Abstract
Purpose: Radiofrequency ablation (RFA) is the most common percutaneous technique applied to treat painful spinal metastasis; however, experience in this field is somehow still limited. A systematic literature research was performed to understand the effects of RFA in terms of analgesia and safety.
Materials and methods: Inclusion criteria for the studies were as follows: (1) randomised controlled or non-randomised studies with a prospective or retrospective design; (2) population made up of adults with spinal metastasis; (3) spinal metastasis treated with RFA alone or in combination/comparison with other treatments; (4) studies reporting about patients’ pain before and at least one time-point following RFA; and (5) English-language studies.
Results: Seven hundred and thirty-three articles were screened and 8 (4 prospective, 4 retrospective) matched the inclusion criteria. Study population ranged between 10 and 92 patients across studies. Five out of eight studies reported a highly effective pain management (≥4 points of pain reduction between baseline and the last time-point available); 2/8 studies reported moderate results (≥2 points of pain reduction between baseline and the last time-point available). All studies combined RFA with cement augmentation in the vast majority of patients (40–100%) or metastasis (94–95.8%). Grade I–IIIa neural complications were reported in up to 16% of the cases and were always managed conservatively or with steroids.
Conclusions: RFA, combined with vertebral augmentation in most of the cases, is effective and safe in achieving short- to mid-term (from 1 week to 6 months) analgesia in patients affected by painful spinal metastasis.
Introduction
Bone is the third most common site for cancer metastasis after lung and liver; and spine accounts for around 70% of all osseous metastasis [Citation1,Citation2]. Metastatic spinal involvement is associated with significant morbidity and reduction in health-related quality of life due to pain, pathological fractures and spinal cord compression [Citation3].
Treatment of patients with spinal metastasis is often palliative and primarily involves painkillers and radiation therapy alone or in combination. However, many patients do not benefit at all from these treatments and many only achieve partial pain relief [Citation4,Citation5]. On the other hand, invasive surgical interventions are of limited value for multi-metastatic cancer patients just needing pain management. On a large scale, the considerable post-surgical morbidity and the reduced life expectancy of such a fragile population prevents the application of surgery. Therefore, surgery is often reserved to oligometastastic patients needing a curative treatment, especially if they present a slow-evolving disease.
Recently, some percutaneous minimally invasive image-guided ablative treatments have been introduced for the management of painful bone metastasis. Results were very promising [Citation6,Citation7], and were also confirmed in the spine [Citation8,Citation9]. Basically, ablative treatments provide variable extent of tumour destruction by energy delivery into the tumour through a percutaneously-inserted needle. Amongst all the available ablative techniques, radiofrequency ablation (RFA) is the most commonly applied due to its historical availability [Citation8–10]. Nevertheless, experience with such technique in the setting of painful spinal metastasis is still somehow limited. Accordingly, a literature search was performed to understand the effects of percutaneous RFA in terms of analgesia and safety for the management of painful spinal metastasis.
Materials and methods
Literature search
Informed consent was not required for this retrospective literature review. The present study was conducted in accordance with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. This study was realised following the guidelines of the Cochrane Collaboration for systematic reviews of interventions [Citation11].
PubMed Epub Ahead of Print, MEDLINE In-Process & Other Non-Indexed Citations, Ovid MEDLINE® Daily and Ovid MEDLINE® (Ovid SP), Embase (Ovid SP) and the Cochrane Central Register of Controlled trials (CENTRAL) were screened on 27 March 2017 in order to search for the study papers. To complete the search, conference abstracts presented in the last 2 years in the proceedings of the American Society for Clinical Oncology (ASCO 2015–2016) and the European Society for Medical Oncology (ESMO 2015–2016) were also screened. In all cases, the search strategies combined free text and controlled vocabulary terms (Medical Subject Headings in MEDLINE and CENTRAL and Emtree terms in Embase) for spinal/bone metastasis and RFA.
Selection criteria
Studies matching all the following criteria were included in the final analysis:
Randomised controlled or non-randomised studies with a prospective or retrospective design;
Population made up of patients ≥18 years and affected by spinal metastasis;
Spinal metastasis treated with RFA alone or in combination/comparison with other treatments;
Studies reporting patients’ pain before and at least one time-point following RFA;
English-language studies.
