Abstract
Aim: To investigate the analgesic properties and the safety of low power bipolar radiofrequency ablation (RFA) performed with internally cooled electrodes and vertebral augmentation for the treatment of painful spinal malignancies.
Materials and methods: Consent was waived for retrospective study participation. Review of electronic records identified 11 consecutive patients (6 females; 5 males; mean age 61.3 ± 11.6 years) with one-index painful spinal tumour, who were treated between June 2016 and October 2017 with bipolar RFA and vertebral augmentation. Patients were treated if they presented with focal pain (≥4/10 on a 0–10 visual analogic scale in the 24-h period) corresponding to a metastatic vertebral level on cross sectional imaging. The Wilcoxon test was used to evaluate the significance of the post-operative pain.
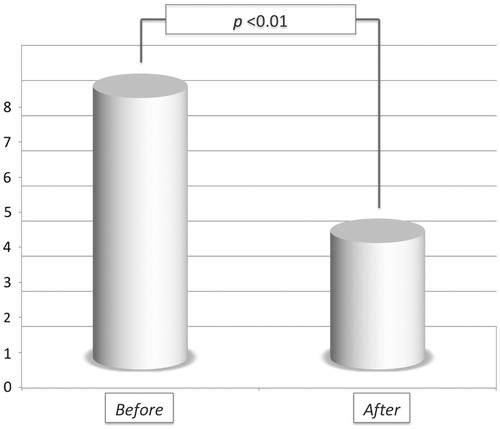
Results: Lumbar levels were treated in 72.7% cases; metastatic epidural involvement was noted in 81.8% cases; 54.5% patients received associated treatments in addition to RFA, which was coupled to vertebral augmentation in all cases. Two (18.2%) complications were noted. Mean pain score measured at last clinical follow-up available (mean 1.9 ± 1.4 months) was 3.5 ± 2 (versus 7.8 ± 1.1 at baseline; p <0.01).
Conclusions: Low-power bipolar RFA performed with internally cooled electrodes and coupled to vertebral augmentation provides safe and effective early analgesia in patients affected by painful spinal malignancies.
Introduction
Spine accounts for 70% of all bone metastases [Citation1,Citation2], and spinal metastatic involvement highly impair patients’ quality of life due to pain, pathological fractures and spinal cord compression [Citation3]. The palliative treatment of spinal metastases primarily relies on analgesic drugs and radiation therapy (RT). Nevertheless, many patients suffer the side effects of analgesic drugs or do not obtain effective pain management from them [Citation4]; on the other hand, RT provides long-lasting complete pain relief in only 15–30% patients [Citation5,Citation6].
Recently, percutaneous image-guided thermal treatments proved to be effective for the management of painful bone tumours [Citation7,Citation8] with promising results reported also for the spine, where ablation is often combined with vertebral augmentation [Citation9,Citation10]. In order to limit temperature-mediated nervous injuries, new low-power bipolar RFA systems have been recently designed for the specific need of spinal ablation [Citation9,Citation10]. Such systems provide small and predictable ablations by limited thermal energy diffusion beyond the vertebral body [Citation11] thus, significantly reducing the risk of iatrogenic nervous injuries.
The aim of the present study was to investigate the analgesic properties and the safety of low-power bipolar RFA performed with internally cooled electrodes and coupled with vertebral augmentation for the treatment of painful spinal malignancies.
Materials and method
No specific funding was received for this study, which was approved by the local Institutional Review Board. Consent was waived for retrospective study participation.
Patients’ selection and interventional procedure
Review of electronic records identified 11 consecutive patients (6 females, mean age 61.2 ± 13.4 years, range 40–77; 5 males, mean age 61.5 ± 11.2 years, range 41–73) with one painful spinal tumour, who were treated with bipolar RFA and vertebral augmentation between June 2016 and October 2017 in a tertiary referral university hospital. Patients were selected for primary treatment or following the failure of other palliative therapies (e.g. analgesic drugs, RT) provided a focal pain (≥4/10 on a 0–10 visual analogic scale – VAS – in the 24-h period) corresponding to a vertebral body tumour on cross sectional imaging. Patients were not treated in the following cases: cervical levels; radicular or neurological symptoms; uncorrectable coagulation disturbances; pregnancy; local/systemic infection.
