Abstract
Objective: To evaluate the shrinkage rate, symptom relief and improvement of the quality of life following ultrasound-guided high intensity focussed ultrasound (USgHIFU) for multiple uterine fibroids.
Methods: From October 2015 to November 2016, 81 black women with multiple symptomatic fibroids underwent USgHIFU. The number of the fibroids ranged from 3 to 9. The shrinkage rate of fibroids, symptom severity score and quality of life were evaluated following USgHIFU. Magnetic resonance imaging (MRI), the uterine fibroid symptom and quality of life (UFS-QOL) questionnaire were used for evaluation.
Results: The mean age of patients was 35.3 ± 5.9 years. The average weight of these patients was 68.4 ± 11.4 kg, with the median abdominal subcutaneous fat thickness of 38.0 ± 11.4 mm. The median fibroid volume was 36.0 (range: 1.8–1220.1) cm³. During HIFU, 60.5% of the patients reported lower abdominal pain, 43.2% sciatic/buttock pain, 60.5% skin “burning” sensation, 6.2% abnormal vaginal discharge and 13.6% transient leg pain. No severe complications were observed. The average volume reduction rate of fibroids in 21 patients who completed the follow-up was 32.5 ± 24.0, 42.3 ± 32.2 and 52.5 ± 36.3% 1, 3 and 6 months after HIFU, respectively. The UFS score decreased and the QOL values significantly increased during the follow-up period. Re-intervention treatment occurred in two of the 21 patients 6 months after HIFU. One patient conceived 3 months after HIFU, and she had a term vaginal delivery without any obstetrical complications.
Conclusions: Based on our results, USgHIFU is safe and effective in treating patients with multiple uterine fibroids.
Introduction
Uterine fibroids are the most common benign tumours in the female genital tract. The prevalence of this benign tumour is generally reported as 20–40% among reproductive-age women [Citation1]. It is reported that the prevalence of fibroids is three times higher in black women than white women [Citation2,Citation3]. Half of them may have heavy and prolonged menstrual bleeding, urinary frequency or urgency, constipation and/or infertility [Citation4]. The prevalence of multiple uterine fibroids is not clear, but it seems that half of the patients have multiple fibroids [Citation5]. Since different types of fibroids (submucosal, intramural and subserosal fibroids) coexist or the fibroids exist in multiple locations (some submucous myomas are partially in the cavity and partially in the wall of the uterus), the effects of multiple fibroids on the anatomical structures of the uterus are often larger than those of a single fibroid. Therefore, patients with multiple fibroids may often complain of symptoms and those symptoms may be more severe than those with a solitary fibroid [Citation6].
The definitive treatment for multiple uterine fibroids is hysterectomy. However, hysterectomy is not suitable for patients who wish to have children. Myomectomy is an option for patients wishing to retain their uterus, but the surgical damage to uterus is dramatic in treating patients with multiple fibroids, and the cumulative recurrence rate of fibroids at 12 and 24 months after myomectomy was 12.4 and 46.0%, respectively, with the risk of further treatment being high [Citation7,Citation8]. As a minimally invasive treatment, uterine artery embolization (UAE) has been used to treat multiple uterine fibroids. Severe side effects and complications including postembolization syndrome, infection of the uterus, ovary damage, temporary amenorrhoea, problems with future pregnancies and possibly infertility have limited its application [Citation9].
Over the last decade, high intensity focussed ultrasound (HIFU) has been widely used in clinical practice to treat uterine fibroids. As a non-invasive treatment technique, HIFU is performed under the guidance of ultrasound or magnetic resonance imaging, so the fibroids can be selectively ablated, even fibroids smaller than 2 cm in diameter, without damaging adjacent structures [Citation10,Citation11]. Many studies from Asia, Europe and North America have demonstrated that HIFU is a safe and cost-effective alternative for the treatment of uterine fibroids [Citation12–14]. However, the subjects in these studies were of Asian, Caucasian or African descent. There is no study investigating the application of ultrasound-guided HIFU (USgHIFU) in Africa. This study retrospectively analysed 81 black women with multiple uterine fibroids treated with USgHIFU at Chris Hani Baragwanath Academic Hospital in South Africa. It evaluated the therapeutic effect and safety of USgHIFU in the treatment of multiple uterine fibroids and compared the results we have observed in this study with the outcomes of patients of African descent in previous papers.