To ensure consistency, included studies were labelled for design according to definitions listed in the Centre for Reviews and Dissemination (CRD) guidance for systematic reviews [Citation12]. Prospective studies are those that monitor patients for an outcome before it has occurred. These have been classed as either interventional, in which patients have been enrolled on a pre-specified protocol to receive a particular intervention, or case series where patients have been followed without interference in the type of intervention prescribed with no control arm. Retrospective studies are those investigating analysis outcomes, which have already occurred in patients. These studies were also further categorised as case series.
Two reviewers screened all abstracts independently for paper selection. All conflicts were resolved in consensus. In duplicate cases (i.e. series published twice or more by the same authors), only the series with the largest sample size and/or longest follow-up for pain assessment were included.
Risk of bias in non-randomised studies was not assessed due to a large amount of heterogeneity in non-randomised study designs with no single critical appraisal tool suitable for all.
Data collection and analysis
The full text of all included papers was reviewed in order to collect specific data in a dedicated electronic spreadsheet (Excel 2011; Microsoft, Seattle, WA). For each included study, the following data were extracted, when available:
Study characteristics (design; aim and inclusion/exclusion criteria; total sample size; time-point for pain assessment following RFA);
Patients’ baseline characteristics (sample size; proportion and type of primary tumour; size and location of the index spinal tumour; associated treatments);
RFA-related variables (ablation time, zone and temperature; ablation system used; comparator procedure and/or additional intervention linked to the index procedure and the time-point at which it was performed; number and proportion of patients receiving additional intervention);
Pain experienced by the population at each reported time point and the system used to measure it. Such measurements were used to calculate clinical efficacy. In particular, pain improvement was rated as moderate when ≥2 points of pain reduction were noted between baseline and the last time-point available; on the other hand, pain improvement was rated as high when ≥4 points of pain reduction were noted between baseline and the last time-point available. Since RFA + cement augmentation was the most common therapeutic strategy adopted, in studies presenting results separately for different groups of treatments (i.e. RFA alone and RFA + cement augmentation), only clinical efficacy reported for the group RFA + cement augmentation was taken into account to determine clinical efficacy. In the end, when available, also pain reduction/relief and use of pain medications after RFA as compared to baseline were reported;
Procedure-related complications were reported according to the Clavidien–Dindo Classification [Citation13]. Cement leakage was not considered as a complication unless it resulted into a clinically relevant episode;
Patients’ quality of life and disability as well as RFA aptitude to achieve local tumour control were also reported if scores or similar instruments were applied.
Statistical analysis
Descriptive statistics were used to present results. When percentages were not reported these were calculated from the number of responders and the total number of patients. If not reported, the difference between pre-operative and post-operative pain scores was calculated.
Results
Seven hundred and thirty-three articles were identified from the systematic literature search. Following the review of abstracts and titles, 63 full-text articles were ordered for assessment. On full paper review, 8 non-randomised studies (3 prospective case series, 1 prospective interventional study and 4 retrospective case series) that investigated RFA alone or in combination with another treatment were identified and included for the final analysis [Citation8,Citation10,Citation14–19]. No randomised controlled trials met the inclusion criteria. No relevant papers were identified from the search of the conference proceedings. Aim, inclusion/exclusion criteria and other relevant features of each included study are summarised in .
Table 1. Design and main features of each included study.
Although 9 patients from the series by Greenwood et al. [Citation17] were already included in the series by Anchala et al. [Citation10], the former series was not excluded from the present analysis due to the particularity of the study aiming at evaluating the safety and efficacy of percutaneous ablation (RFA or cryoablation in one case) combined to radiation therapy for pain management.
Baseline characteristics of the population/target lesions of each included study are provided in . Sample size ranged between 10 and 50 patients in prospective studies and between 21 and 92 in retrospective ones. In most cases, targeted metastases were likely to origin from breast, lung and renal cancers. In 4 out of 5 studies reporting the distribution of the treated levels, lumbar ones were the most commonly treated. Only three studies reported the size of the target metastasis: Nakatsuka et al. [Citation14] reported a mean size of 4.9 cm (range 3–8); Proschek et al. [Citation15] reported a mean size of 1.9 cm (range 3–8); in the end, Greenwood et al. [Citation17] described sizes ranging from 1.6 cm to metastasis involving the entire vertebral body and extending into the para-vertebral space.
Table 2. Baseline characteristics of the study population.