Ablations were performed under Cone-Beam CT- or CT/fluoroscopy-guidance with the Osteocool RFA System (Medtronic, Minneapolis, Minnesota, USA) by five interventional radiologists with different experience (2–25 years) in bone tumours ablation. Procedures were always performed under general anaesthesia. Antibiotic prophylaxis (2 g cefazolin) was systematically administered i.v. before starting the procedure.
Based on the size, shape and location of the metastasis and the risk of iatrogenic neural damage, one or two internally cooled electrodes were deployed and activated with variable number of ablation zones, target temperatures and times. Osteocool is a temperature-based, low-power (20 W per electrode with up to two electrodes being activated simultaneously) RFA system provided with three different ablative protocols based on the size (7, 10 or 20 mm, ) of the active tip of the selected electrode. RFA energy is progressively delivered by the system until the target temperature is reached and kept constant during the remaining ablation time. The system regulates energy deliverance based on the impendence feedback.
Figure 1. (A) Osteocool RFA system provides three different ablative protocols with standard ablation temperature set at 70 °C; the protocol is automatically selected by the system based on the size of the active tip (7, 10 or 20 mm) of the selected electrode. (B) When two electrodes are activated simultaneously, based on the distance between the two active tips, different sizes and shapes of the ablation area are obtained. The widest ablation area, roughly reproducing the shape of the vertebral body, is obtained when the two tips of the electrodes are spaced away 8–10 mm.
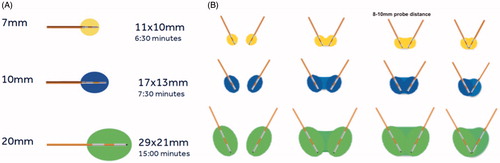
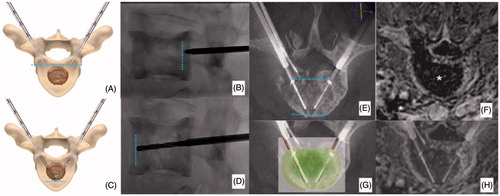
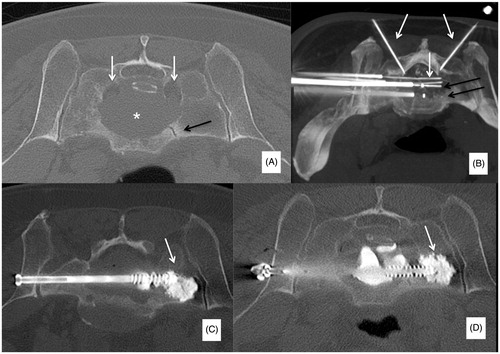
Modifications to all the different variables regulating the ablation protocol are allowed before starting the ablation. According to the distance between the tips of the two electrodes, different sizes and shapes of the ablation area are obtained (); the widest ablation zone, roughly reproducing the shape of the vertebral body, is obtained when a bilateral double-electrode approach is performed with the tips of the two electrodes spaced away 8–10 mm. In this case, the vertebral body is bilaterally accessed with 10–13 G bone trocars and their distal tips are deployed just behind the posterior margin of the target tumour: the line connecting the tips of both trocars corresponds to the posterior margin of the ablation area. Thereafter, bone drilling is coaxially performed to allow allocation of the electrodes. The distal tips of the drills are advanced just anteriorly to the anterior margin of the tumour with a “drill-kissing” configuration: the line passing through the distal tips of the drills corresponds to the anterior border of the ablation area ().
Figure 2. Bilateral double-probe approach performed with the “drill-kissing” technique. (A, B) The vertebral body is bilaterally accessed with 10–13 G bone trocars; the distal tips of the trocars are deployed just behind the posterior margin of the tumour; and the line connecting the tips of both trocars corresponds to the posterior margin of the ablation area. (C, D) Bone drilling is thereafter performed coaxially to create a channel to allocate the electrodes; the distal tip of the drills is advanced just anteriorly to the anterior margin of the tumour with a “drill-kissing” configuration; the line passing through the distal tips of the drills corresponds to the anterior border of the ablation area. (E) Final configuration of electrodes deployment with their distal tips spaced away 8–10 mm. (F) Axial T1-weighted CE-MRI sequence showing the size of the ablation area (*) roughly reproducing the shape of the vertebral body. (G) Reconstructed image showing the expected ablation area based on the configuration of electrodes deployment. (H) Reconstructed image showing the ablation area obtained at 24-h CE-MRI based on the configuration of electrodes deployment.