Materials and methods
Patients
Between October 2015 and November 2016, 132 black women from 176 consecutive cases in our HIFU unit underwent USgHIFU treatment. Among them, 83 patients had multiple uterine fibroids. In this study, 81 patients who had three or more fibroids were retrospectively analysed.
Inclusion criteria were as follows: (1) patients were premenopausal; (2) patients had heavy and prolonged menstrual bleeding, urinary frequency or urgency, constipation; (3) three or more fibroids and a dominant fibroid less than 15 cm in diameter; (4) patients able to lie in a prone position for at least 1 h and (5) patients able to communicate with the nurse or physician during the procedure.
Exclusion criteria were as follows: (1) pregnant or lactating women; (2) contraindications to MR imaging; (3) metallic foreign bodies in treatment area; (4) uterine malignancy or ovarian tumours; (5) abnormal Pap smear and (6) abdominal subcutaneous fat thickness greater than 60 mm.
Pre-treatment magnetic resonance imaging (MRI)
A pelvic contrast-enhanced MR imaging examination (1.5 T TESLA, GE, Boston, MA) was performed in every patient before HIFU treatment. T2-weighted imaging and T1-weighted imaging before and after administration of gadolinium (dose, 0.1 mmol per-kilogram of body weight) were obtained in three planes. The tumour numbers, location (anterior wall, posterior wall or uterine fundus), type (intramural, subserosal or submucous), size and blood supply were recorded. The targeted fibroids were measured in three dimensions: longitudinal (D1), anteroposterior (D2) and transverse (D3). Fibroid volume was measured on contrast-enhanced T1WI using software which was programmed by engineers from Chongqing Haifu Medical Co., Ltd. 9 (Chongqing, China) to contour lesions in every slice of MR imaging, calculated by the same programme.
Pre-treatment preparation
All patients were required to ingest liquid and a half liquid diet prior to the procedure, fast for 12 h before treatment and undergo a cleansing enema 2 h prior to procedure to reduce the risk of bowel injury. Before the procedure, the anterior abdominal wall (from the umbilicus to the superior border of the symphysis pubis) was shaved. Cleaning of the skin performed with degassed water and 70% ethanol to decrease the risk of skin burn.
A urinary catheter was inserted to control the bladder volume during treatment. A degassed water balloon was prepared for each patient, to compress or push the bowel away from the acoustic pathway (), thus preventing bowel injury.
Figure 1. Real-time ultrasound monitoring images from a 42 years old patient. (A). An ultrasound image obtained before HIFU. A degassed water balloon (arrow) was used to push bowel away, the small loops of bowel (arrow) was detected outside the acoustic field. (B). A significant grey scale change was observed in the fibroid (arrow).
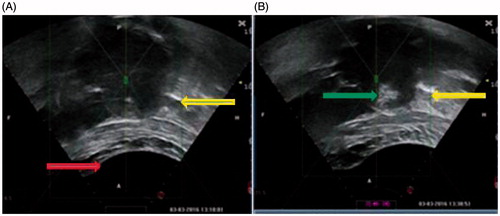
The procedure of HIFU ablation
HIFU treatment was performed using an ultrasound-guided HIFU tumour therapeutic system (JC200 HIFU, Chongqing Haifu (HIFU) Tech Co., Ltd., Chongqing, China). This system is integrated with an ultrasound probe (MyLab 70, Esaote, Genoa, Italy), providing real-time ultrasound monitoring. The focussed ultrasound energy was produced by a 20 cm diameter transducer with a focal region of 8 × 3×3 mm3.
