Abstract
Objective: To investigate the effects of a microbubble ultrasound contrast agent on high-intensity focused ultrasound (HIFU) treatment of uterine fibroids.
Methods: A total of 120 patients with solitary uterine fibroid were randomly assigned into Groups A, B, C and D. Patients in Groups A and B received 1.5 ml of SonoVue, Groups C and D received 1.5 ml of saline before HIFU ablation. HIFU sonication started at 6 min after administration of SonoVue or saline in Groups A and C, whereas it started at 10 min in Groups B and D. On day 1 after HIFU, magnetic resonance imaging was performed. Patients were followed up via phone or clinic visit during the first week after HIFU.
Results: No significant difference was observed in terms of age, fibroid location, diameter of fibroids, signal intensity on T2-weighted imaging, or tumour volume among the four groups (p > 0.05). The use of SonoVue significantly shortened the treatment time and sonication time. The sonication start time of 6 min, relative to 10 min, had significant effects on the treatment time and sonication time. The use of intravenous SonoVue followed by HIFU ablation 6 min later significantly increased the rate of significant grey-scale changes (55.9%) and the non-perfused volume ratio (94.2% ± 10.6%). No significant differences were observed in the incidence of intra-procedure and post-HIFU adverse effects among the four groups (p > 0.05).
Conclusions: SonoVue could be safely used to enhance the ablation effects of HIFU treatment of uterine fibroids.
Introduction
Uterine fibroids are the most common benign tumours of female reproductive system. The prevalence is reported as 20–40% among reproductive age women, and it is generally more frequently seen in women aged 30–50 years [Citation1]. The conventional treatments for uterine fibroids include both medical and surgical treatment. With the advancement of science and technology, minimally invasive and non-invasive treatments are now being used to treat uterine fibroids. As a non-invasive treatment, high-intensity focused ultrasound (HIFU) has become of great interest in recent years. The main mechanisms of HIFU involve thermal and cavitation effects [Citation2]. Many studies have shown the effectiveness and safety of HIFU [Citation3,Citation4]. Despite its efficacy in killing tumour tissue, HIFU may also cause skin injury, nerve and periosteal injury, bladder injury and endometrial damage. How can we not only ensure the efficacy of HIFU ablation, but also reduce its complications, is the focus of the current clinical research [Citation5,Citation6].
Currently, contrast-enhanced ultrasound has been widely used to evaluate the treatment results of HIFU in China. Several studies have also shown that contrast-enhanced ultrasound agents could enhance the ablation effect because the microbubbles change the acoustic characteristics of tissues, improve the thermal and cavitation effects, and thus the energy deposition in the target area is easier and the therapeutic effect increases [Citation7,Citation8]. However, if the microbubble concentration is too high, it will damage the outside tissue. On the other hand, if the microbubble concentration is too low, the microbubble-enhancing effect will not be obvious. Therefore, it is necessary to choose the appropriate dose of microbubble to enhance the treatment effects of HIFU safely and effectiveness. Peng et al. [Citation9] retrospectively analysed 291 patients and showed that SonoVue enhanced the effect of HIFU ablation of uterine fibroids when HIFU treatment started 10 min after SonoVue injection (2 ml). SonoVue has a short half-life. Following intravenous administration, the blood level curve showed an elimination half-life of 6 min. More than 80% of the administered SonoVue was removed after 11 min [Citation10]. In this study, we conducted a randomised controlled trial to investigate the enhancement effects of ultrasound contrast agent SonoVue on HIFU, to see if higher the concentration of contrast agent has a more obvious enhancement effect, and to provide a clinical value for further study on the optimal concentration of contrast agent and the safest time for starting sonication.
Materials and methods
This study was approved by the Ethics Committees at the Affiliated Hospital of North Sichuan Medical College, Nanchong, Sichuan.
Subjects
From January 2014 to December 2014, a total of 120 patients with solitary uterine fibroid from the Affiliated Hospital of North Sichuan Medical College were enrolled. The diagnosis of uterine fibroids was confirmed with Doppler ultrasound and magnetic resonance imaging (MRI). All patients were willing to accept HIFU treatment and signed an informed consent for HIFU treatment.