In the present series, target temperature was fixed at 70 °C for the first three cases in accordance to the manufacturer’s protocol. Then, following four ablation tests performed with the most powerful manufacturer’s protocol (two 20 mm active tip electrodes; 8–10 mm of distance between the two tips; 15 min. ablation time; 70 °C target temperature) on ex vivo porcine tissue, showing that measured temperature at the lateral external borders of the ablation area hardly reached 45 °C, the target temperature was always set at >70 °C.
Based on operators’ choice, neural protective measures were applied during RFA to prevent injuries to the spinal cord or nerve roots; two systems were applied alone or in combination: (a) thermocouples to monitor temperature rise in critical areas such as the posterior wall; (b) hydro-dissection of the epidural space performed with room-temperature electrically inert 5% dextrose solution. Thermocouples and/or hydro-dissection needles were deployed through an inter-laminar, trans-foraminal or trans-pedicular approach.
At the end of the ablation, through the bone trocars applied to deploy the electrodes, poly-methyl-methacrylate (PMMA, Osteopal, Heraeus Medical GmbH, Wehrheim, Germany) was injected under continuous fluoroscopic guidance to achieve vertebral augmentation. No kyphoplasty balloons were inflated before PMMA injection.
Clinical assessment and follow-up
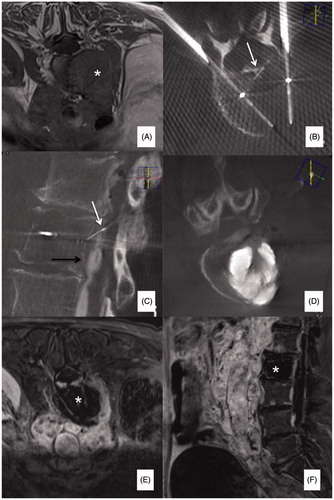
The physician performing the procedure examined the patients 1–2 weeks before the procedure with their most recent cross sectional imaging; 1–2 months clinical follow-up was systematically scheduled to assess the intensity of focal pain according to a 0–10 VAS, and to compare it to baseline. Contrast-enhanced MRI (CE-MRI) was systematically scheduled 24 h following RFA to assess the size of the ablation area ().
Figure 3. (A) Painful metastatic involvement of L2 (*) in a 77 years old female patient affected by bladder cancer. (B) Two 20 mm active tip RFA electrodes were deployed through a trans-pedicular approach and several different impacts were performed. (B, C) A thermocouple was deployed through a descending trans-foraminal approach (white arrow); epidural hydro-dissection was performed through the same approach (black arrow). (D) Vertebral augmentation was subsequently performed. (E, F) Contrast enhanced T1-weighted sequence obtained 24 h after RFA shows the extension of the necrotic area (*).
After the first clinical/radiological follow-up, patients were not systematically followed-up, unless suspicion of complication and/or new unexpected symptoms occurred. Nevertheless, some patients underwent routine imaging follow-up for systemic disease restaging; accordingly, local tumour control granted by RFA was investigated, despite the pure palliative role of the procedure.
Data collection and analysis
All patients’ electronic records were reviewed and the following data collected:
Patients demographics (sex; age; histology of the index spinal tumour);
Lesion-related variables (involved vertebral level; radiological aspect of the index tumour; metastatic epidural involvement; associated treatments);
Procedure-related variables (number and type of applied electrodes; approaches applied to get access to the target vertebra; protective measures; ablation time and temperature; vertebral augmentation; technical success; complications; size of the ablation area);
Follow-up (pain intensity after RFA; evolution of the index spinal tumour).
A procedure was considered technically successful if the index vertebra was accessed with the RFA electrodes; adequate ablation was performed (according to the pre-operative plan), followed by vertebral augmentation.
Complications were assessed according to CIRSE classification [Citation12].
Basic descriptive statistics were used to present results. The Wilcoxon test was used to evaluate the significance of the post-operative pain.