Details of RFA treatments across all the included studies are provided in . In none of the prospective or retrospective studies, RFA was compared with another intervention. Mean ablation time ranged between 6 and 9.75 min. across four studies. Bipolar RFA was applied in 4 out of the 8 studies. Only four studies reported previous treatments that included chemotherapy, radiation therapy, surgery or a combination.
Table 3. RFA-related variables.
All studies applied cement augmentation following RFA in some (40–60%) or all (100%) patients, or in the vast majority of the treated vertebrae (94–95.8%). In 6 out of 8 studies and in the majority of patients (95.8%) treated by Anchala et al. [Citation10], cement augmentation followed RFA during the same interventional session. Interestingly, two of the 4 patients not receiving vertebral augmentation in Anchala et al. [Citation10] went on to bone insufficiency fractures and subsequently needed cement augmentation at 3 and 12 months, respectively. In the end, 4 patients in Grönemeyer et al. [Citation19] underwent cement augmentation 3 to 7 days after RFA.
Clinical efficacy (pain relief)
The efficacy of RFA was quantified based on pain scales: VAS was the applied method in 6 out of 8 studies; the remaining two studies applied the NRS scale. Mean baseline VAS scores ranged between 5.9 and 8.6 across studies; mean baseline NRS score between 5.9 and 8. Results related to pain measurement are provided in .
Table 4. Pain evaluation across studies.
The last time-point of pain assessment largely varied across studies and ranged between 1 week and 6 months. Only, Yang et al. [Citation18] reported a follow-up >6 months in all cases (range 7–15 months; mean 7.8 months). Despite such discrepancy of time intervals at which pain was evaluated, 5/8 studies reported a highly effective analgesia (mean pain reduction ranging between 4 and 5.76); 2/8 studies reported a moderate analgesia (mean reported pain reduction 3.3 and 3.8, respectively). Such measurements were not available in Yang et al. [Citation18].
Six studies out of 8 provided a statistical analysis between mean baseline pain and mean pain assessed at different intermediate time-points: for each considered time-point and included study, a statistically significant drop of pain was noticed. Interestingly, in the two studies reporting results separately for different groups of treatment, at each time interval, the group receiving RFA + cement augmentation had better pain scores compared to RFA alone. In particular, Nakatsuka et al. [Citation14] reported a drop in mean pain score of 2.6 for the RFA group vs. 4.9 for the RFA + cement augmentation at 1 week. Proschek et al. [Citation15] reported a drop in mean pain score of 2.4 and 2.6 for the RFA group vs. 3.9 and 4 for the RFA + cement augmentation one, at 6 h and at the last follow-up available, respectively. However, in both studies, there were no statistically significant differences between the RFA and the RFA + cement augmentation since RFA alone was able to achieve a statistically relevant pain relief compared to baseline.
The three series combining radiation therapy to RFA and cement augmentation in most of the included patients (43%, 50% and 100%) were those reporting the best absolute pain drop (ranging between 4.9 and 5.76) at their last follow-up [Citation10,Citation14,Citation17]. Similarly, Bagla et al. [Citation8] reported a significant pain relief (pre-treatment: 5.5; 90 days; 2.1, p < 0.01) in a sub-group of 16 patients (32%) who had received radiation therapy prior to RFA.
Modification of pain medication intake following RFA was reported by two studies: Greenwood et al. [Citation17] noticed 62% of patients reducing their intake, 19% increasing it and 19% not changing it compared to baseline. Similarly, Anchala et al. [Citation10] reported that 54% of patients reduced their intake, 16% increased it and 30% kept it stable at the 4-week follow-up.
Safety
No patient reported grade IV–V complications. Grade I–IIIa complications were reported in up to 16% of the cases. In the series by Nakatsuka et al. [Citation14], 1 out of 10 patients reported a grade II complication consistent with a transient neural damage related to the high temperature raise during RFA; such complication completely resolved 2 days after the procedure with intravenous administration of steroids. On the other hand, Yang et al. [Citation18] reported side effects related to RFA in 4 out of 25 patients (16%). Of these, two experienced contralateral lower limb pain and numbness during the procedure; however, such symptomatology spontaneously disappeared with temperature decrease after RFA (grade I complication). The remaining 2 patients needed high doses of corticosteroids; one of them experiencing heaviness in the legs 1 day after RFA without any subsequent consequence at 1-week follow-up (grade II–IIIa complications).
In the end, Georgy et al. [Citation16] reported PMMA leakage outside the vertebral body in 70.5% of the cases; however, in only 2.7% of them, such leakage resulted in a radicular pain needing selective nerve block (grade IIIa complication).