Inclusion criteria were as follows: (1) the diagnosis of uterine fibroids was made by Doppler ultrasound and MRI, and the diameter of uterine fibroid was ≥3 cm; (2) patients were premenopausal adult women (at least 18 years old); (3) patients were able to communicate with the nurse or physician during the procedure; and (4) all had symptomatic fibroids requiring treatment.
Exclusion criteria: (1) pregnant women; (2) multiple fibroids; (3) patients with suspected or confirmed uterine malignancy or degenerating fibroids; and (4) patients were unable to communicate with the nurse or physician during the procedure.
One hundred and twenty eligible patients were randomly assigned to four study groups according to a computer-generated schedule. Randomisation was performed in a 1:1:1:1 ratio for the four groups. The patients in Groups A and B received a bolus of 1.5 ml of SonoVue (Bracco, Milan, Italy, one vial of SonoVue was reconstituted with 5 ml of normal saline) solution via hand vein. The patients in Groups C and D received 1.5 ml of normal saline via hand vein before HIFU treatment. All procedures were performed by the same nurse. HIFU sonication started at 6 min after administration of SonoVue or normal saline in Groups A and C and at 10 min after administration of SonoVue or saline in Groups B and D ().
Table 1. Contributing factors and group assignments.
MRI evaluation
Before HIFU treatment, MRI was performed to determine the size, location and volume of each target fibroid. Enhanced MRI was performed to determine the blood supply of the fibroid (). In addition, the length (D1), thickness (D2) and width (D3) of each target fibroid were determined, and the fibroid volume was calculated with the following formula: V = (1/6) × π × D1 × D2 × D3 [Citation9,Citation11]. One day after HIFU treatment, MRI was performed to evaluate the non-perfused volume (NPV) ratio. The length (d1), thickness (d2) and width (d3) of the non-perfused area in each treated fibroid were measured, and the NPV ratio was calculated with the following formula: V = (1/6) × π × d1 × d2 × d3. The NPV ratio was defined as NPV/fibroid volume ×100%.
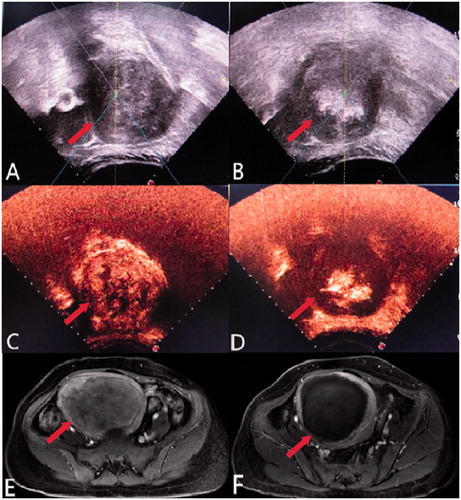
Figure 1. A 45-year-old patient with an 8.7 × 8.5 × 8.3 cm uterine fibroid and the significant grey-scale change during HIFU treatment. (A) Before HIFU treatment, ultrasound showed a hypoechoic fibroid (arrow). (B) Immediately after HIFU treatment, ultrasound showed a lumpy grey-scale change in the treatment area (arrow). (C) Before HIFU treatment, contrast-enhanced ultrasound showed the tumour perfusion area (arrow). (D) Immediately after HIFU treatment, contrast-enhanced ultrasound showed no enhancement surrounding the fibroid (arrow), indicating complete ablation. (E) Pre-treatment enhanced T1-weighted MRI showed tumour perfusion (arrow). (F) Post-treatment enhanced T1-weighted MRI showed a small area of enhancement along the tumour edges, with an ablation rate of 94% (arrow).
HIFU treatment
All treatments were performed using the JC200 focused ultrasound tumour therapeutic system (Chongqing HIFU Technology Co., Ltd., Chongqing, China). This system is integrated with real-time monitoring ultrasound (MyLab 70, Esaote, Genova, Italy). Focused ultrasound energy was produced by a 20-cm diameter transducer with frequency of 1.0 MHz.