Results
Lumbar levels were treated in 8/11 (72.7%) cases, thoracic levels in 2/11 (18.2%) and a sacral level was treated in 1/11 (9.1%) case. Seven out of 11 tumours (63.6%) were lytic and 4/11 (36.4%) mixed. Metastatic epidural involvement was noted in 9/11 (81.8%) cases.
RT was associated before or after RFA in 3/11 (27.3%) cases. One (9.1%) patient presenting with a large hyper-vascular L1 metastasis from hepatocellular carcinoma underwent trans-arterial embolisation before RFA in order to minimise the “heat-sink effect” during RFA. In the end, the first patient of the series (9.1%) underwent lumbar laminectomy and surgical stabilisation two days following RFA, as previously planned, due to a primary chondrosarcoma of L3 (). Baseline characteristics of the population are summarised in .
Figure 4. (A, B) Primary painful L3 chondrosarcoma (*) severely compressing the dural sac in a 61 years old female patient confined to a wheelchair; a bi-pedicular involvement is also present (white arrows) thus, making the lumbar spine instable. (C) Two 20 mm active tip RFA electrodes were deployed through a trans-pedicular approach and several different impacts were performed. A thermocouple was also deployed through a posterior inter-laminar approach (black arrow) to monitor the temperature rise at the level of the posterior wall; during ablation some gas bubbles increasing the impedance and thus limiting RFA energy delivery were noted (white arrows); accordingly, some drops of saline were injected to boost RFA, which was then conducted uneventfully. (D) Vertebral augmentation was subsequently performed, followed by (E) surgical laminectomy and stabilisation. The patient was pain free and able to walk again for 5 months until new local tumour progression occurred.
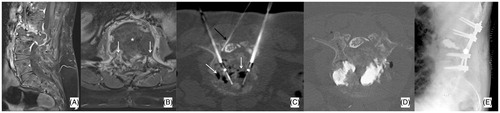
Table 1. Study population.
A double-electrode approach was used in 10/11 (90.9%) cases; in one (9.1%) case, a single-electrode unilateral approach was used. In 10/11 (90.9%) cases, neural protective measures were applied: a combined system made up of an independent thermocouple and of a hydro-dissection system was used in 8/10 (80%) cases; in the remaining two (20%) cases, a simple thermocouple was deployed coaxially at the level of the posterior wall through a trans-pedicular approach.
After the first three cases, the mean RFA target temperature was 86.2 °C ± 5.1 (99% CI: 79.85–92.65; median 90; range 80–90). Mean RFA treatment time in the whole population was 24.7 ± 9.5 min. (99% CI: 15.62–33.83; median 25; range 15–45). RFA procedural data are summarised in .
Table 2. RFA data.
Following RFA, all the treated levels received vertebral augmentation: simple PMMA augmentation was applied in 10/11 (90.9%) cases; one patient presenting with a pathologic vertical fracture of S1 underwent percutaneous osteosynthesis coupled to PMMA injection to fill the lytic cavity and to fix the tip of the screws in healthy distal bone (). Technical success was reached in all cases.
Figure 5. (A) Slow-evolving S1 lytic metastasis (*) in a 58 years old male patient affected by a neuroendocrine cancer of the lung; the patient presented with low back pain and bilateral sciatica due to bilateral involvement of S1 neuroforamina (white arrows); a vertical pathologic fracture was also noted (black arrow). (B) Two 20 mm active tip RFA electrodes were deployed (black arrows) and several different impacts were performed. Furthermore, four thermocouples were deployed (white arrows): one in each neuroforamen and one behind each electrode to monitor the temperature rise in both neuroforamina and at the level of the posterior wall. (C, D) Following RFA, two cannulated screws were deployed to fix the fracture; moreover, PMMA was also injected to fill the lytic cavity and to anchor the tip of the screws in distal healthy bone (white arrows).
Two (18.2%) complications were noted: the first was a grade 1 complication consistent with some PMMA lost in the para-vertebral soft-tissues while retrieving the bone trocar; the lost PMMA was safely removed in the same interventional session as described elsewhere [Citation13]; the second was a grade 6 complication consistent with a sepsis in an end-stage lung cancer patient who was treated despite a subclinical para-vertebral abscess, which was inadvertently misdiagnosed the day of the procedure. The abscess was drained two days following the procedure, bacterial culture obtained and subsequent antibiotic therapy established. Nevertheless, the patient died 3 weeks later due to septic complications.