Quality of life and other variables
Quality of life was assessed in two studies. Bagla et al. [Citation8] used two cancer-specific health-related quality-of-life instruments (the abbreviated form and the bone specific form of the Functional Assessment of Cancer Therapy-General; FACT-G7 and FACT-BP, respectively). Additionally, in the same study, the Modified Oswestry Disability Index (MODI) was used to assess back-related disability. The mean FACT-G7 score improved from 11.0 at baseline to 16.2 at 3 months (p < 0.0001); the mean FACT-BP score improved from 22.6 at baseline to 38.9 at 3 months (p < 0.0001); in the end, the mean MODI passed from 52.9 at baseline to 37.0 at 3 months (p < 0.01). Similarly, Proschek et al. [Citation15] applied the Oswestry Disability Index (ODI) to assess quality of life in both groups of the study (RFA alone and RFA + cement augmentation). In the first group, after treatment, the mean ODI score improved significantly to 34% (range 28–38%; p = 0.014) and this result was almost unchanged three to six months later (p = 0.06). Similarly, the mean ODI significantly improved in the second group (36%; range 31–39%; p = 0.003). However, 15–36 months after treatment, quality of life showed only minor differences compared to the results obtained after treatment (mean 35%; range 26–38%; p = 0.071).
Three studies evaluated also the RFA ability to achieve local tumour control. The Anchala et al. [Citation10] series reported a locally stable or improved disease in 76.9% of the cases on average 92 days after RFA. On the contrary, local progressive disease was noted in the remaining 23.1%, 82 days (on average) following RFA. Greenwood et al. [Citation17] noticed a locally stable disease in 12 out of 13 (92.3%) patients at 3 months, and in 10 out of 10 (100%) at 6 months, despite systemic disease progression. In the end, Yang et al. [Citation18] reported that along a 2-year follow-up, 66.67% of patients did not present tumour progression.
Discussion
Despite the large amount of screened papers, following the application of the inclusion criteria, only eight studies entered the final analysis. Such paucity of papers confirms the lack of experience on a large scale for RFA applied to treat painful spinal metastasis. Nevertheless, the majority of the included studies (7/8) reported moderate to significant results in terms of analgesia at the last available time-point. Moreover, when intermediate time-points of assessment were considered, RFA always resulted in a statistically significant pain relief compared to baseline. The last time-point for pain assessment largely varied across studies and hardly passed the 6-month follow-up. Therefore, at the moment, it is possible to state that RFA is likely to provide effective short- and mid-term (≤6 months) pain relief. Studies with longer follow-ups are desirable in order to investigate the long-term analgesic properties of RFA even though it may be difficult to assess pain at longer time-points due to the generally short life expectancy of the multi-metastatic population.
Since the 90s, radiation therapy has been the gold-standard for pain management of bone metastasis although reported results are suboptimal. In fact, up to 30% patients do no benefit at all from the treatment [Citation4]; the mean time to response is around 3 weeks and up to 50% patients getting partial or complete pain relief following radiation therapy are likely to present with recurring pain 20–24 weeks following the end of the treatment [Citation5]. These results are slightly better when stereotactic body radiotherapy is applied, even though only 50% of treated patients get a complete pain relief [Citation20]. Accordingly, many patients may potentially benefit from percutaneous treatments after failure of radiation therapy. Although direct comparative studies between percutaneous treatments and radiation therapy are substantially lacking [Citation21], some studies proved that when percutaneous treatments are coupled to radiation therapy for the treatment of painful bone metastasis, high rates of pain relief are achieved compared to radiation therapy alone [Citation22,Citation23]. In the present literature analysis, there were no comparative studies among RFA and RFA plus radiation therapy. Nevertheless, the three series combining radiation therapy to RFA and vertebral augmentation in most of their patients were the ones reporting the best absolute pain drop at the last time-point available [Citation10,Citation14,Citation17] thus confirming the synergic effect of percutaneous treatments to radiation therapy.