Patients were in prone position on the HIFU table, with the abdominal wall in contact with degassed water. Conscious sedation was administered during the HIFU procedure. During treatment, patients were requested to lie still and to report any discomfort, including burning of the skin, lower abdominal, leg, sciatic or buttock pain. The treatment was terminated when significant grey-scale change was observed in the entire treatment area on the ultrasound B image () or the therapeutic dosage was reached. At the end of treatment, 1.5 ml (17.7 mg) of SonoVue was injected via hand vein to further evaluate the ablation effect (). Two experienced radiologists measured and compared the contrast-enhanced ultrasound B results. The volume of the target fibroid tumour and the volume of the non-perfused region after treatment were measured on MRI (). In addition, sonication time (s; time of HIFU ablation), intensity of treatment (s/h; irradiation time ×60/treatment time), NPV ratio (%; volume of non-perfused region/volume of target fibroid tumour ×100%), rate of the significant grey-scale change (number of cases that showed a significant grey-scale change/total number of cases), and the sonication time to reach significant grey-scale change (s) were recorded.
Statistical analysis
SPSS 22.0 was used for statistical analysis. If the data feature met the normal distribution, means ± SD were reported; if it was an abnormal distribution, median and inter-quartile range (P25–P75) were reported. Factorial analysis was performed to compare treatment time, sonication time, intensity of treatment and NPV ratio. Treatment time, sonication time and non-perfusion volume were then log-transformed for statistical analysis. Qualitative data were analysed with the χ2 test. Quantitative data with normal distributions were analysed with randomised block analysis of variance. Quantitative data without normal distribution were analysed with the rank sum test. p < 0.05 was considered statistically significant.
Results
Baseline characteristics
HIFU was successfully performed in all 120 patients. The mean age of patients was 40.7 ± 5.6 (25–54) years; the maximum tumour diameter was ranged from 31.0 to 114.0 (median: 50.5) mm; the tumour volume ranged from 14.0 to 558.0 (median: 49.0) cm3. The maximum tumour diameters were 53.6 ± 17.3 mm, 50.4 ± 13.3 mm, 54.3 ± 16.0 mm and 57.1 ± 16.6 mm in Groups A, B, C and D, respectively. No significant difference was observed between either of the two groups (p > 0.05) ().
Table 2. General information for thefour groups (X¯ ± S).
Treatment outcomes
showed the HIFU treatment results. Factorial analysis showed that the sonication time and treatment time were significantly shorter in the groups using SonoVue than in the groups without (FSonoVue = 37.955, p < 0.01; FSonoVue = 33.982, p < 0.01). In addition, treatment start time after administration of SonoVue had a significant effect on treatment time (FTime = 6.389, p = 0.013) and sonication time (FTime = 4.335, p = 0.04). The group using SonoVue with a 6-min sonication start time after administration of SonoVue had the shortest HIFU treatment time and sonication time. No significant differences were observed between either two groups in the intensity of treatment or the NPV ratio (p > 0.05) ().
Table 3. Comparison of the treatment outcomes among the four groups.
Furthermore, significant differences among Groups A, B, C and D in the rates of significant grey-scale change (χ2 = 22.540, p < 0.01) and NPV ratio (χ2 = 20.729, p < 0.01) were observed. No significant difference was observed in the time to significant grey-scale change among the four groups (p > 0.05) ().
Table 4. Comparison of the treatment outcomes among the four groups.
Adverse effects and complications
During the procedure, the patients from the four groups reported radiation pain, sacrococcygeal pain, a “hot” sensation on the skin, lower abdominal pain, groin pain and buttock pain. However, the pain was transient and the score was lower than 4 points. No significant difference was observed in the rate of adverse effects among the four groups. No severe complications occurred in this study.