The patients were placed in a prone position on the therapeutic table, with the anterior abdominal wall in contact with degassed water. During treatment, a degassed water balloon was placed against the abdominal wall if bowel was seen in the acoustic pathway. The procedure of HIFU treatment was performed under conscious sedation. The goal of conscious sedation was to reduce pain and discomfort while allowing the patient to be sufficiently awake to communicate. During the procedure, the patients’ vital signs (respiratory rate, heart rate, blood pressure and oxygen saturation level) were monitored.
Real-time ultrasonographic imaging was used to observe the target area and adjacent structures. The sagittal ultrasound scanning mode was chosen for both pre-treatment planning and sonication. The fibroid was divided into 5 mm sections. Energy was delivered via point sonication during treatment. Treatment began from the deep aspect of the fibroid working towards the superficial surface while preserving at least a 1 cm boundary to the junctional zone and 1.5 cm from the endometrium. A low power level (200 W) was used in the beginning of the procedure and this was slowly increased to a maximum of 400 W if the patient was able to tolerate the increase. Treatment power ranging from 200–400 W was used determined by different types and sites of fibroids. During the procedure, the therapeutic energy was adjusted based on feedback from the patients and changes in grey scale on ultrasonographic imaging. This process was repeated on a slice by slice basis, to achieve complete ablation of the fibroid. Once the grey scale covered most of the lesion, the treatment was terminated.
Follow-up
Patients were followed-up at 1, 3 and 6 months intervals after HIFU treatment. All patients underwent post-treatment MR imaging with the same standardised protocol as the pre-treatment MRI (. Clinical assessment was performed to compare the changes in fibroid-related symptoms. The uterine fibroid symptom and quality of life (UFS-QOL) questionnaire were used [Citation15], which was designed to assess symptom severity of uterine fibroids and the quality of life. All eligible patients completed a 37 item (eight for symptoms (USF) and 29 for quality of life (QOL)) questionnaire before and 1, 3 and 6 months after HIFU. A 10-point improvement in SSS of UFS-QOL at 6 months after treatment was considered to be significant [Citation16]. The questionnaires were collected when patients returned for outpatient examination. Responses for each item were scored from 1 (no symptoms) to 5 (major symptoms). The sum of the scores was analysed as a 0–100 scale for comparison. Higher scores of UFS indicated worse symptoms and lower scores of QOL indicated worse quality of life.
Figure 2. Contrast-enhanced MR images from a 36 years old patient with multiple uterine fibroids. (A). A sagittal view image obtained before HIFU treatment showed the size of uterus was 9.6 × 7.6 × 7.0 cm3, multiple uterine fibroids were detected (arrows). (B). A sagittal view image obtained one day after HIFU showed the fibroids were completely ablated (arrows). (C). A sagittal view image obtained 6 months after HIFU showed the size of uterus was 8.0 × 7.3 × 6.4 cm3, the rate of fibroid volume reduction was 89.6% (arrows).
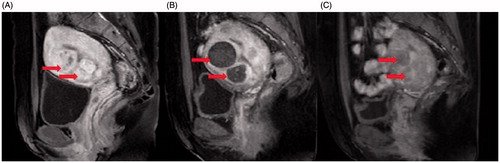
Statistical analysis
SPSS version 21.0 software (SPSS, IBM Company, New York City, NY) was used for statistical analysis. Normally distributed data were reported as mean ± one standard deviation (SD), data that is not normally distributed were reported as median and interquartile range. Mann–Whitney test was used for statistical comparisons of fibroid volume between baseline and 1-, 3- and 6-month after HIFU treatment. A p value of <0.05 was considered to be statistically significant.