Another significant advantage of the combined strategy (RFA + vertebral augmentation) compared to radiation therapy alone is that during the same interventional session, vertebral augmentation can be easily achieved through the same coaxial working cannula used to deploy the ablation devices. In fact, it is well known that a significant risk of pathologic fractures exists in weight-bearing bones infiltrated by tumoral deposits [Citation21,Citation24,Citation25], and that additional energy deposition in the form of radiation therapy or thermal ablation may significantly increase such risk [Citation20,Citation26]. For these reasons, consolidation is highly desirable following ablation, and cement (i.e. poly-methyl-methacrylate) is highly adapted for such goal due to its high resistance to compression stresses acting on a vertebral body [Citation27,Citation28]. Such idea is well represented in all the studies included in the present literature review. Most of the patients (40–100%) or treated vertebrae (94–95.8%) received vertebral augmentation following RFA; and, interestingly, among the 4 patients not receiving vertebral augmentation in Anchala et al. [Citation10], two presented with a secondary bone insufficiency fracture on the target level thus, requiring subsequent cement augmentation. Nevertheless, due to the lack of dedicated studies, it is not clear what is the real advantage of combining ablation and vertebral augmentation as compared to vertebral augmentation alone, which had already proved to be highly effective in achieving both pain relief and consolidation in cases of vertebral body lytic disease [Citation27], even when epidural involvement is noted [Citation29]. One possible indication for ablation therapies without augmentation could be the treatment of mixed/sclerotic vertebral metastasis since in such cases, cement injection alone has no or limited benefit (). Moreover, according to the Cardiovascular and Interventional Radiological Society of Europe, quality improvement guidelines for bone tumour management [Citation30], ablation should be proposed in case of:
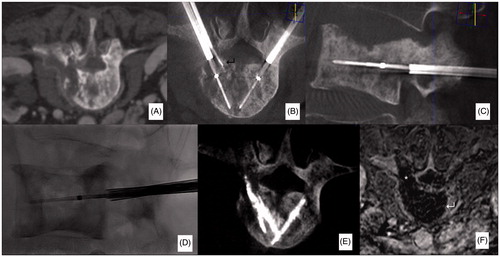
Figure 1. Case from authors’ personal series. Sixty-year-old man affected by a multi-metastatic lung cancer. The patient was referred for local treatment of a painful mixed metastasis of L3 (A). Bipolar RFA was performed with a bi-pedicular approach under CBCT-guidance (B–D). Ablation was conducted following the deployment of a thermometer at the level of the posterior wall to monitor temperature rise at this level (↵). Following RFA, due to the mixed aspect of the metastasis, the quantity of cement injected was minimal and limited to fill the bone trocars pathways. Contrast-enhancement MRI obtained 48 h following RFA shows a large necrotic area roughly reproducing the shape of the vertebral body (↵); a necrotic area is also noted in the left pedicle since track-ablation was performed on this side (*, F).
Oligometastatic patients with few (<3) small (<3 cm) metastasis for complete tumour eradication;
Metastasis extending to the nearby soft tissues or to the canal.
These indications can also be applied in the setting of the metastatic spine. However, due to the particular configuration of the spine, it should be noted that in case of curative treatments, the threshold size of the target lesion should probably be reduced to <2 cm. In addition, extension outside the vertebral body and most of all, metastatic epidural involvement highly impairs local tumour control (). When local tumour control was investigated in the two studies included in this literature analysis, a high rate of success (76.9%–92.3%) was observed at 3-months follow-up. Although these results were obtained in only two studies including few patients and with short follow-ups, a potential curative role of ablation in selected oligometastatic patients is suggested. Further dedicated studies are definitively needed to corroborate these results.
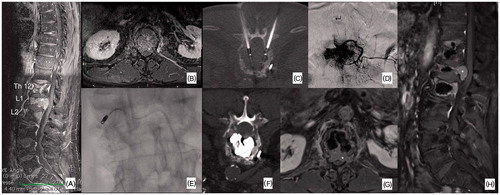
Figure 2. Case from authors’ personal series. Seventy-year-old man affected by a multi-metastatic hepatocellular carcinoma. The patient was referred for local treatment of painful lytic metastases of T12, L1 and L2 (A). Clinical and radiological evaluation revealed a more important involvement of L1 where epidural disease extension was noted (*, B); L1 was also the most painful level. Accordingly, bipolar RFA was proposed to treat L1 (C). Due to the hyper-vascular nature of the metastasis, in order to maximise the RFA effect, an embolisation was performed just before the ablation (D, E) in order to reduce the “heat-sink effect”; nevertheless, due to the origin of the anterior spinal artery (↵) at the L1 level, only a partial unilateral embolisation was performed (E). All the 3 vertebral bodies underwent subsequent vertebral augmentation (F). Contrast-enhancement MRI obtained at 3-months follow-up showed epidural disease progression on L1 (*).
