrequency treatment relieves chronic knee osteoarthritis pain: a double-blind randomized controlled trial. Pain 152:481–7.
- Masala S, Fiori R, Raguso M, et al. (2014). Pulse-dose radiofrequency for knee osteoartrithis. Cardiovasc Intervent Radiol 37:482–7.
- Mystakidou K, Mendoza T, Tsilika E, et al. (2001). Greek brief pain inventory: validation and utility in cancer pain. Oncology 60:35–42.
- Filippiadis DK, Binkert C, Pellerin O, et al. (2017). Cirse quality assurance document and standards for classification of complications: the cirse classification system. Cardiovasc Intervent Radiol 40:1141–6.
- Karaman H, Tüfek A, Kavak GÖ, et al. (2011). Intra-articularly applied pulsed radiofrequency can reduce chronic knee pain in patients with osteoarthritis. J Chin Med Assoc 74:336–40.
- Erdine S, Bilir A, Cosman ER, Cosman ER Jr. (2009). Ultrastructural changes in axons following exposure to pulsed radiofrequency fields. Pain Pract 9:407–17.
- Tun K, Cemil B, Gurcay AG, et al. (2009). Ultrastructural evaluation of pulsed radiofrequency and conventional radiofrequency lesions in rat sciatic nerve. Surg Neurol 72:496–500.
- Protasoni M, Reguzzoni M, Sangiorgi S, et al. (2009). Pulsed radiofrequency effects on the lumbar ganglion of the rat dorsal root: a morphological light and transmission electron microscopy study at acute stage. Eur Spine J 18:473–8.
- Jackson DW, Sheer MJ, Simon TM. (2001). Cartilage substitutes: overview of basic science and treatment options. J Am Acad Orthop Surg 9:37–52.
- Goto M, Hanyu T, Yoshio T, et al. (2001). lntra-articular injection of hyaluronate (SI-66010) improves joint pain and synovial fluid prostaglandin E2 levels in rheumatoid arthritis: a multicenter clinical trial. Clin Exp Rheumatol 19:377–83.
- Serban MA, Yang G, Prestwich GD. (2008). Synthesis, characterisation and chondroprotective properties of a hyaluronan thioethyl ether derivative. Biomaterials 29:1388–99.