Results
Patients and lesions
Eighty-one patients with 346 fibroids were treated with USgHIFU. The average age of patients was 35.3 ± 5.9 years (range: 19–46 years). The average weight of patients was 68.4 ± 11.4 kg (range: 43–99 kg), with an average abdominal subcutaneous fat thickness of 38.0 ± 11.4 mm (range: 7.5–65 mm) and the average body mass index (BMI) was 26.3 ± 4.6 (range, 15.8–39.3). Based on MRIs, the number of fibroids in patients ranged from 3 to 9. The median number of fibroids in these patients was 6. Among the 346 fibroids, 127 were located in the anterior wall of the uterus, 72 in the posterior wall of the uterus, 67 in the fundus and 80 in the lateral wall. Among them, 38 were submucosal, 166 were intramural and 142 were subserosal. Based on the signal intensity on T2WI, 61.6% (213/346) of fibroids were hypointense, 19.9% (69/346) of them were isointense and 18.5% (64/346) were hyperintense. The median volume of the uterus was 36.0 (range, 1.8–1220.1) cm3. The median size of the fibroids was 4.8 (range, 1.5–14.4) cm in diameter ().
Table 1. Baseline characteristics of patients with multiple uterine fibroids.
HIFU treatment evaluation
Details of the procedure are as follows: The overall treatment time, which was defined as the time from the first sonication to the last sonication, ranged from 40 to 240 min (mean ± SD, 97.3 ± 61.3 min). The sonication time was defined as the exposure time, which depended on the size and blood supply of the fibroids. It ranged from 280 to 2020 s (mean ± SD, 549.2 ± 454.2 s). The sonication intensity was defined as the sonication time given in 1 h, which ranged from 134 to 863.6 s in 1 h (mean ± SD, 411.0 ± 137.1 s/h). The average sonication power was 386.6 ± 24.1 (range, 300–400) W and the median non-perfused volume (NPV) ratio was 85.2% ().
Table 2. USgHIFU treatment results of patients with multiple uterine fibroids.
During the procedure, real-time ultrasound imaging was used to monitor the grey scale changes in the treated area. Of all the treated fibroids, 52.9% of them had significant grey scale changes () and 47.1% had general grey scale changes. However, no statistically significant difference was observed in rate of significant grey scale changes between hypointense, Isointense and hyperintense fibroids.
Side effects and complications
Common side effects reported during HIFU treatment included transient leg pain, sciatica/buttock pain, heat on the skin, lower abdominal pain and abnormal vaginal discharge. Patients may have experienced more than one of the aforementioned side effects. In this study, common side effects during the procedure included sciatic/buttock pain (43.2%), “burning” skin sensation (61.7%), lower abdominal pain (60.5%), transient leg pain (13.6%) and abnormal vaginal discharge (6.2%) (). The lower abdominal pain and sciatic/buttock pain that occurred during the procedure disappeared immediately after treatment in 90% of patients and in the remaining 10%, the pain subsided within three days after treatment. The transient leg pain which occurred during treatment was as a result of the fibroids being close to the sciatic nerves. When the patient complained of leg pain, treatment ceased and the focus point moved to another safe area to avoid nerve injury. In this study, no permanent nerve injury occurred. Abnormal vaginal discharge occurred in patients with submucosal fibroids, the patients with abnormal vaginal discharge subsided in one week after HIFU.
Table 3. Side effects and complications during or after the procedure.
Overnight stay is a requirement of the institution. All patients went home the following day after 1 d post HIFU evaluation. Utilising the standardised SIR grading system of complications [Citation17], no major complications occurred.
Follow-up results
Clinical symptoms and quality of life evaluation
Among the 81 patients underwent USgHIFU, 65 completed 1-month follow-up, 35 completed 3 months follow-up, 23 completed 6 months follow-up. Only 21 patients completed all 1, 3 and 6 months follow-ups. The follow-up results of the 21 patients are presented in . The average score of UFS and QOL pre-treatment was 56.3 ± 16.7 points and 41.3 ± 21.2 points, respectively. The score of UFS significantly decreased and the score of QOL improved 1-, 3- and 6-month after HIFU treatment.
Table 4. Comparison of UFS and QOL pre-HIFU and post-HIFU.