Regarding the RFA technology, bipolar systems were used in half of the included studies. Such choice is justified by the fact that highly controllable ablation areas are obtained with this technology since the electrical current strictly flows between two dipoles located at the distal tip of the electrode. Therefore, high levels of safety are granted in a delicate environment such as the spine. This perspective was confirmed in the present literature analysis since no neural damage was reported in studies applying bipolar RFA. Nevertheless, it should be noted that, the safety profile of RFA (mono- and bipolar systems altogether) is overall high since major complications were not reported in the included studies. On the other hand, minor neural complications were reported in up to 16% of the cases in one series not specifying the type of RFA technology applied [Citation18]. However, such adverse events resolved always spontaneously or with steroids. From a technical standpoint, the safety profile of spinal ablation may be still increased if extensive protective measures such as thermometers and hydro- or carbo-dissection are applied [Citation31]. These manoeuvres aim at keeping the anterior epidural space and/or foramina within physiological temperature intervals (i.e. between 10° and 44 °C) [Citation32]. For these reasons, a combination of CT/fluoroscopy- or CBCT-guidance should be preferred over the sole fluoroscopy guidance to facilitate the application of such manoeuvres. In the end, when RFA is coupled to cement augmentation, the risk of complication is not substantially increased since, although the risk of cement leakage may be high, it rarely results into a clinically significant event.
Although the present study reported encouraging results, some limitations should be highlighted and are mainly related to the fact that the included studies were few, non-randomised and applied similar but not identical treatment strategies in small populations.
In conclusion, RFA, often in combination with vertebral augmentation, is effective and safe in achieving short- to mid-term (from 1 week to 6 months) analgesia in patients with painful spinal metastasis. When radiation therapy is added to RFA and vertebral augmentation, a synergic effect is achieved with a significant benefit in terms of pain drop. Further prospective randomised trials are needed to prove the long-term efficacy of RFA especially in comparison/combination with radiation therapy.
Disclosure statement
Authors Roberto Luigi Cazzato and Julien Garnon are proctors for Medtronic; Authors Julien Garnon and Afshin Gangi are Proctors for Galil Medical.
References
- Delank KS, Wendtner C, Eich HT, et al. (2011). The treatment of spinal metastases. Dtsch Arztebl Int 108:71–80.
- Sutcliffe P, Connock M, Shyangdan D, et al. (2013). A systematic review of evidence on malignant spinal metastases: natural history and technologies for identifying patients at high risk of vertebral fracture and spinal cord compression. Health Technol Assess 17:1–274.
- Harel R, Angelov L. (2010). Spine metastases: current treatments and future directions. Eur J Cancer 46:2696–707.
- Lutz S, Balboni T, Jones J, et al. (2017). Palliative radiation therapy for bone metastases: update of an ASTRO Evidence-Based Guideline. Pract Radiat Oncol 7:4–12.
- Steenland E, Leer JW, van Houwelingen H, et al. (1999). The effect of a single fraction compared to multiple fractions on painful bone metastases: a global analysis of the Dutch bone metastasis study. Radiother Oncol 52:101–9.
- Dupuy DE, Liu D, Hartfeil D, et al. (2010). Percutaneous radiofrequency ablation of painful osseous metastases: a multicenter American college of radiology imaging network trial. Cancer 116:989–97.
- Callstrom MR, Dupuy DE, Solomon SB, et al. (2013). Percutaneous image-guided cryoablation of painful metastases involving bone: multicenter trial. Cancer 119:1033–41.
- Bagla S, Sayed D, Smirniotopoulos J, et al. (2016). Multicenter prospective clinical series evaluating radiofrequency ablation in the treatment of painful spine metastases. Cardiovasc Intervent Radiol 39:1289–97.
- Wallace AN, Greenwood TJ, Jennings JW. (2015). Radiofrequency ablation and vertebral augmentation for palliation of painful spinal metastases. J Neurooncol 124:111–18.
- Anchala PR, Irving WD, Hillen TJ, et al. (2014). Treatment of metastatic spinal lesions with a navigational bipolar radiofrequency ablation device: a multicenter retrospective study. Pain Physician 17:317–27.
- Higgins JPT, Green S, Cochrane handbook for systematic reviews of interventions version 5.1.0 (updated 2011). In: Cochrane Collab. Available from: www.cochrane-www.handbook.org.