Discussion
As a novel non-invasive treatment, HIFU provides a new option for patients who are unwilling to undergo surgery and wish to keep their uterus. Researchers are looking for methods to improve the effects of HIFU ablation and to shorten treatment time [Citation11]. Ferrara et al. [Citation12] showed that ultrasound contrast agent microbubbles contained gas, resulting in a significant mismatch in the acoustic impedance of tumour tissue, thereby strengthening the ultrasonic scattering and reflectivity of the target area and improving HIFU energy deposition. Luo et al. [Citation13] showed that the necrotic volume achieved in rabbit liver pre-treated with sulphur hexafluoride microbubbles before HIFU was larger than that pre-treated with saline because the microbubbles reduced the cavitation threshold in the tissue. As ultrasonic beams focused on the lesion, the energy deposition increases significantly, leading to more extensive cell necrosis in the target area. To date, few studies have been conducted. Jiang et al. [Citation14] randomly assigned 80 patients with solitary uterine fibroids into two groups, the experimental group received SonoVue + HIFU, and the control group received HIFU alone. The rate of significant grey-scale change was 82.5% in the experimental group and 50.0% in the control group. Moreover, the energy efficiency factor was also significantly smaller in the experimental group than in the control group (p = 0.029), whereas the NPV ratio was significantly higher in the experimental group than that in the control group (p = 0.006), suggesting that sulphur hexafluoride microbubbles enhanced the effect of HIFU ablation of uterine fibroids. Peng et al. [Citation9] retrospectively analysed 291 patients from three centres and showed that SonoVue enhanced the effect of HIFU ablation of uterine fibroids. When HIFU treatment started 10 min after SonoVue injection (2 ml), the rate of significant grey-scale change was higher, the total ablation time was significantly shorter, and the sonication time for ablating 1 cm3 of tumour was significantly shorter in the experimental group than those in the control group.
In this study, 120 patients were randomly assigned into Group A (6 min + 1.5 ml SonoVue), Group B (10 min + 1.5 ml SonoVue), Group C (6 min + 1.5 ml saline) and Group D (10 min + 1.5 ml saline). No significant differences were observed in terms of age, tumour location, maximum tumour diameter, tumour signal intensity on T2-weighted imaging, or tumour volume among the groups (p > 0.05) (). Factorial analysis revealed that SonoVue, relative to saline, significantly shortened the HIFU treatment (FSonoVue = 37.955, p < 0.01) and sonication time (FSonoVue = 33.982, p < 0.01). Moreover, starting HIFU ablation sooner after SonoVue injection significantly decreased the treatment time (FTime = 6.389, p = 0.013) and sonication time (FTime = 4.335, p = 0.04) (). In addition, significant interactions were observed between treatment start time and the usage of SonoVue (FSonoVue × time = 4.564, p = 0.035; FSonoVue × time = 4.107, p = 0.045), suggesting that the interaction between intravenous SonoVue (1.5 ml) and HIFU ablation 6 min after injection had more significant effects on treatment time and sonication time than either of the two factors alone. In addition, intravenous administration of SonoVue (1.5 ml) followed by HIFU ablation 6 min later significantly increased the rate of significant grey-scale change and NPV ratio (p < 0.05) (). SonoVue is constantly metabolised and eliminated from the body; its concentration in the body decreases over time after SonoVue injection. Thus, the concentration of SonoVue in the fibroids was higher at 6 min after injection than that at 10 min. These effects were more significant when HIFU ablation started sooner after SonoVue injection than later (i.e. at a higher SonoVue concentration). It is reported that the effectiveness of HIFU ablation on hyperintense fibroids is inferior to hypointensive uterine fibroids. Unfortunately, we had few cases of high signal uterine fibroids in this study and could not conclude whether the microbubbles can enhance the effectiveness of HIFU more on hyperintense fibroids than hypointense fibroids. Further studies are needed.
Sulphur hexafluoride microbubbles are mainly metabolised in the lungs. At present, no method has been established to determine their dispersion parameters. Piscaglia and Bolondi [Citation15] summarised the safety of SonoVue in 23 188 patients from 28 centres in Italy. The incidence of adverse effects was 0.0086%, and no deaths were reported. A total of 29 patients had adverse effects, wherein two patients had serious adverse effects that were resolved after treatment. These results suggested that SonoVue had a good safety profile and was safer than radiological contrast agents. Peng et al. [Citation11] started HIFU ablation 10 min after SonoVue injection (2 ml) and showed that SonoVue safely enhanced the effect of HIFU ablation with no significant side effects. In this study, one group started HIFU ablation 6 min after SonoVue injection with 1.5 ml. The results showed no significant difference among the groups in the incidence of intra-procedure and post-HIFU adverse effects (p > 0.05, ), suggesting that it is safe to start HIFU ablation 6 min after SonoVue injection (initial dose: 1.5 ml). By shortening the HIFU treatment time and sonication time, SonoVue was expected to reduce HIFU-related side effects. However, this benefit was not observed in this study, it is likely because of the small sample size. Further studies with a larger number of subjects are needed to confirm these results.