Fibroid volume changes after HIFU
shows the average shrinkage rate of 120 treated fibroids in 21 patients. The overall shrinkage rate of the fibroids 1, 3 and 6 months after HIFU was 32.5 ± 24.0, 42.3 ± 32.2 and 52.5 ± 36.3%, respectively. The fibroid volumes decreased after treatment. No significant difference was observed in shrinkage rate between hypointense, isointense and hyperintense fibroids (). shows the changes of fibroids on MRI before and after treatment.
Table 5. Shrinkage rate of 120 fibroids in 21 followed to 6 months during follow-up interval.
Pregnancy and re-intervention
A 27-year-old patient who underwent HIFU treatment in March 2016 was pregnant three months after HIFU and gave birth to a baby in January 2017. This patient had three fibroids, the largest one located at left lateral of the uterus, two small fibroids located at anterior wall of the uterus. The size of these three fibroids was 14, 2 and 2 cm in diameter, respectively. The NPV ratio was 86.2, 92.0 and 100%, respectively. The shrinkage rate of the three fibroids at 1 month was around 20%.
During the follow-up period, 2 of the 21 patients for which a 6 month follow up was available required reintervention. These two patients were 32 and 37 years old, respectively; both had submucosal fibroids with hyperintensity on T2WI. A myomectomy was performed 6 months post-treatment because there was no reduction in menstrual bleeding.
Discussion
Black women tend to have more fibroids and greater symptomatology at a younger age [Citation18,Citation19]. In this study, we found 83 (62.9%) patients had multiple fibroids and 81 (61.4%) had three or more fibroids with a median number of six. The average age of the patients was 35.3 ± 5.9 years and all patients had fibroid-related symptoms. The abdominal subcutaneous fat thickness and BMI in black women are larger than those of Asian or Caucasian women in previous studies [Citation20,Citation21]. In this study, the average abdominal subcutaneous fat thickness was 38.0 ± 11.4 mm and the average BMI was 26.3 ± 4.6 kg/m2, which is similar to those of previous studies [Citation21,Citation22].
This study also found that many fibroids in South African black women had a poor blood supply. Based on MR imaging, we found 61.6% (213/346) of the fibroids were hypointense on T2WI and most of them exhibited poor enhancement in both arterial phase and venous phase. The follow-up results showed that the average shrinkage rate of the treated fibroids was 32.5 ± 24.0, 42.3 ± 32.2 and 52.5 ± 36.3% at 1, 3 and 6 months intervals post-HIFU, respectively. The shrinkage rate of the treated fibroids was greater compared to that in previous studies with different ethnic groups [Citation16,Citation23–25]. We further compared the shrinkage over time in hypointense, isointense and hyperintense fibroids and there was no significant difference. Our results similar to earlier studies that the symptoms associated with uterine fibroids gradually improved as the volume of the fibroid reduced [Citation23–25]. After HIFU treatment, the symptom severity score (SSS) decreased and the score of QOL significantly improved. The mean change score of SSS and QOL at 6 months after USgHIFU was larger compared to that of African descent in previous studies [Citation26,Citation27]. Therefore, USgHIFU is effective in treating black women with multiple uterine fibroids.
In South Africa, most of the black women with uterine fibroids tend to have more children. Myomectomy has been an option for patients with uterine fibroids, who wish to have children. Pregnancy after myomectomy may increase the risk of uterine rupture, abnormal placentation, intrauterine adhesions and miscarriage [Citation28,Citation29]. Gambacorti et al. reviewed 11 studies and reported the overall incidence of uterine rupture was 0.93% [Citation30]. Pregnancy after myomectomy may also increase the risks of C-section. In this study, the average age of the patients was 35.3 ± 5.9 years. Most of them wished to have children after HIFU treatment. One patient conceived three months after HIFU and gave birth to a healthy term baby by vaginal delivery. An earlier study evaluated the ovarian function following HIFU and found no change in oestrogen levels [Citation31]. Qin et al. reported that a group of patients conceived within one year after USgHIFU and had no complications with their deliveries [Citation32]. Recently, Zou et al. reported 78 patients with 80 pregnancies. The average time to conceive was just 5.6 ± 2.7 months after HIFU. The foetuses of all the patients developed well during both the pregnancy and child-bearing without uterine rupture or perinatal and postpartum complications. The C-section rate seems to be high in this study: 56 (78.9%) cases had C-section. However, 50 of them had caesarean sections for social reasons [Citation33]. In another study, where 51 patients conceived after MRgFUS treatment of uterine fibroids, the vaginal delivery rate was 64% [Citation34]. Compared to the average interval of 16.0 ± 8.7 months between myomectomy and pregnancy, the time between HIFU and conception was significantly shorter [Citation33–35]. Therefore, for patients with multiple fibroids who wish to conceive, HIFU is preferable to myomectomy because it causes less damage to the myometrium.