When considering RFA performed with the “drill-kissing” technique, the following mean diameters of the ablation area were measured at the 24-h CE-MRI: axial 38.43 ± 9.62 mm (99% CI: 24.94–51.91; median 35; range 28–56); cranio-caudal: 23.86 ± 5.61 mm (99% CI: 16.00–31.72; median 23; range 17–34); antero-posterior: 32.86 ± 7.52 mm (99% CI: 22.33–43.39; median 32; range 23–44). The first case of the series was excluded from this calculation since saline injection was performed during the ablation to increase the electrical conductivity ().
A statistically significant pain drop () was recorded in the entire population at follow-up (mean time interval for post-operative pain assessment: 1.9 ± 1.4 months; 99% CI: 0.49–3.31; median 1; range 1–5): mean pre-operative VAS was 7.8 ± 1.1 (99% CI: 6.63–8.97; median 8; range 6–9) versus mean post-operative VAS 3.5 ± 2 (99% CI: 1.49–5.51; median 3.5; range 0–6; p < 0.01). For 6/11 (54.5%) patients a post-treatment imaging follow-up (mean 3.5 ± 2.9 months; 99% CI: 1.24–8.24; median 2.5; range 1–8) was available: local disease was stable in four cases and progressed in the remaining two.
Discussion
The present series showed that low-power bipolar RFA performed with internally cooled electrodes and followed by vertebral augmentation is safe and effective in achieving early pain management in patients with painful spinal malignancies. Such outcome was safely achieved even when the target ablation temperature was increased; in fact, no iatrogenic nervous injuries were noted and the two reported complications were not RFA-related. Nevertheless, effective pain management was reported also with the standard protocol (70 °C over 7–15 min) [Citation14] and with another commercially available bipolar RFA system (DFINE; Merit Medical, South Jordan, UT), which is provided of a distal articulated electrode [Citation9,Citation10,Citation15]. The main features differentiating the Osteocool from the DFINE are: (a) Osteocool works with internally cooled electrodes that avoid tissue charring; (b) up to two electrodes may be simultaneously activated; (c) exact dimension of the ablation area can be predicted according to probes deployment; (d) Osteocool is a temperature-based system, which is not the case for the DFINE that is power-based thus, not granting constant temperatures during ablation. However, the DFINE system has the advantage of the articulated electrode, which permits more direct ablation in the posterior median aspect of the vertebral body [Citation15] through a single unilateral vertebral access. Currently, Osteocool provides only intra-electrode bipolar RFA and the posterior margin of the ablation area is indirectly obtained by the fusion of the two ablation areas independently produced by each activated electrode. A potential future development to obtain a more direct ablation of the posterior margin relies on the development of inter-electrodes bipolar RFA through the two proximal dipoles of the each deployed electrode.
Despite the configuration of the device applied, all the published studies coupled bipolar RFA with vertebral augmentation [Citation9,Citation10] to prevent secondary vertebral collapse. However, in terms of pain relief, it is not clear what is the real advantage of this double approach as compared to vertebral augmentation alone, which had already proved to be highly effective in achieving both pain relief and consolidation in case of vertebral body lytic disease [Citation16]. Possible indications favouring ablation could be: (a) the treatment of mixed or blastic vertebral tumours (the latter ideally treated with cryoablation to avoid impedance-related drawbacks that are typically seen while performing RFA of blastic bone tumours); (b) the treatment of bone metastases in oligometastatic patients or in those presenting with extra-vertebral tumour deposits [Citation17]. However, interventional treatments combining in the same session ablation and augmentation have a clear advantage compared to RT alone, which provides only potential pain relief without any prevention of vertebral collapse. Moreover, when ablation, vertebral augmentation and RT were combined to treat the same vertebral level, significant pain relief was achieved [Citation18]; which is in line with Di Staso et al. who proved that the 12-week rate of complete and overall pain response and the rate of recurrent pain favour the combined treatment (RFA + RT) as compared to RT alone for the treatment of painful bone metastases [Citation19]. The same team reported also similar results when cryoablation was combined with RT [Citation20].