Table 5. Comparison of intra-procedure and post-procedure adverse effects.
Conclusions
In short, the ultrasound contrast agent sulphur hexafluoride microbubbles (SonoVue) safely enhanced the effect of HIFU ablation for uterine fibroids. SonoVue shortened HIFU treatment time and sonication time and increased the rate of significant grey-scale change and NPV ratio. Moreover, its effect was more obvious when HIFU ablation started sooner after SonoVue injection. This study also demonstrated that it was safe to start HIFU ablation 6 min after SonoVue injection. Nevertheless, large, randomised controlled clinical case studies are needed to further clarify the safe dosage range and other treatment parameters.
Disclosure statement
The authors have no funding or conflicts of interest to disclose.
References
- Khan AT, Shehmar M, Gupta JK. (2014). Uterine fibroids: current perspectives. Int J Women Health 6:95–114.
- Kim YS, Rhim H, Choi MJ, et al. (2008). High-intensity focused ultrasound therapy: an overview for radiologists. Korean J Radiol 9:291–302.
- Zhang L, Chen WZ, Liu YJ, et al. (2010). Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol 73:396–403.
- Kim HS, Baik JH, Pham LD, Jacobs MA. (2011). MR-guided high-intensity focused ultrasound treatment for symptomatic uterine leiomyomata: long-term outcomes. Acad Radiol 18:970–6.
- Wang W, Wang Y, Tang J. (2009). Safety and efficacy of high intensity focused ultrasound ablation therapy for adenomyosis. Acad Radiol 16:1416–23.
- Zhou XD, Ren XL, Zhang J, et al. (2007). Therapeutic response assessment of high intensity focused ultrasound therapy for uterine fibroid: utility of contrast-enhanced ultrasonography. Eur J Radiol 62:289–94.
- Luo W, Zhou X, Tian X, et al. (2006). Enhancement of ultrasound contrast agent in high-intensity focused ultrasound ablation. Adv Ther 23:861–8.
- Orsi F, Monfardini L, Bonomo G, et al. (2015). Ultrasound guided high intensity focused ultrasound (USgHIFU) ablation for uterine fibroids: do we need the microbubbles? Int J Hypertherm 31:233–9.
- Peng S, Xiong Y, Li K, et al. (2012). Clinical utility of a microbubble-enhancing contrast (“SonoVue”) in treatment of uterine fibroids with high intensity focused ultrasound: a retrospective study. Eur J Radiol 81:3832–8.
- Schneider M. (1999). Characteristics of SonoVuetrade mark. Echocardiography 16:743–6.
- Orsini LF, Salardi S, Pilu G, et al. (1984). Pelvic organs in premenarcheal girls: real-time ultrasonography. Radiology 153:113–6.
- Ferrara KW, Borden MA, Zhang H. (2009). Lipid-shelled vehicles: engineering for ultrasound molecular imaging and drug delivery. Acc Chem Res 42:881–92.
- Luo W, Zhou X, Ren X, et al. (2007). Enhancing effects of SonoVue, a microbubble sonographic contrast agent, on high-intensity focused ultrasound ablation in rabbit livers in vivo. J Ultrasound Med 26:469–76.
- Jiang N, Xie B, Zhang X, et al. (2014). Enhancing ablation effects of a microbubble-enhancing contrast agent (“SonoVue”) in the treatment of uterine fibroids with high-intensity focused ultrasound: a randomized controlled trial). Cardiovasc Intervent Radiol 37:1321–8.
- Piscaglia F, Bolondi L. (2006). The safety of SonoVue in abdominal applications: retrospective analysis of 23188 investigations. Ultrasound Med Biol 32:1369–75.