- Centre for Reviews and Dissemination. (2009). Systematic reviews: CRD’s guidance for undertaking reviews in healthcare. Available from: https://www.york.ac.uk/media/crd/Systematic_Reviews.pdf.
- Clavien PA, Barkun J, de Oliveira ML, et al. (2009). The Clavien-Dindo classification of surgical complications: five-year experience. Ann Surg 250:187–96.
- Nakatsuka A, Yamakado K, Takaki H, et al. (2009). Percutaneous radiofrequency ablation of painful spinal tumors adjacent to the spinal cord with real-time monitoring of spinal canal temperature: a prospective study. Cardiovasc Intervent Radiol 32:70–5.
- Proschek D, Kurth A, Proschek P, et al. (2009). Prospective pilot-study of combined bipolar radiofrequency ablation and application of bone cement in bone metastases. Anticancer Res 29:2787–92.
- Georgy BA. (2009). Bone cement deposition patterns with plasma-mediated radio-frequency ablation and cement augmentation for advanced metastatic spine lesions. Am J Neuroradiol 30:1197–202.
- Greenwood TJ, Wallace A, Friedman MV, et al. (2015). Combined ablation and radiation therapy of spinal metastases: a novel multimodality treatment approach. Pain Physician 18:573–81.
- Yang PL, He XJ, Li HP, et al. (2017). Image-guided minimally invasive percutaneous treatment of spinal metastasis. Exp Ther Med 13:705–9.
- Grönemeyer DH, Schirp S, Gevargez A. (2002). Image-guided radiofrequency ablation of spinal tumors: preliminary experience with an expandable array electrode. Cancer J 8:33–9.
- Husain ZA, Sahgal A, De Salles A, et al. (2017). Stereotactic body radiotherapy for de novo spinal metastases: systematic review. J Neurosurg Spine 27:295–302.
- Cazzato RL, Buy X, Grasso RF, et al. (2015). Interventional Radiologist’s perspective on the management of bone metastatic disease. Eur J Surg Oncol 41:967–74.
- Di Staso M, Zugaro L, Gravina GL, et al. (2011). A feasibility study of percutaneous radiofrequency ablation followed by radiotherapy in the management of painful osteolytic bone metastases. Eur Radiol 21:2004–10.
- Di Staso M, Gravina GL, Zugaro L, et al. (2015). Treatment of solitary painful osseous metastases with radiotherapy, cryoablation or combined therapy: propensity matching analysis in 175 patients. PLoS One 10:e0129021
- Cazzato RL, Garnon J, Tsoumakidou G, et al. (2017). Percutaneous image-guided screws mediated osteosnthesis of impending and pathological/insufficiency fractures of the femoral neck in non-surgical cancer patients. Eur J Radiol 90:1–5.
- Cazzato RL, Koch G, Buy X, et al. (2016). Percutaneous image-guided screw fixation of bone lesions in cancer patients: double-centre analysis of outcomes including local evolution of the treated focus. Cardiovasc Intervent Radiol 39:1455–63.
- Tsoumakidou G, Borensztein M, Zini C, et al. (2014). Postablation insufficiency fracture of the iliac crest: management by percutaneous screw fixation. Cardiovasc Intervent Radiol 37:1126–8.
- Tsoumakidou G, Too CW, Koch G, et al. (2017). CIRSE Guidelines on Percutaneous Vertebral Augmentation. Cardiovasc Intervent Radiol 40:331–42.
- Belkoff SM, Mathis JM, Jasper LE, et al. (2001). The biomechanics of vertebroplasty. The effect of cement volume on mechanical behavior. Spine (Phila Pa 1976) 26:1537–41.
- Saliou G, Kocheida El M, Lehmann P, et al. (2010). Percutaneous vertebroplasty for pain management in malignant fractures of the spine with epidural involvement. Radiology 254:882–90.
- Gangi A, Tsoumakidou G, Buy X, et al. (2010). Quality improvement guidelines for bone tumour management. Cardiovasc Intervent Radiol 33:706–13.
- Tsoumakidou G, Buy X, Garnon J, et al. (2011). Percutaneous thermal ablation: how to protect the surrounding organs. Tech Vasc Interv Radiol 14:170–6.
- Tsoumakidou G, Koch G, Caudrelier J, et al. (2016). Image-guided spinal ablation: a review. Cardiovasc Intervent Radiol 39:1.