Limitations of the present study are mainly related to the small sample size of the study population and to its retrospective nature, which did not permit larger measurements of outcomes other than short-term pain relief and size of the ablation area. Moreover, the absence of a control group undergoing vertebral augmentation alone did not allow definitive understanding of the real benefit of RFA.
In conclusion, low-energy bipolar RFA performed with internally cooled electrodes and coupled with vertebral augmentation safely provides effective early analgesia in patients presenting with painful spinal malignancies. Further prospective studies are needed to confirm these preliminary results also at mid-term follow-up.
Disclosure statement
No potential conflict of interest was reported by the authors. Authors 1, 2 and 6 are proctors for Medtronic. The present paper received no funding.
References
- Delank KS, Wendtner C, Eich HT, et al. (2011). The treatment of spinal metastases. Dtsch Arztebl Int 108:71–80.
- Sutcliffe P, Connock M, Shyangdan D, et al. (2013). A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol Assess 17:1–274.
- Harel R, Angelov L. (2010). Spine metastases: current treatments and future directions. Eur J Cancer 46:2696–707.
- Hara S. (2008). Opioids for metastatic bone pain. Oncology 74 Suppl 1:52–4.
- Lutz S, Balboni T, Jones J, et al. (2017). Palliative radiation therapy for bone metastases: update of an ASTRO evidence-based guideline. Pract Radiat Oncol 7:4–12.
- Steenland E, Leer JW, van Houwelingen H, et al. (1999). The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch Bone Metastasis Study. Radiother Oncol 52:101–9.
- Dupuy DE, Liu D, Hartfeil D, et al. (2010). Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American College of Radiology Imaging Network trial. Cancer 116:989–97.
- Callstrom MR, Dupuy DE, Solomon SB, et al. (2013). Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer 119:1033–41.
- Bagla S, Sayed D, Smirniotopoulos J, et al. (2016). Multicenter prospective clinical series evaluating radiofrequency ablation in the treatment of painful spine metastases. Cardiovasc Intervent Radiol 39:1289–97.
- Wallace AN, Greenwood TJ, Jennings JW. (2015). Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J Neurooncol 124:111–18.
- Wallace AN, Hillen TJ, Friedman MV, et al. (2017). Percutaneous spinal ablation in a sheep model: protective capacity of an intact cortex, correlation of ablation parameters with ablation zone size, and correlation of postablation MRI and pathologic findings. Am J Neuroradiol 38:1653.
- Filippiadis DK, Binkert C, Pellerin O, et al. (2017). Cirse quality assurance document and standards for classification of complications: the Cirse Classification System. Cardiovasc Interv Radiol 40:1141–6.
- Cazzato RL, Garnon J, Ramamurthy N, et al. (2016). Percutaneous management of accidentally retained foreign bodies during image-guided non-vascular procedures: novel technique using a large-bore biopsy system. Cardiovasc Interv Radiol 39:1050–6.
- David E, Kaduri S, Yee A, et al. (2017). Initial single center experience: radiofrequency ablation assisted vertebroplasty and osteoplasty using a bipolar device in the palliation of bone metastases. Ann Palliat Med 6:118–24.
- Hillen TJ, Anchala P, Friedman MV, Jennings JW. (2014). Treatment of metastatic posterior vertebral body osseous tumors by using a targeted bipolar radiofrequency ablation device: technical note. Radiology 273:261–7.
- Tsoumakidou G, Too CW, Koch G, et al. (2017). CIRSE guidelines on percutaneous vertebral augmentation. Cardiovasc Interv Radiol 40:331–42.
- Gangi A, Tsoumakidou G, Buy X, Quoix E. (2010). Quality improvement guidelines for bone tumour management. Cardiovasc Intervent Radiol 33:706–13.
- Greenwood TJ, Wallace A, Friedman MV, et al. (2015). Combined ablation and radiation therapy of spinal metastases: a novel multimodality treatment approach. Pain Physician 18:573–81.
- Di Staso M, Zugaro L, Gravina GL, et al. (2011). A feasibility study of percutaneous Radiofrequency Ablation followed by Radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol 21:2004–10.
- Di Staso M, Gravina GL, Zugaro L, et al. (2015). Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: propensity matching analysis in 175 patients. PLoS One 10:e0129021.