Various long-term follow-up studies reported the re-intervention rate following myomectomy was 3.2% at 2 years and 23.5% at 5 years [Citation36,Citation37]. However, following MRgFUS, the re-intervention rate was between 8 and 28% within 12 months [Citation38,Citation39]. The high re-intervention rate following MRgFUS could be explained by the low NPV ratio. In this study, two of 21 patients underwent a myomectomy at 6 months post-HIFU because of heavy menstrual bleeding. We further reviewed these patients and found that both had submucosal fibroids with hyperintensity on T2WI. The re-intervention rate in this study was lower than some other studies because as most of the fibroids were hypointense and these fibroids respond better to HIFU, so a high NPV ratio is easy to achieve. Likewise, since the small fibroids (smaller than 2 cm in diameter) could be visualised on the monitoring ultrasound imaging of HIFU, they could be ablated and reduce the risk of recurrence.
Safety is always the most important concern when using USgHIFU to treat uterine fibroids. In this study, patients reported lower abdominal pain, sciatica/buttock pain, burning skin sensation, abnormal vaginal discharge and leg pain during the procedure. However, the pain is often transient and the score is generally less than four points. Sciatic/buttock pain and lower abdominal pain were observed in approximately 10% of patients following HIFU, but the pain subsided without any specific treatment within 7 d. Abnormal vaginal discharge occurred in five (6.2%) patients. This vaginal discharge occurred because the fibroids treated were close to the endometrium and the heat was transferred to the endometrium. Abnormal vaginal discharge was minimal and subsided a few weeks after HIFU. In this study, no patient required urgent surgical intervention, no permanent nerve injury occurred, no bowel injury occurred and no skin burn was observed. The results from this study showed that HIFU treatment for black women with multiple uterine fibroids has a satisfactory safety profile.
This study is limited because it is retrospective and the follow-up time is short. This study is also limited because many patients didn’t complete the follow-up. Factors that led patients to not come back for further follow-up included the hospital budget and socioeconomic issues. We could not do a post-HIFU MRI for many patients because of the limited hospital budget and because some patients could not afford to come back. In addition, we measured the abdominal subcutaneous fat thickness on MRI while the patients were supine, while patient was treated in a prone position and a degassed water balloon was used to compress the abdominal wall. Thus, the values of abdominal subcutaneous fat thickness vary between MRI and real-time ultrasound. Future studies with larger number of subjects and a longer follow-up period, as well as a comparison between HIFU and myomectomy, are needed to investigate the long-term recurrence rate and the re-intervention rate, as well as the pregnancy outcomes after treatment.
Conclusions
In summary, this study demonstrated that USgHIFU can be used safely to treat black women with multiple uterine fibroids. The short-term follow up results showed that the symptoms of the patients were improved after HIFU treatment and maintained during the follow-up period. Future studies with a larger number of subjects and a longer follow-up time to compare HIFU with myomectomy are required to investigate the long-term recurrence rate and the re-intervention rate, as well as the pregnancy outcomes after treatment.
Disclosure statement
L. Z. is a senior consultant to Chongqing Haifu Company. The other authors report no conflict of interest to declare. The authors alone are responsible for the content and writing of the article.
Additional information
Funding
References
- Chabbert-Buffet N, Esber N, Bouchard P. (2014). Fibroid growth and medical options for treatment. Fertil Steril 102:630–9.
- Day Baird D, Dunson DB, Hill MC, et al. (2003). High cumulative incidence of uterine leiomyoma in black and white women: ultrasound evidence. Am J Obstet Gynaecol 188:100–7.
- Lurie S, Piper I, Woliovitch I, Glezerman M. (2005). Age-related prevalence of sonographically confirmed uterine myomas. J Obstet Gynaecol 25:42–4.
- Stewart EA. (2015 ). Clinical practice. Uterine fibroids. N Engl J Med 372:1646–55.
- Fonseca-Moutinho JA, Barbosa LS, Torres DG, Nunes SM. (2013). Abnormal uterine bleeding as a presenting symptom is related to multiple uterine leiomyoma: an ultrasound-based study. Int J Womens Health 5:689–94.
- Benson CB, Chow JS, Chang-Lee W, et al. (2001). Outcome of pregnancies in women with uterine leiomyomas identified by sonography in the first trimester. J Clin Ultrasound 29:261–4.
- Nezhat FR, Roemisch M, Nezhat CH, et al. (1998). Recurrence rate after laparoscopic myomectomy. J Am Assoc Gynecol Laparosc 5:237–40.
- Nishiyama S, Saito M, Sato K, et al. (2006). High recurrence rate of uterine fibroids on trans-vaginal ultrasound after abdominal myomectomy in Japanese women. Gynecol Obstet Invest 61:155–9.
- Prollius A, de Vries C, Loggenberg E, et al. (2004). Uterine artery embolization for symptomatic fibroids. Int J Gynaecol Obstet 84:236–40.
- Humphrey VF. (2007). Ultrasound andmatter-physical interactions. Prog Biophys Mol Biol 93:195–211.
- Liu Z, Gong C, Liu Y, Zhang L. (2018). Establishment of a scoring system for predicting the difficulty level of high-intensity focussed ultrasound ablation of uterine fibroids. Int J Hyperthermia 34:77–86. [Epub ahead of print]. doi: 10.1080/02656736.2017.1325015
- Xie B, Zhang C, Xiong C, et al. (2015). High intensity focused ultrasound ablation for submucosal fibroids: a comparison between type I and type II. Int J Hyperthermia 31:593–9.
- Zhang L, Chen WZ, Liu YJ, et al. (2010). Feasibility of magnetic resonance imaging-guided high intensity focused ultrasound therapy for ablating uterine fibroids in patients with bowel lies anterior to uterus. Eur J Radiol 73:396–403.
- O’Sullivan AK, Thompson D, Chu P, et al. (2009). Cost-effectiveness of magnetic resonance guided focused ultrasound for the treatment of uterine fibroids. Int J Technol Assess Health Care 25:14–25.
- Spies JB, Coyne K, Guaou Guaou N, et al. (2002). The UFS-QOL, a new disease-specific symptom and health-related quality of life questionnaire for leiomyoma. Obstet Gynecol 99:290–300.
- Stewart EA, Rabinovici J, Tempany CM, et al. (2006). Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 85:22–9.
- Sacks D, Mcclenny TE, Cardella JF, Lewis CA. (2003). Society of interventional radiology clinical guidelines. J Vasc Interv Radiol 14:199–202.
- Stewart EA, Cookson CL, Gandolfo RA, Schulze-Rath R. (2017). Epidemiology of uterine fibroids: a systematic review. BJOG 124:1501–12.
- Moorman PG, Leppert P, Myers ER, Wang F. (2013). Comparison of characteristics of fibroids in African American and white women undergoing premenopausal hysterectomy. Fertil Steril 99:768–76.
- Kronenfeld LW, Reba-Harrelson L, Von Holle A, et al. (2010). Ethnic and racial differences in body size perception and satisfaction. Body Image 7:131–6.
- Faerstein E, Szklo M, Rosenshein N. (2001). Risk factors for uterine leiomyoma: a practice-based case-control study. I. African-American heritage, reproductive history, body size, and smoking. Am J Epidemiol 153:1–10.
- Rush EC, Goedecke JH, Jennings C, et al. (2007). BMI, fat and muscle differences in urban women of five ethnicities from two countries. Int J Obes (Lond) 31:1232–9.
- Funaki K, Fukunishi H, Sawada K. (2009). Clinical outcomes of magnetic resonance-guided focused ultrasound surgery for uterine myomas: 24-month follow-up. Ultrasound Obstet Gynecol 34:584–9.
- Rabinovici J, Inbar Y, Revel A, et al. (2007). Clinical improvement and shrinkage of uterine fibroids after thermal ablation by magnetic resonance-guided focused ultrasound surgery. Ultrasound Obstet Gynecol 30:771–7.
- Dobrotwir A, Pun E. (2012). Clinical 24 month experience of the first MRgFUS unit for treatment of uterine fibroids in Australia. J Med Imaging Radiat Oncol 56:409–16.
- Stewart EA, Rabinovici J, Tempany CM, et al. (2006). Clinical outcomes of focused ultrasound surgery for the treatment of uterine fibroids. Fertil Steril 85:22–9.
- Thiburce AC, Frulio N, Hocquelet A, et al. (2015). Magnetic resonance-guided high-intensity focused ultrasound for uterine fibroids: mid-term outcomes of 36 patients treated with the sonalleve system. Int J Hyperthermia 31:764–70.
- Parazzini F, Tozzi L, Bianchi S. (2016). Pregnancy outcome and uterine fibroids. Best Pract Res Clin Obstet Gynaecol 34:74–84.
- Klatsky PC, Tran ND, Caughey AB, Fujimoto VY. (2008). Fibroids and reproductive outcomes: a systematic literature review from conception to delivery. Am J Obstet Gynecol 198:357–66.
- Gambacorti-Passerini Z, Gimovsky AC, Locatelli A, Berghella V. (2016). Trial of labor after myomectomy and uterine rupture: a systematic review. Acta Obstet Gynecol Scand 95:724–34.
- Wang X, Qin J, Wang L, et al. (2013). Effect of high-intensity focused ultrasound on sexual function in the treatment of uterine fibroids: comparison to conventional myomectomy. Arch Gynecol Obstet 288:851–8.
- Qin J, Chen JY, Zhao WP, et al. (2012). Outcome of unintended pregnancy after ultrasound-guided high-intensity focused ultrasound ablation of uterine fibroids. Int J Gynaecol Obstet 117:273–7.
- Zou M, Chen L, Wu C, et al. (2017). Pregnancy outcomes in patients with uterine fibroids treated with ultrasound-guided high-intensity focused ultrasound. BJOG 3:30–5.
- Rabinovici J, David M, Fukunishi H, et al. (2010). Pregnancy outcome after magnetic resonance-guided focused ultrasound surgery (MRgFUS) for conservative treatment of uterine fibroids. Fertil Steril 93:199–209.
- Zhang Y, Hua KQ. (2014). Patients’ age, myoma size, myoma location, and interval between myomectomy and pregnancy may influence the pregnancy rate and live birth rate after myomectomy. J Laparoendosc Adv Surg Tech A 24:95–9.
- Mara M, Maskova J, Fucikova Z, et al. (2008). Midterm clinical and first reproductive results of a randomized controlled trial comparing uterine fibroid embolization and myomectomy. Cardiovasc Intervent Radiol 31:73–85.
- Reed SD, Newton KM, Thompson LB, et al. (2006). The incidence of repeat uterine surgery following myomectomy. J Womens Health (Larchmt) 15:1046–52.
- Stewart EA, Gostout B, Rabinovici J, et al. (2007). Sustained relief of leiomyoma symptoms by using focused ultrasound surgery. Obstet Gynecol 110:279–87.
- Morita Y, Ito N, Hikida H, et al. (2008). Non-invasive magnetic resonance imaging-guided focused ultrasound treatment for uterine fibroids–early experience. Eur J Obstet Gynecol Reprod Biol 139:199–203.
